Adoption of Total Neoadjuvant Therapy in the Treatment of Locally Advanced Rectal Cancer
Abstract
1. Introduction
2. Historical Approaches to LARC
2.1. Moving Radiation to the Neoadjuvant Setting
2.2. Role of Neoadjuvant Chemoradiation
2.3. Timing of Neoadjuvant Radiation in Relation to Surgery Matters
2.4. Adjuvant Chemotherapy following Neoadjuvant Chemoradiation
2.5. Role of Oxaliplatin as Concurrent Chemotherapy with Radiation
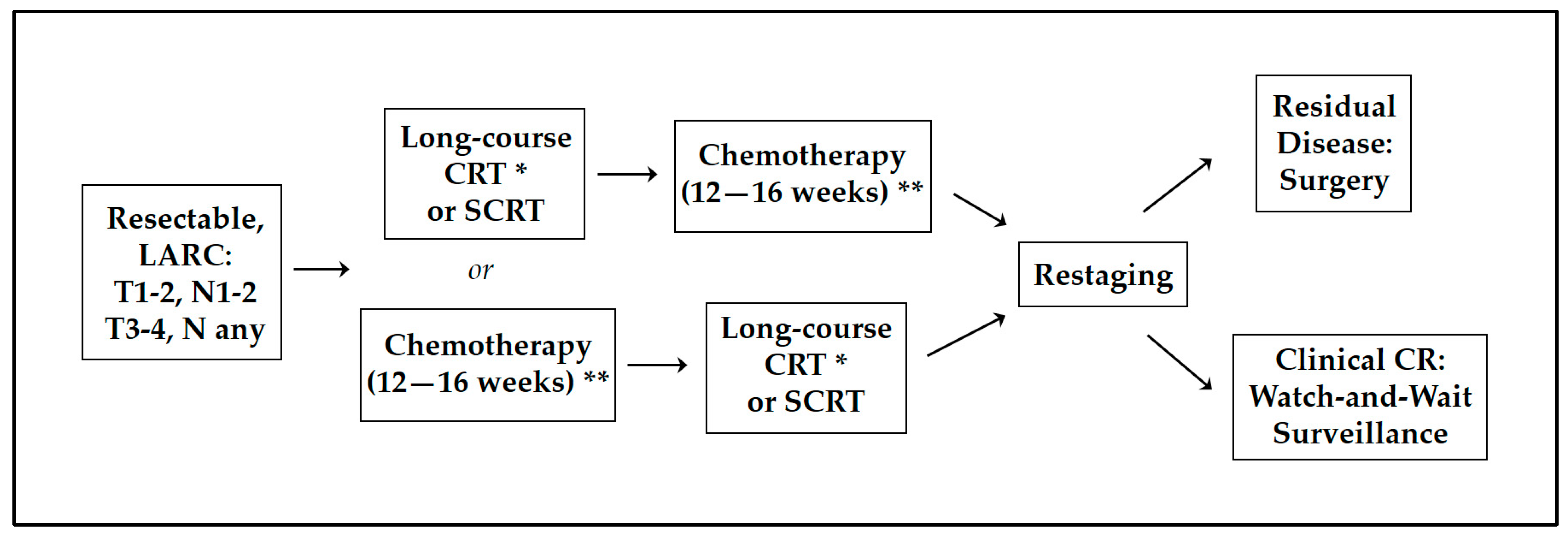
3. Total Neoadjuvant Therapy: New Standard of Care for LARC
3.1. Addressing Locoregional Failure and Systemic Relapse in LARC
3.2. Neoadjuvant Induction vs. Consolidation Chemotherapy
3.3. Chemotherapy Completion Improves with TNT
4. Watch-and-Wait Method
4.1. Morbidity of Surgery
4.2. Organ Preservation
5. Organ Preservation following TNT
6. TNT Is Not for Everyone
6.1. Mismatch-Repair Deficiency
6.2. Morbidity of Radiation
6.3. Intermediate-Risk LARC
7. Role of Additional Agents in TNT
8. Rise in Early Onset Rectal Cancer
9. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef]
- Bernstein, T.E.; Endreseth, B.H.; Romundstad, P.; Wibe, A.; Norwegian Colorectal Cancer, G. Circumferential resection margin as a prognostic factor in rectal cancer. Br. J. Surg. 2009, 96, 1348–1357. [Google Scholar] [CrossRef] [PubMed]
- Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 29–42. [Google Scholar] [CrossRef]
- Conroy, T.; Bosset, J.F.; Etienne, P.L.; Rio, E.; Francois, E.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouche, O.; Gargot, D.; et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Etienne, P.-L.; Rio, E.; Evesque, L.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouche, O.; Boileve, A.; Delaye, M.; et al. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: 7-year results of PRODIGE 23 phase III trial, a UNICANCER GI trial. JCO 2023, 41, LBA3504. [Google Scholar] [CrossRef]
- Dijkstra, E.A.; Nilsson, P.J.; Hospers, G.A.P.; Bahadoer, R.R.; Meershoek-Klein Kranenbarg, E.; Roodvoets, A.G.H.; Putter, H.; Berglund, A.; Cervantes, A.; Crolla, R.; et al. Locoregional Failure During and After Short-course Radiotherapy followed by Chemotherapy and Surgery Compared to Long-course Chemoradiotherapy and Surgery—A Five-year Follow-up of the RAPIDO Trial. Ann. Surg. 2023, 278, e766–e772. [Google Scholar] [CrossRef]
- Fisher, B.; Wolmark, N.; Rockette, H.; Redmond, C.; Deutsch, M.; Wickerham, D.L.; Fisher, E.R.; Caplan, R.; Jones, J.; Lerner, H.; et al. Postoperative Adjuvant Chemotherapy or Radiation Therapy for Rectal Cancer: Results From NSABP Protocol R-011. J. Natl. Cancer Inst. 1988, 80, 21–29. [Google Scholar] [CrossRef]
- Wolmark, N. Randomized Trial of Postoperative Adjuvant Chemotherapy with or without Radiotherapy for Carcinoma of the Rectum: National Surgical Adjuvant Breast and Bowel Project Protocol R-02. J. Natl. Cancer Inst. 2000, 92, 388–396. [Google Scholar] [CrossRef]
- Frykholm, G.J.; Glimelius, B.; Pahlman, L. Preoperative or postoperative irradiation in adenocarcinoma of the rectum: Final treatment results of a randomized trial and an evaluation of late secondary effects. Dis. Colon Rectum 1993, 36, 564–572. [Google Scholar] [CrossRef]
- Sebag-Montefiore, D.; Stephens, R.J.; Steele, R.; Monson, J.; Grieve, R.; Khanna, S.; Quirke, P.; Couture, J.; de Metz, C.; Myint, A.S.; et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): A multicentre, randomised trial. Lancet 2009, 373, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Folkesson, J.; Birgisson, H.; Pahlman, L.; Cedermark, B.; Glimelius, B.; Gunnarsson, U. Swedish Rectal Cancer Trial: Long lasting benefits from radiotherapy on survival and local recurrence rate. J. Clin. Oncol. 2005, 23, 5644–5650. [Google Scholar] [CrossRef] [PubMed]
- Swedish Rectal Cancer, T.; Cedermark, B.; Dahlberg, M.; Glimelius, B.; Pahlman, L.; Rutqvist, L.E.; Wilking, N. Improved survival with preoperative radiotherapy in resectable rectal cancer. N. Engl. J. Med. 1997, 336, 980–987. [Google Scholar] [CrossRef]
- Kapiteijn, E.; Marijnen, C.A.; Nagtegaal, I.D.; Putter, H.; Steup, W.H.; Wiggers, T.; Rutten, H.J.; Pahlman, L.; Glimelius, B.; van Krieken, J.H.; et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N. Engl. J. Med. 2001, 345, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Peeters, K.C.; Marijnen, C.A.; Nagtegaal, I.D.; Kranenbarg, E.K.; Putter, H.; Wiggers, T.; Rutten, H.; Pahlman, L.; Glimelius, B.; Leer, J.W.; et al. The TME trial after a median follow-up of 6 years: Increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann. Surg. 2007, 246, 693–701. [Google Scholar] [CrossRef] [PubMed]
- van Gijn, W.; Marijnen, C.A.; Nagtegaal, I.D.; Kranenbarg, E.M.; Putter, H.; Wiggers, T.; Rutten, H.J.; Pahlman, L.; Glimelius, B.; van de Velde, C.J.; et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011, 12, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Gerard, J.P.; Conroy, T.; Bonnetain, F.; Bouche, O.; Chapet, O.; Closon-Dejardin, M.T.; Untereiner, M.; Leduc, B.; Francois, E.; Maurel, J.; et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: Results of FFCD 9203. J. Clin. Oncol. 2006, 24, 4620–4625. [Google Scholar] [CrossRef]
- Sauer, R.; Liersch, T.; Merkel, S.; Fietkau, R.; Hohenberger, W.; Hess, C.; Becker, H.; Raab, H.R.; Villanueva, M.T.; Witzigmann, H.; et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J. Clin. Oncol. 2012, 30, 1926–1933. [Google Scholar] [CrossRef]
- Akgun, E.; Ozkok, S.; Tekin, M.; Yoldas, T.; Caliskan, C.; Kose, T.; Karabulut, B.; Sezak, M.; Elmas, N.; Ozutemiz, O. The effects of chemoradiotherapy on recurrence and survival in locally advanced rectal cancers with curative total mesorectal excision: A prospective, nonrandomized study. World J. Surg. Oncol. 2017, 15, 205. [Google Scholar] [CrossRef]
- Roh, M.S.; Colangelo, L.H.; O’Connell, M.J.; Yothers, G.; Deutsch, M.; Allegra, C.J.; Kahlenberg, M.S.; Baez-Diaz, L.; Urisny, C.S.; Petrilli, N.J.; et al. Preoperative Multimodality Therapy Improves Disease-Free Survival in Patients with Carcinoma of the Rectum: NSABP R-03. J. Clin. Oncol. 2009, 27, 5124–5130. [Google Scholar] [CrossRef]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rodel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Habr-Gama, A.; Perez, R.O.; Sao Juliao, G.P.; Proscurshim, I.; Fernandez, L.M.; Figueiredo, M.N.; Gama-Rodrigues, J.; Buchpiguel, C.A. Consolidation chemotherapy during neoadjuvant chemoradiation (CRT) for distal rectal cancer leads to sustained decrease in tumor metabolism when compared to standard CRT regimen. Radiat. Oncol. 2016, 11, 24. [Google Scholar] [CrossRef]
- Erlandsson, J.; Lorinc, E.; Ahlberg, M.; Pettersson, D.; Holm, T.; Glimelius, B.; Martling, A. Tumour regression after radiotherapy for rectal cancer—Results from the randomised Stockholm III trial. Radiother. Oncol. 2019, 135, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Erlandsson, J.; Holm, T.; Pettersson, D.; Berglund, A.; Cedermark, B.; Radu, C.; Johansson, H.; Machado, M.; Hjern, F.; Hallbook, O.; et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): A multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017, 18, 336–346. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Azad, N.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. Rectal Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 1139–1167. [Google Scholar] [CrossRef] [PubMed]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv22–iv40. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.S.; Kim, S.Y.; Lee, J.S.; Nam, B.H.; Kim, K.P.; Kim, J.E.; Park, Y.S.; Park, J.O.; Baek, J.Y.; Kim, T.Y.; et al. Oxaliplatin-Based Adjuvant Chemotherapy for Rectal Cancer After Preoperative Chemoradiotherapy (ADORE): Long-Term Results of a Randomized Controlled Trial. J. Clin. Oncol. 2019, 37, 3111–3123. [Google Scholar] [CrossRef]
- Breugom, A.J.; van Gijn, W.; Muller, E.W.; Berglund, A.; van den Broek, C.B.M.; Fokstuen, T.; Gelderblom, H.; Kapiteijn, E.; Leer, J.W.H.; Marijnen, C.A.M.; et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: A Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann. Oncol. 2015, 26, 696–701. [Google Scholar] [CrossRef]
- Breugom, A.J.; Swets, M.; Bosset, J.F.; Collette, L.; Sainato, A.; Cionini, L.; Glynne-Jones, R.; Counsell, N.; Bastiaannet, E.; van den Broek, C.B.; et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: A systematic review and meta-analysis of individual patient data. Lancet Oncol. 2015, 16, 200–207. [Google Scholar] [CrossRef]
- Carvalho, C.; Glynne-Jones, R. Challenges behind proving efficacy of adjuvant chemotherapy after preoperative chemoradiation for rectal cancer. Lancet Oncol. 2017, 18, e354–e363. [Google Scholar] [CrossRef]
- Aschele, C.; Cionini, L.; Lonardi, S.; Pinto, C.; Cordio, S.; Rosati, G.; Artale, S.; Tagliagambe, A.; Ambrosini, G.; Rosetti, P.; et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: Pathologic results of the STAR-01 randomized phase III trial. J. Clin. Oncol. 2011, 29, 2773–2780. [Google Scholar] [CrossRef] [PubMed]
- Gerard, J.P.; Azria, D.; Gourgou-Bourgade, S.; Martel-Lafay, I.; Hennequin, C.; Etienne, P.L.; Vendrely, V.; Francois, E.; de La Roche, G.; Bouche, O.; et al. Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J. Clin. Oncol. 2012, 30, 4558–4565. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.J.; Colangelo, L.H.; Beart, R.W.; Petrelli, N.J.; Allegra, C.J.; Sharif, S.; Pitot, H.C.; Shields, A.F.; Landry, J.C.; Ryan, D.P.; et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: Surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J. Clin. Oncol. 2014, 32, 1927–1934. [Google Scholar] [CrossRef] [PubMed]
- Allegra, C.J.; Yothers, G.; O’Connell, M.J.; Beart, R.W.; Wozniak, T.F.; Pitot, H.C.; Shields, A.F.; Landry, J.C.; Ryan, D.P.; Arora, A.; et al. Neoadjuvant 5-FU or Capecitabine Plus Radiation with or without Oxaliplatin in Rectal Cancer Patients: A Phase III Randomized Clinical Trial. J. Natl. Cancer Inst. 2015, 107, djv248. [Google Scholar] [CrossRef]
- Deng, Y.; Chi, P.; Lan, P.; Wang, L.; Chen, W.; Cui, L.; Chen, D.; Cao, J.; Wei, H.; Peng, X.; et al. Neoadjuvant Modified FOLFOX6 with or without Radiation Versus Fluorouracil Plus Radiation for Locally Advanced Rectal Cancer: Final Results of the Chinese FOWARC Trial. J. Clin. Oncol. 2019, 37, 3223–3233. [Google Scholar] [CrossRef]
- Zhang, J.; Chi, P.; Lan, P.; Cui, L.; Wei, H.; Zhao, R.; Huang, Z.; Zhang, H.; Cai, Y.; Wang, J.; et al. Long-term outcome of neoadjuvant mFOLFOX6 with or without radiation versus fluorouracil plus radiation for locally advanced rectal cancer: A multicenter, randomized phase III trial. JCO 2023, 41, 3505. [Google Scholar] [CrossRef]
- Jiao, D.; Zhang, R.; Gong, Z.; Liu, F.; Chen, Y.; Yu, Q.; Sun, L.; Duan, H.; Zhu, S.; Liu, F.; et al. Fluorouracil-based preoperative chemoradiotherapy with or without oxaliplatin for stage II/III rectal cancer: A 3-year follow-up study. Chin. J. Cancer Res. 2015, 27, 588–596. [Google Scholar] [CrossRef]
- Schmoll, H.J.; Stein, A.; Van Cutsem, E.; Price, T.; Hofheinz, R.D.; Nordlinger, B.; Daisne, J.F.; Janssens, J.; Brenner, B.; Reinel, H.; et al. Pre- and Postoperative Capecitabine without or with Oxaliplatin in Locally Advanced Rectal Cancer: PETACC 6 Trial by EORTC GITCG and ROG, AIO, AGITG, BGDO, and FFCD. J. Clin. Oncol. 2021, 39, 17–29. [Google Scholar] [CrossRef]
- Rodel, C.; Graeven, U.; Fietkau, R.; Hohenberger, W.; Hothorn, T.; Arnold, D.; Hofheinz, R.D.; Ghadimi, M.; Wolff, H.A.; Lang-Welzenbach, M.; et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): Final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015, 16, 979–989. [Google Scholar] [CrossRef]
- Diefenhardt, M.; Ludmir, E.B.; Hofheinz, R.-D.; Ghadimi, M.; Minsky, B.D.; Rödel, C.; Fokas, E. Association of Treatment Adherence with Oncologic Outcomes for Patients with Rectal Cancer: A Post Hoc Analysis of the CAO/ARO/AIO-04 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1416. [Google Scholar] [CrossRef]
- Cisel, B.; Pietrzak, L.; Michalski, W.; Wyrwicz, L.; Rutkowski, A.; Kosakowska, E.; Cencelewicz, A.; Spalek, M.; Polkowski, W.; Jankiewicz, M.; et al. Long-course preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for clinical T4 and fixed clinical T3 rectal cancer: Long-term results of the randomized Polish II study. Ann. Oncol. 2019, 30, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Akiyoshi, T.; Fukunaga, Y.; Sakamoto, T.; Mukai, T.; Hiyoshi, Y.; Nagasaki, T.; Taguchi, S.; Chino, A.; Shinozaki, E.; et al. Adding Induction Chemotherapy Before Chemoradiotherapy with Total Mesorectal Excision and Selective Lateral Lymph Node Dissection for Patients with Poor-Risk, Locally Advanced, Mid-to-Low Rectal Cancer May Improve Oncologic Outcomes: A Propensity Score-Matched Analysis. Ann. Surg. Oncol. 2023, 30, 4716–4724. [Google Scholar] [CrossRef] [PubMed]
- Verheij, F.S.; Omer, D.M.R.; Williams, H.; Buckley, J.T.; Lin, S.T.; Qin, L.-X.; Thompson, H.M.; Yuval, J.B.; Gollub, M.J.; Wu, A.J.-C.; et al. Sustained organ preservation in patients with rectal cancer treated with total neoadjuvant therapy: Long-term results of the OPRA trial. J. Clin. Oncol. 2023, 41, 3520. [Google Scholar] [CrossRef]
- Jin, J.; Tang, Y.; Hu, C.; Jiang, L.M.; Jiang, J.; Li, N.; Liu, W.Y.; Chen, S.L.; Li, S.; Lu, N.N.; et al. Multicenter, Randomized, Phase III Trial of Short-Term Radiotherapy Plus Chemotherapy Versus Long-Term Chemoradiotherapy in Locally Advanced Rectal Cancer (STELLAR). J. Clin. Oncol. 2022, 40, 1681–1692. [Google Scholar] [CrossRef]
- Voogt, E.L.K.; Schaap, D.P.; van den Berg, K.; Nieuwenhuijzen, G.A.P.; Bloemen, J.G.; Creemers, G.J.; Willems, J.; Cnossen, J.S.; Peulen, H.M.U.; Nederend, J.; et al. Improved response rate in patients with prognostically poor locally advanced rectal cancer after treatment with induction chemotherapy and chemoradiotherapy when compared with chemoradiotherapy alone: A matched case-control study. Eur. J. Surg. Oncol. 2021, 47, 2429–2435. [Google Scholar] [CrossRef]
- Cercek, A.; Roxburgh, C.S.D.; Strombom, P.; Smith, J.J.; Temple, L.K.F.; Nash, G.M.; Guillem, J.G.; Paty, P.B.; Yaeger, R.; Stadler, Z.K.; et al. Adoption of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer. JAMA Oncol. 2018, 4, e180071. [Google Scholar] [CrossRef]
- Fernandez-Martos, C.; Pericay, C.; Aparicio, J.; Salud, A.; Safont, M.; Massuti, B.; Vera, R.; Escudero, P.; Maurel, J.; Marcuello, E.; et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J. Clin. Oncol. 2010, 28, 859–865. [Google Scholar] [CrossRef]
- Fernandez-Martos, C.; Garcia-Albeniz, X.; Pericay, C.; Maurel, J.; Aparicio, J.; Montagut, C.; Safont, M.J.; Salud, A.; Vera, R.; Massuti, B.; et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: Long-term results of the Spanish GCR-3 phase II randomized trialdagger. Ann. Oncol. 2015, 26, 1722–1728. [Google Scholar] [CrossRef]
- Diefenhardt, M.; Fleischmann, M.; Martin, D.; Hofheinz, R.-D.; Piso, P.; Germer, C.-T.; Hambsch, P.; Grützmann, R.; Kirste, S.; Schlenska-Lange, A.; et al. Clinical outcome after total neoadjuvant treatment (CAO/ARO/AIO-12) versus intensified neoadjuvant and adjuvant treatment (CAO/ARO/AIO-04) a comparison between two multicenter randomized phase II/III trials. Radiother. Oncol. 2023, 179, 109455. [Google Scholar] [CrossRef]
- Fokas, E.; Allgauer, M.; Polat, B.; Klautke, G.; Grabenbauer, G.G.; Fietkau, R.; Kuhnt, T.; Staib, L.; Brunner, T.; Grosu, A.L.; et al. Randomized Phase II Trial of Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: CAO/ARO/AIO-12. J. Clin. Oncol. 2019, 37, 3212–3222. [Google Scholar] [CrossRef]
- Fokas, E.; Schlenska-Lange, A.; Polat, B.; Klautke, G.; Grabenbauer, G.G.; Fietkau, R.; Kuhnt, T.; Staib, L.; Brunner, T.; Grosu, A.L.; et al. Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Patients with Locally Advanced Rectal Cancer: Long-term Results of the CAO/ARO/AIO-12 Randomized Clinical Trial. JAMA Oncol. 2022, 8, e215445. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Aguilar, J.; Patil, S.; Gollub, M.J.; Kim, J.K.; Yuval, J.B.; Thompson, H.M.; Verheij, F.S.; Omer, D.M.; Lee, M.; Dunne, R.F.; et al. Organ Preservation in Patients with Rectal Adenocarcinoma Treated with Total Neoadjuvant Therapy. J. Clin. Oncol. 2022, 40, 2546–2556. [Google Scholar] [CrossRef] [PubMed]
- Bosset, J.F.; Collette, L.; Calais, G.; Mineur, L.; Maingon, P.; Radosevic-Jelic, L.; Daban, A.; Bardet, E.; Beny, A.; Ollier, J.C.; et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N. Engl. J. Med. 2006, 355, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Perry, W.R.G.; Abd El Aziz, M.A.; Duchalais, E.; Grass, F.; Behm, K.T.; Mathis, K.L.; Kelley, S.R. Sexual dysfunction following surgery for rectal cancer: A single-institution experience. Updates Surg. 2021, 73, 2155–2159. [Google Scholar] [CrossRef] [PubMed]
- Celentano, V.; Cohen, R.; Warusavitarne, J.; Faiz, O.; Chand, M. Sexual dysfunction following rectal cancer surgery. Int. J. Colorectal Dis. 2017, 32, 1523–1530. [Google Scholar] [CrossRef]
- Hendren, S.K.; O’Connor, B.I.; Liu, M.; Asano, T.; Cohen, Z.; Swallow, C.J.; Macrae, H.M.; Gryfe, R.; McLeod, R.S. Prevalence of male and female sexual dysfunction is high following surgery for rectal cancer. Ann. Surg. 2005, 242, 212–223. [Google Scholar] [CrossRef]
- Da Silva, G.M.; Hull, T.; Roberts, P.L.; Ruiz, D.E.; Wexner, S.D.; Weiss, E.G.; Nogueras, J.J.; Daniel, N.; Bast, J.; Hammel, J.; et al. The Effect of Colorectal Surgery in Female Sexual Function, Body Image, Self-Esteem and General Health: A Prospective Study. Ann. Surg. 2008, 248, 266–272. [Google Scholar] [CrossRef]
- Lange, M.M.; Marijnen, C.A.; Maas, C.P.; Putter, H.; Rutten, H.J.; Stiggelbout, A.M.; Meershoek-Klein Kranenbarg, E.; van de Velde, C.J.; Cooperative clinical investigators of the Dutch. Risk factors for sexual dysfunction after rectal cancer treatment. Eur. J. Cancer 2009, 45, 1578–1588. [Google Scholar] [CrossRef]
- Lange, M.M.; Maas, C.P.; Marijnen, C.A.; Wiggers, T.; Rutten, H.J.; Kranenbarg, E.K.; van de Velde, C.J.H. Urinary dysfunction after rectal cancer treatment is mainly caused by surgery. Br. J. Surg. 2008, 95, 1020–1028. [Google Scholar] [CrossRef]
- Chen, T.Y.; Wiltink, L.M.; Nout, R.A.; Meershoek-Klein Kranenbarg, E.; Laurberg, S.; Marijnen, C.A.; van de Velde, C.J. Bowel function 14 years after preoperative short-course radiotherapy and total mesorectal excision for rectal cancer: Report of a multicenter randomized trial. Clin. Color. Cancer 2015, 14, 106–114. [Google Scholar] [CrossRef]
- Sturiale, A.; Martellucci, J.; Zurli, L.; Vaccaro, C.; Brusciano, L.; Limongelli, P.; Docimo, L.; Valeri, A. Long-term functional follow-up after anterior rectal resection for cancer. Int. J. Color. Dis. 2017, 32, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Atallah, S.; Martin-Perez, B.; Albert, M.; deBeche-Adams, T.; Nassif, G.; Hunter, L.; Larach, S. Transanal minimally invasive surgery for total mesorectal excision (TAMIS–TME): Results and experience with the first 20 patients undergoing curative-intent rectal cancer surgery at a single institution. Tech. Coloproctol. 2014, 18, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Paun, B.C.; Cassie, S.; MacLean, A.R.; Dixon, E.; Buie, W.D. Postoperative complications following surgery for rectal cancer. Ann. Surg. 2010, 251, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Swellengrebel, H.A.; Marijnen, C.A.; Verwaal, V.J.; Vincent, A.; Heuff, G.; Gerhards, M.F.; van Geloven, A.A.; van Tets, W.F.; Verheij, M.; Cats, A. Toxicity and complications of preoperative chemoradiotherapy for locally advanced rectal cancer. Br. J. Surg. 2011, 98, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Habr-Gama, A.; Perez, R.O.; Nadalin, W.; Sabbaga, J.; Ribeiro, U., Jr.; Silva e Sousa, A.H., Jr.; Campos, F.G.; Kiss, D.R.; Gama-Rodrigues, J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: Long-term results. Ann. Surg. 2004, 240, 711–717; discussion 717–718. [Google Scholar] [CrossRef] [PubMed]
- Habr-Gama, A.; Gama-Rodrigues, J.; Sao Juliao, G.P.; Proscurshim, I.; Sabbagh, C.; Lynn, P.B.; Perez, R.O. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: Impact of salvage therapy on local disease control. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 822–828. [Google Scholar] [CrossRef]
- van der Valk, M.J.M.; Hilling, D.E.; Bastiaannet, E.; Meershoek-Klein Kranenbarg, E.; Beets, G.L.; Figueiredo, N.L.; Habr-Gama, A.; Perez, R.O.; Renehan, A.G.; van de Velde, C.J.H.; et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): An international multicentre registry study. Lancet 2018, 391, 2537–2545. [Google Scholar] [CrossRef]
- Fernandez, L.M.; Sao Juliao, G.P.; Figueiredo, N.L.; Beets, G.L.; van der Valk, M.J.M.; Bahadoer, R.R.; Hilling, D.E.; Meershoek-Klein Kranenbarg, E.; Roodvoets, A.G.H.; Renehan, A.G.; et al. Conditional recurrence-free survival of clinical complete responders managed by watch and wait after neoadjuvant chemoradiotherapy for rectal cancer in the International Watch & Wait Database: A retrospective, international, multicentre registry study. Lancet Oncol. 2021, 22, 43–50. [Google Scholar] [CrossRef]
- Temmink, S.J.D.; Peeters, K.; Bahadoer, R.R.; Kranenbarg, E.M.; Roodvoets, A.G.H.; Melenhorst, J.; Burger, J.W.A.; Wolthuis, A.; Renehan, A.G.; Figueiredo, N.L.; et al. Watch and wait after neoadjuvant treatment in rectal cancer: Comparison of outcomes in patients with and without a complete response at first reassessment in the International Watch & Wait Database (IWWD). Br. J. Surg. 2023, 110, 676–684. [Google Scholar] [CrossRef]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef]
- Hasan, S.; Renz, P.; Wegner, R.E.; Finley, G.; Raj, M.; Monga, D.; McCormick, J.; Kirichenko, A. Microsatellite Instability (MSI) as an Independent Predictor of Pathologic Complete Response (PCR) in Locally Advanced Rectal Cancer: A National Cancer Database (NCDB) Analysis. Ann. Surg. 2020, 271, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Alatise, O.I.; Knapp, G.C.; Sharma, A.; Chatila, W.K.; Arowolo, O.A.; Olasehinde, O.; Famurewa, O.C.; Omisore, A.D.; Komolafe, A.O.; Olaofe, O.O.; et al. Molecular and phenotypic profiling of colorectal cancer patients in West Africa reveals biological insights. Nat. Commun. 2021, 12, 6821. [Google Scholar] [CrossRef] [PubMed]
- Farchoukh, L.F.; Celebrezze, J.; Medich, D.; Cunningham, K.; Holder-Murray, J.; Holtzman, M.; Lee, K.; Choudry, H.; Pai, R.K. DNA Mismatch Repair-deficient Rectal Cancer Is Frequently Associated with Lynch Syndrome and with Poor Response to Neoadjuvant Therapy. Am. J. Surg. Pathol. 2022, 46, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Papke, D.J., Jr.; Yurgelun, M.B.; Noffsinger, A.E.; Turner, K.O.; Genta, R.M.; Redston, M. Prevalence of Mismatch-Repair Deficiency in Rectal Adenocarcinomas. N. Engl. J. Med. 2022, 387, 1714–1716. [Google Scholar] [CrossRef] [PubMed]
- Dahlberg, M.; Glimelius, B.; Graf, W.; Pahlman, L. Preoperative irradiation affects functional results after surgery for rectal cancer: Results from a randomized study. Dis. Colon Rectum 1998, 41, 543–549; discussion 549–551. [Google Scholar] [CrossRef]
- Birgisson, H.; Pahlman, L.; Gunnarsson, U.; Glimelius, B. Late gastrointestinal disorders after rectal cancer surgery with and without preoperative radiation therapy. Br. J. Surg. 2008, 95, 206–213. [Google Scholar] [CrossRef]
- Peeters, K.C.; van de Velde, C.J.; Leer, J.W.; Martijn, H.; Junggeburt, J.M.; Kranenbarg, E.K.; Steup, W.H.; Wiggers, T.; Rutten, H.J.; Marijnen, C.A. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: Increased bowel dysfunction in irradiated patients--a Dutch colorectal cancer group study. J. Clin. Oncol. 2005, 23, 6199–6206. [Google Scholar] [CrossRef]
- Marijnen, C.A.; van de Velde, C.J.; Putter, H.; van den Brink, M.; Maas, C.P.; Martijn, H.; Rutten, H.J.; Wiggers, T.; Kranenbarg, E.K.; Leer, J.W.; et al. Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer: Report of a multicenter randomized trial. J. Clin. Oncol. 2005, 23, 1847–1858. [Google Scholar] [CrossRef]
- Bruheim, K.; Guren, M.G.; Skovlund, E.; Hjermstad, M.J.; Dahl, O.; Frykholm, G.; Carlsen, E.; Tveit, K.M. Late side effects and quality of life after radiotherapy for rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1005–1011. [Google Scholar] [CrossRef]
- Pollack, J.; Holm, T.; Cedermark, B.; Altman, D.; Holmstrom, B.; Glimelius, B.; Mellgren, A. Late adverse effects of short-course preoperative radiotherapy in rectal cancer. Br. J. Surg. 2006, 93, 1519–1525. [Google Scholar] [CrossRef]
- Birgisson, H.; Pahlman, L.; Gunnarsson, U.; Glimelius, B. Occurrence of second cancers in patients treated with radiotherapy for rectal cancer. J. Clin. Oncol. 2005, 23, 6126–6131. [Google Scholar] [CrossRef] [PubMed]
- Baxter, N.N.; Habaermann, E.; Tepper, J.; Durham, S.; Virnig, B. Risk of Pelvic Fractures in Older Women Following Pelvic Irradiation. JAMA 2005, 294, 2587. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Oden, A.; Johnell, O.; De Laet, C.; Jonsson, B.; Oglesby, A.K. The components of excess mortality after hip fracture. Bone 2003, 32, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Center, J.R.; Nguyen, T.V.; Schneider, D.; Sambrook, P.N.; Eisman, J.A. Mortality after all major types of osteoporotic fracture in men and women: An observational study. Lancet 1999, 353, 878–882. [Google Scholar] [CrossRef]
- Weiss, N.S.; Liff, J.M.; Ure, C.L.; Ballard, J.H.; Abbott, G.H.; Daling, J.R. Mortality in women following hip fracture. J. Chronic Dis. 1983, 36, 879–882. [Google Scholar] [CrossRef]
- Mell, L.K.; Schomas, D.A.; Salama, J.K.; Devisetty, K.; Aydogan, B.; Miller, R.C.; Jani, A.B.; Kindler, H.L.; Mundt, A.J.; Roeske, J.C.; et al. Association Between Bone Marrow Dosimetric Parameters and Acute Hematologic Toxicity in Anal Cancer Patients Treated with Concurrent Chemotherapy and Intensity-Modulated Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1431–1437. [Google Scholar] [CrossRef]
- Dainiak, N. Hematologic consequences of exposure to ionizing radiation. Exp. Hematol. 2002, 30, 513–528. [Google Scholar] [CrossRef]
- Klopp, A.H.; Moughan, J.; Portelance, L.; Miller, B.E.; Salehpour, M.R.; Hildebrandt, E.; Nuanjing, J.; D’Souza, D.; Souhami, L.; Small, W.; et al. Hematologic Toxicity in RTOG 0418: A Phase 2 Study of Postoperative IMRT for Gynecologic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 83–90. [Google Scholar] [CrossRef]
- Wo, J.Y.; Viswanathan, A.N. Impact of Radiotherapy on Fertility, Pregnancy, and Neonatal Outcomes in Female Cancer Patients. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1304–1312. [Google Scholar] [CrossRef]
- Ogilvy-Stuart, A.L.; Shalet, S.M. Effect of radiation on the human reproductive system. Environ. Health Perspect. 1993, 101, 109–116. [Google Scholar] [CrossRef]
- Fabiani, C.; Ferrante, M.G.; Meneghini, C.; Licata, E.; Paciotti, G.; Gallo, M.; Schiavi, M.; Spina, V.; Guarino, A.; Caserta, D.; et al. Female fertility preservation: Impact of cancer on ovarian function and oocyte quality. Int. J. Gynaecol. Obstet. 2021, 156, 166–171. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.; Marchetti, C.; Marampon, F.; Cascialli, G.; Muzii, L.; Tombolini, V. Radiation effects on male fertility. Andrology 2019, 7, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Schrag, D.; Shi, Q.; Weiser, M.R.; Gollub, M.J.; Saltz, L.B.; Musher, B.L.; Goldberg, J.; Al Baghdadi, T.; Goodman, K.A.; McWilliams, R.R.; et al. Preoperative Treatment of Locally Advanced Rectal Cancer. N. Engl. J. Med. 2023, 389, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Gerard, J.-P.; Barbet, N.; Schiappa, R.; Magné, N.; Martel, I.; Mineur, L.; Deberne, M.; Zilli, T.; Dhadda, A.; Myint, A.S. Neoadjuvant chemoradiotherapy with radiation dose escalation with contact X-ray brachytherapy boost or external beam radiotherapy boost for organ preservation in early cT2–cT3 rectal adenocarcinoma (OPERA): A phase 3, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2023, 8, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Brodin, N.P.; English, K.; Ohri, N.; Chuy, J.W.; Rajdev, L.N.; Narang, R.; Kalnicki, S.; Guha, C.; Garg, M.K.; et al. Comparing outcomes following total neoadjuvant therapy and following neoadjuvant chemoradiation therapy in patients with locally advanced rectal cancer. eClinicalMedicine 2019, 16, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Masi, G.; Vivaldi, C.; Fornaro, L.; Lonardi, S.; Buccianti, P.; Sainato, A.; Marcucci, L.; Martignetti, A.; Luca Urso, E.D.; Castagna, M.; et al. Total neoadjuvant approach with FOLFOXIRI plus bevacizumab followed by chemoradiotherapy plus bevacizumab in locally advanced rectal cancer: The TRUST trial. Eur. J. Cancer 2019, 110, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Dewdney, A.; Cunningham, D.; Tabernero, J.; Capdevila, J.; Glimelius, B.; Cervantes, A.; Tait, D.; Brown, G.; Wotherspoon, A.; Gonzalez de Castro, D.; et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C). J. Clin. Oncol. 2012, 30, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Sclafani, F.; Gonzalez, D.; Cunningham, D.; Hulkki Wilson, S.; Peckitt, C.; Tabernero, J.; Glimelius, B.; Cervantes, A.; Dewdney, A.; Wotherspoon, A.; et al. TP53 mutational status and cetuximab benefit in rectal cancer: 5-year results of the EXPERT-C trial. J. Natl. Cancer Inst. 2014, 106, dju121. [Google Scholar] [CrossRef]
- Patel, S.G.; Karlitz, J.J.; Yen, T.; Lieu, C.H.; Boland, C.R. The rising tide of early-onset colorectal cancer: A comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol. Hepatol. 2022, 7, 262–274. [Google Scholar] [CrossRef]
- Vuik, F.E.; Nieuwenburg, S.A.; Bardou, M.; Lansdorp-Vogelaar, I.; Dinis-Ribeiro, M.; Bento, M.J.; Zadnik, V.; Pellise, M.; Esteban, L.; Kaminski, M.F.; et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 2019, 68, 1820–1826. [Google Scholar] [CrossRef]
- Bailey, C.E.; Hu, C.Y.; You, Y.N.; Bednarski, B.K.; Rodriguez-Bigas, M.A.; Skibber, J.M.; Cantor, S.B.; Chang, G.J. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015, 150, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Oh, S.-T.; Ahn, C.-H.; Lee, J.; Lee, K.-Y.; Chae, H.S.; Kim, S.S.; Kim, S.W.; Seo, K.J. Simultaneous Integrated Boost Volumetric Modulated Arc Therapy for Rectal Cancer: Long-Term Results after Protocol-Based Treatment. J. Oncol. 2022, 2022, 6986267. [Google Scholar] [CrossRef] [PubMed]
- Stanojevic, A.; Samiotaki, M.; Lygirou, V.; Marinkovic, M.; Nikolic, V.; Stojanovic-Rundic, S.; Jankovic, R.; Vlahou, A.; Panayotou, G.; Fijneman, R.J.A.; et al. Data-Independent Acquisition Mass Spectrometry Analysis of FFPE Rectal Cancer Samples Offers In-Depth Proteomics Characterization of the Response to Neoadjuvant Chemoradiotherapy. Int. J. Mol. Sci. 2023, 24, 15412. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Kang, B.M.; Kim, I.Y.; Kim, J.Y.; Park, S.J.; Park, W.C.; Bae, K.B.; Bae, B.N.; Baek, S.K.; Baik, S.H.; et al. Korean Society of Coloproctology (KSCP) trial of cONsolidation Chemotherapy for Locally advanced mid or low rectal cancer after neoadjUvant concurrent chemoraDiothErapy: A multicenter, randomized controlled trial (KONCLUDE). BMC Cancer 2018, 18, 538. [Google Scholar] [CrossRef]
- Smith, J.J.; Dasari, A.; Shi, Q.; Garcia-Aguilar, J.; Sanoff, H.K.; George, T.J.; Hong, T.S.; Yothers, G.; Philip, P.A.; Nelson, G.D.; et al. Alliance A022104/NRG-GI010: A randomized phase II trial testing the efficacy of triplet versus doublet chemotherapy to achieve clinical complete response in patients with locally advanced rectal cancer—The Janus Rectal Cancer trial. J. Clin. Oncol. 2023, 41, TPS3640. [Google Scholar] [CrossRef]
- Matsuda, C.; Kudo, T.; Morimoto, Y.; Kagawa, Y.; Tei, M.; Ide, Y.; Miyoshi, N.; Takahashi, H.; Uemura, M.; Takemasa, I.; et al. A phase II study of neoadjuvant capecitabine, oxaliplatin, and irinotecan (XELOXIRI) in patients with locally advanced rectal cancer. Ann. Gastroent Surg. 2023, 7, 81–90. [Google Scholar] [CrossRef]
| Trial | Therapy | Follow-up (Years, Median) | pCR, % | DFS, % | LRR, % | DM, % | OS, % | CSS, % |
|---|---|---|---|---|---|---|---|---|
| MRC CR07 and NCIC-CTG C016 [11] | Neoadjuvant RT | 5 | NA | 73.6 | 4.7 | 19 | 70.3 | NA |
| Selective Adjuvant CRT | 66.7 | 11.5 | 21 | 67.9 | ||||
| Swedish Rectal Cancer Trial [12] | Neoadjuvant SCRT | 13 | NA | NA | 9 | 34 | 38 | 72 |
| Surgery alone | 26 | 34 | 30 | 62 | ||||
| TME Dutch Study [16] | Neoadjuvant SCRT | 10 | NA | 74 | 5 | 25 | 48 | NA |
| TME alone | 68 | 11 | 28 | 49 | ||||
| FFCD 9203 [17] | Neoadjuvant RT | 5 | 3.9 | 55.5 | 16.5 | NA | 67.9 | NA |
| Neoadjuvant CRT | 12.1 | 59.4 | 8.1 | 67.4 | ||||
| CAO/ARO/AIO-94 (German Trial) [18] | Neoadjuvant CRT | 10 | 9 | 68.1 | 7.1 | 29.8 | 59.6 | NA |
| Adjuvant CRT | NA | 67.8 | 10.1 | 29.6 | 59.9 | |||
| Akgun et al. [19] * | Neoadjuvant CRT | 5 | 13 | 75.2 | 7.4 | 13.6 | 79.8 | 87.5 |
| Adjuvant CRT | NA | 64.8 | 13.4 | 18.4 | 74.7 | 80 | ||
| NSABP-03 [20] | Neoadjuvant CRT | 5 | 15 | 64.7 | 10.7 | NA | 74.5 | NA |
| Adjuvant CRT | NA | 53.4 | 10.7 | 65.6 |
| Trial | Therapy | Follow-Up (Years, Median) | pCR, % | DFS, % | LRR, % | LRFS, % | DM, % | DMFS, % | DrTF, % | OS, % | CSS, % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| UNICANCER-PRODIGE 23 [5,6] * | CRT | 7 | 12 | 62.5 | 8.1 | NA | NA | 72 | NA | 76.1 | 79.6 |
| INCT + CRT | 28 | 67.6 | 5.3 | 79 | 81.9 | 84.9 | |||||
| RAPIDO [4,7] * | SCRT + CNCT | 5 | 28 | NA | 10 | NA | 23.0 | NA | 27.8 | 81.7 | NA |
| CRT | 14 | 6 | 30.4 | 34.0 | 80.2 | ||||||
| STELLAR [41] | SCRT + CNCT | 3 | 16.6 | 64.5 | 8.4 | NA | 22.9 | NA | NA | 86.5 | NA |
| CRT | 11.8 | 62.3 | 11.0 | 24.7 | 75.1 | ||||||
| Polish II [42] | SCRT + CNCT | 8 | 16 | 43 | 35 | NA | 36 | NA | NA | 49 | NA |
| CRT | 12 | 41 | 32 | 34 | 49 | ||||||
| OPRA [43] | INCT-CRT | 5 | 8 × | 72 | NA | 94 | NA | 82 | NA | 88 | NA |
| CRT-CNCT | 9 × | 71 | NA | 90 | NA | 79 | NA | 88 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conces, M.L.; Mahipal, A. Adoption of Total Neoadjuvant Therapy in the Treatment of Locally Advanced Rectal Cancer. Curr. Oncol. 2024, 31, 366-382. https://doi.org/10.3390/curroncol31010024
Conces ML, Mahipal A. Adoption of Total Neoadjuvant Therapy in the Treatment of Locally Advanced Rectal Cancer. Current Oncology. 2024; 31(1):366-382. https://doi.org/10.3390/curroncol31010024
Chicago/Turabian StyleConces, Madison L., and Amit Mahipal. 2024. "Adoption of Total Neoadjuvant Therapy in the Treatment of Locally Advanced Rectal Cancer" Current Oncology 31, no. 1: 366-382. https://doi.org/10.3390/curroncol31010024
APA StyleConces, M. L., & Mahipal, A. (2024). Adoption of Total Neoadjuvant Therapy in the Treatment of Locally Advanced Rectal Cancer. Current Oncology, 31(1), 366-382. https://doi.org/10.3390/curroncol31010024





