Abstract
Intensified preoperative chemotherapy after (chemo)radiotherapy, (Total Neoadjuvant Therapy–TNT), increases pathological complete response (pCR) rates and local control. In cases of clinically complete response (cCR) and close follow-up, non-operative management (NOM) is feasible. We report early outcomes and toxicities of a long-term TNT regime in a single-center cohort. Fifteen consecutive patients with distal or middle-third locally advanced rectal cancer (UICC stage II–III) were investigated, who received neoadjuvant chemoradiotherapy (total adsorbed dose: 50.4 Gy in 28 fractions and two concomitant courses 5-fluorouracil (250 mg/m2/d)/oxaliplatin (50 mg/m2), followed by consolidating chemotherapy (nine courses of FOLFOX4). NOM was offered if staging revealed cCR 2 months after TNT, with resection performed otherwise. The primary endpoint was complete response (pCR + cCR). Treatment-related side effects were quantified for up two years after TNT. Ten patients achieved cCR, of whom five opted for NOM. Ten patients (five cCR and five non-cCR) underwent surgery, with pCR confirmed in the five patients with cCR. The main toxicities comprised leukocytopenia (13/15), fatigue (12/15) and polyneuropathy (11/15). The most relevant CTC °III + IV events were leukocytopenia (4/15), neutropenia (2/15) and diarrhea (1/15). The long-term TNT regime resulted in promising response rates that are higher than the response rates of short TNT regimes. Overall tolerability and toxicity were comparable with the results of prospective trials.
1. Introduction
Until recently, the standard of care for locally advanced rectal cancer (LARC, UICC stage II and III) comprised neoadjuvant chemoradiotherapy (CRT) followed by resection and adjuvant chemotherapy (CTx). Recent trials have demonstrated the beneficial effects of intensified preoperative CTx regarding pathological complete response (pCR) rates and progression-free survival (PFS) [1,2,3,4,5]. The so-called “total neoadjuvant therapy” (TNT, intensified preoperative treatment) often comprises concomitant 5-Fluorouracil (5-FU)- and oxaliplatin-based chemoradiotherapy (CRT) regimes in combination with 3–6 cycles of fluoropyrimidine-based consolidating CTx [1,2]. Alternatively, less intensive concomitant CRT protocols (including 5-FU monotherapy) were followed by nine, more intense cycles of FOLFOX [3]. A non-operative approach (NOM) appears to be safe in cases of clinical complete response (cCR) in highly selected cases [5,6], translating into a gain in quality of life (QoL) compared to mandatory surgery and frequent colostomy [7,8].
The cumulative CTx dose in current TNT protocols is lower than the cumulative dose patients received in the intervention group of the ACO/ARO/AIO-04 trial [9]. This protocol was applied regularly at our institution before TNT was introduced. A pre-operative CTx with similar doses (Preoperative, concomitant CRT: oxaliplatin 50 mg/sqm d1, 8, 22, 29; 5-FU 250 mg/sqm/d d1–14 + d22–35) was followed by adjuvant CTx for eight cycles. Oxaliplatin was dosed higher (100 mg/sqm, q2w for eight cycles) than currently common for consolidation CTx of current TNT protocols (mostly 50–85 mg/sqm, q2w for up to nine cycles). However, long-term data beyond three years’ follow-up are not yet available for current TNT protocols. So, this de-escalation compared to adjuvant CTx protocols should be further investigated regarding long-term overall survival (OS) and progression-free survival (PFS).
In a subanalysis of the results of the ARO-04 trial [9], Hofheinz et al. demonstrated that, in particular, patients below 60 years of age benefited from adding oxaliplatin through lower local and systemic recurrence rates and even improved overall survival [10]. However, there are only a few prospective trials, such as the ADORE trial [11], demonstrating the benefits on disease-free survival (DFS) through adding oxaliplatin to adjuvant CTx. A Cochrane Review of further trials comparing the outcome of pre- and postoperative CTx [12] and the EORTC 22921 trial [13] indicated that the combination of pre- and postoperative CTx might not improve survival. Lim et al. demonstrated that, especially, patients obtaining pCR after preoperative CRT do not significantly benefit from adjuvant CTx [14].
Thus, intensification of neoadjuvant treatment by CTx appears to be beneficial compared to adjuvant CTx. Nevertheless, TNT seems to be a demanding therapy and not suitable for every patient due to treatment-related side effects. We, therefore, suppose a tolerability-guided two-step protocol to maximize patient benefits while simultaneously avoiding undertreatment in patients not tolerating consolidating CTx. This protocol combines long-term CRT, which is followed by consolidating CTx in cases of good tolerability of CRT and has been routinely applied at our institution for three years (Figure 1). This work is intended to report on first experiences with long-term consolidating CTx by this protocol in a single-center cohort and to compare these early results with other TNT protocols.
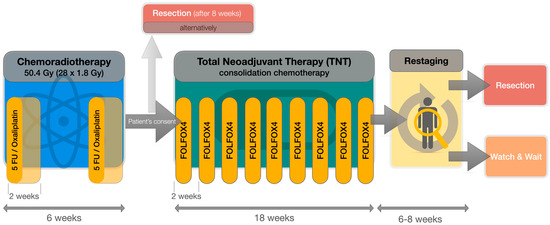
Figure 1.
Flowchart of the applied total neoadjuvant therapy (TNT) protocol, consisting of simultaneous chemoradiotherapy (CRT) with 5-Fluorouracil (5-FU, 250 mg/sqm/d continuously d1–14 and d22–35) and Oxaliplatin (50 mg/sqm on d1, d8 + d21, d28), followed by consolidation chemotherapy with FOLFOX4 (Oxaliplatin 85 mg/sqm on d1; 5-FU 400 mg/sqm d1 + d2; continuous 5-FU 1200 mg/sqm/d d1–2, folinic acid 200 mg/sqm d1 + d2) every 2 weeks (q2w).
2. Materials and Methods
We analyzed 15 consecutive patients who received TNT between March 2020 and April 2022. In accordance with the current recommendation of the German colorectal tumor expert associations (ACO, AIO, ARO) [15,16], patients with LARC received TNT if at least one of the following criteria was fulfilled: T3-tumors with distal location and/or positive lymph nodes (N+), additional risk factors (lateral positive pelvic lymph nodes, extramural vascular invasion-EMVI, infiltration of mesorectal plane-CRM+), or any T4-tumor (Table 1).

Table 1.
Patient characteristics and tumor stages.
Our institutional standard comprises simultaneous CRT with two cycles of 5-FU (250 mg/sqm/d continuously d1–14 and d22–35) and Oxaliplatin (50 mg/sqm on d1, d8 + d21, d28) analogous to the ACO/ARO/AIO-04 protocol [9] (Figure 1). All patients with LARC were undergoing reevaluation at the end of CRT regarding general health condition, treatment-related side effects and patient consent for consolidation CTx (“TNT” regime). Good general health condition (Karnofsky Performance Score, KPS, ≥70%), as well as good compliance, was mandatory for TNT. Patients not fulfilling these criteria underwent routine surgery 6 to 8 weeks after CRT. Adjuvant CTx was recommended to these patients in cases with risk factors, such as unfavorable pTNM assessment following excision.
To allow maximization of tumor regression and further benefits of neoadjuvant therapy, we prefer a longer preoperative interval comprising up to nine cycles of consolidation CTx in case of good tolerance. FOLFOX4 (oxaliplatin 85 mg/sqm on d1; 5-FU 400 mg/sqm d1 + d2; continuous 5-FU 1200 mg/sqm/d d1–2, folinic acid 200 mg/sqm d1 + d2) was chosen for consolidation CTx. The cumulative CTx doses of this protocol range between the less-intense ARO-12 protocol [1] and the, far higher-dosed, PRODIGE-23 protocol [4]. It is comparable with the RAPIDO- [3] and OPRA protocols [5], providing a slightly higher cumulative oxaliplatin dose but less 5-FU overall. Furthermore, the overall cumulated CTx dose of this protocol is similar to the doses patients received by neoadjuvant and adjuvant treatment in the intervention arm of the ACO/AIO/ARO-04 trial.
The CTx was delivered according to the prescribing information of the single agencies and dose reductions were performed if clinically indicated. Hematological toxicity, liver or kidney impairment as well as general condition and neuropathy were considered as measures for dose reduction.
All patients were required to undergo a preoperative restaging 4 to 6 weeks after TNT, including rectoscopy and magnetic resonance imaging for local response assessment, as well as computed tomography for distant metastasis detection. Upon the recommendation of the multidisciplinary tumor board, NOM was offered to all patients obtaining cCR willing to undergo close follow-up. Standard resection (e.g., total mesorectal excision, TME) was conducted in all other cases 6 to 8 weeks after TNT and pCR rates were reported.
This two-step neoadjuvant CRT/CTx-design allows individual therapy adaptions during routine CRT without undertreatment if no additional consolidation CTx (“TNT”) was performed, as this therapy is still equivalent to the established ARO-04 protocol. It simultaneously allows therapy intensification by performing TNT in selected cases (see Table 1 for indications).
General health condition, blood counts and treatment-related side effects (according to EORTC CTCAE v5.0) were monitored weekly during CRT as well as prior to each cycle of consolidating CTx and were analyzed retrospectively for this study. We will further report on early outcomes, such as response rates after TNT and DFS as well.
The early results of this protocol are reported in this article. Due to the small sample size (N = 15) and limited observation period, we focus on descriptive analyzes. IBM SPSS Statistics version 29 (IBM Deutschland GmbH, Ehningen, Germany) was used for statistics.
3. Results
We identified 53 patients with rectal cancer who underwent neoadjuvant CRT between March 2020 and April 2022. Within this group, 15 patients with LARC received TNT and met our eligibility criteria for this analysis (see also Appendix A Figure A2). We included nine male and six female patients with ages ranging between 53 and 79 years (mean: 66.4 years ± 5.84 SD). These patients were followed-up for at least 9 months (mean 18 months, min. 9 months, max. 24 months). The mean follow-up for patients undergoing NOM was 17.4 months (min. 9 months, max. 24 months).
The tumors were mostly staged T3 N+ with additional risk factors (12/15 patients) and located distally (8/15 patients). Table 2 shows the detailed demographic characteristics of the cohort.

Table 2.
Indications for Total Neoadjuvant Therapy (TNT) in this case series.
The TNT regime was near-completely applied in 11/15 patients (i.e., application of at least 8 cycles of FOLFOX4), corresponding to a 73.3% completion rate (Table 3).

Table 3.
Main outcome features.
A dose reduction of CTx was required often due to hematological toxicity or polyneuropathy. Only 3/15 patients received all cycles of FOLFOX4 without dose reduction. On average, five cycles of FOLFOX4 were administered in the scheduled dose. The total cumulative oxaliplatin dose of concomitant and consolidation CTx was 844.6 mg/m2 (±66.8 SD) on average. Termination of consolidation CTx was necessary in 3/15 patients. The reasons were impaired general health condition (2 patients) or hematological toxicity (1 patient).
The majority of patients underwent resection (10/15) with a pCR rate of 50% (5/10). Five patients (5/15) with cCR underwent NOM (“watch and wait”). An exemplary patient, opting for NOM after obtaining cCR, is shown in Figure 2. In total, a cumulative complete response (cCR or/and pCR) was obtained in 10/15 patients (66.7%).
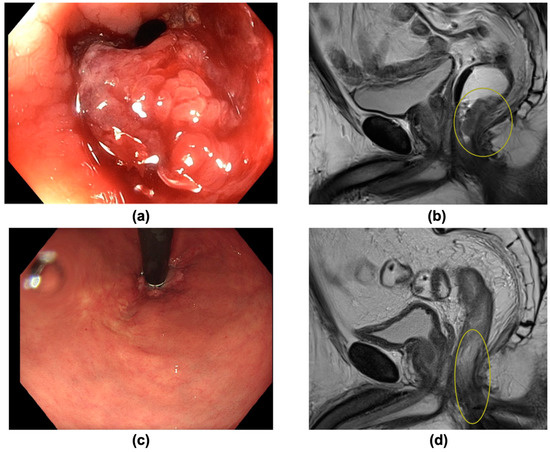
Figure 2.
(a–d): A 63-year-old patient with distal rectal carcinoma (cT4 cN0 cM0) was undergoing Total Neoadjuvant Therapy (TNT), obtaining clinical complete response (cCR). A non-operative management was chosen. There was no recurrence within an 18-month follow-up. (a) Image of endoscopy prior to treatment, showing a vulnerable ulcerated tumor. (b) Pre-treatment sagittal T2-weighted MRI scan, picturing a distal T4 tumor (yellow circle) in minimal distance from the anal sphincter. (c) Endoscopy 8 weeks after TNT, showing a macroscopic good response with a residual scar. (d) Post-treatment sagittal T2-weighted MRI scan confirming clinical complete response 8 weeks after TNT (yellow circle: former tumor bed).
A relapse was diagnosed in two patients. One patient developed multiple distant metastases after 12 months after resection and was lost to follow-up after 24 months. Another patient, undergoing NOM, was diagnosed with liver metastases 24 months after TNT. No local recurrence was reported.
The most common treatment-related side effects of sequential CTx were hematological toxicity and polyneuropathy, as well as impaired general health condition (i.e., fatigue), as summarized in Table 4 and Figure A1. Among these, hematological toxicity was reported most often. Leukocytopenia was observed in 13/15 patients (86.7%), thereof nine patients did not exceed common toxicity criteria grade (CTC °I–II). Anemia and elevated liver enzymes occurred in six patients respectively, never affecting subsequent therapy (CTC °I–II). Thrombocytopenia occurred seldom (2/15 patients).

Table 4.
Overview of most relevant therapy-related side effects, according to EORTC-CTCAE v5.0. “Liver enzymes” comprises ALAT, ASAT, gamma-GT, Bilirubin and AP levels, the highest of which defined the CTC grade. * Including febrile neutropenia.
Overall, CTC grade I or II events were reported in 10/15 patients and CTC grade III or IV events occurred in 5/15 patients. No CTC grade V events were reported.
Therapy limitations were mostly due to hematological toxicity. At least one course was not administered in 4/15 patients. A high rate of any grade of polyneuropathy was observed (11 patients/73.3%), but only two patients developed severe symptoms (CTC °III). One therapy discontinuation was due to simultaneous febrile neutropenia and severe diarrhea (CTC °IV) after two courses; however, the patient recovered completely and underwent resection. Other discontinuations were related to a severe SARS-CoV-2 infection in a single patient, so resection after reconvalescence was favored, and lacking compliance in another patient.
We report postoperative complications in 3/10 patients undergoing resection. An anastomotic insufficiency, delayed wound healing with secondary infection and a vaginal fistula occurred in one patient each. TNT was terminated after two cycles of FOLFOX in the patient who developed the vaginal fistula due to hematological toxicity. In the other two patients, TNT was administered as scheduled.
As displayed in the paired boxplots (Figure 3 and Figure 4), complete response was mostly observed in patients receiving at least eight cycles of consolidating chemotherapy or accordingly cumulative oxaliplatin doses of more than 774 mg/sqm. However, testing for a correlation of higher oxaliplatin doses with complete response is not possible due to the small sample size.
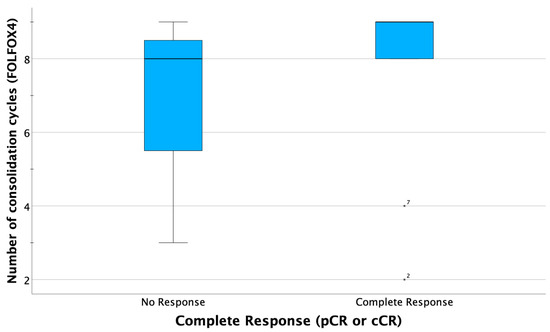
Figure 3.
Boxplots comparing summarized complete response (CR) rates (clinical CR in case of organ preservation and pathological CR in case of resection), indicating that most patients obtaining CR received at least eight courses consolidating chemotherapy. In two patients (ID 2 and ID7), complete response was observed after only 2 or 4 cycles FOLFOX, respectively.
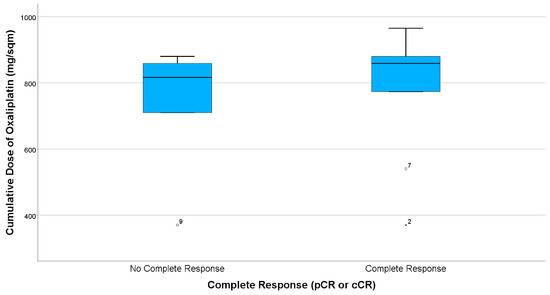
Figure 4.
Boxplots comparing summarized complete response (CR) rates (i.e., clinical CR in case of organ preservation and pathological CR in case of resection), indicating that most patients obtaining CR received cumulative Oxaliplatin doses of more than 774 mg/sqm. There are three patients, having only received two cycles (ID2, ID9) or four cycles (ID7) FOLFOX, respectively.
4. Discussion
Despite the limitations of the small sample size of this retrospective analysis, we are able to report encouraging response rates. Earlier trials evaluating TNT protocols commonly reported the benefit of consolidation CTx compared to induction CTx [1,5]. We, therefore, focused on comparing consolidation CTx regimes of the following studies.
The RAPIDO trial [3] performed nine courses of FOLFOX4 as well, but in total, it was less intense due to the use of short-course radiotherapy only. The regimen resulted in similar therapy-related side effects to our protocol. However, in particular, CTC °I–II neuropathy was slightly higher (79%) in the RAPIDO trial. Overall, pCR rates were substantially lower (28%). However, there is limited comparability between both groups as more patients with high risk factors or more invasive (i.e., T4) tumors were included in the RAPIDO trial.
Another quite intense protocol was applied in the experimental arm of PRODIGE-23 [4]: an induction CTx with six courses of FOLFIRINOX continued by six courses of mFOLFOX6 postoperatively was applied. Despite the limited comparability due to different treatment sequences, PRODIGE-23 resulted in higher cumulative CTx doses and seemed to have caused more toxicities: CTC °I–II neuropathy was higher (83%) and severe diarrhea was reported more often (CTC °I–II: 62%, CTC °III–IV: 11%). Hematological toxicity was within the same range (CTC °I–II about 40–50%, summarized).
The ARO-12 trial [1] was based on the same treatment sequence, comprising simultaneous CRT followed by a shorter consolidation CTX with three cycles of FOLFOX in the experimental arm. Complete response was reported in 25%, but the shorter interval between CRT and resection limits comparisons. Nevertheless, neurological and hematological toxicity were lower, possibly due to less intense consolidation CTx.
The more recent data of the OPRA trial suggested higher response rates compared to our cohort: the approach was similar with long-course CRT (infusional 5-FU monotherapy) followed by eight cycles of FOLFOX4. The majority of the patients received NOM (experimental arm: 120/158 patients, 76%), meaning clinical complete response was achieved after TNT. The cumulative rate of CTC °III–IV events was reported as 38%, but unfortunately, not further specified. This is comparable with the treatment-related side effects observed in our cohort (CTC °III + IV in 5/15 patients–33.3%), suggesting reproducible tolerability of these quite similar protocols.
Another recent trial, ARO-16 [2], investigated intended organ preservation. A similar treatment to the ARO-12 trial was performed, resulting in 15% cCR after 15 weeks according to first results. The cCR rate increased up to 40% after 28 weeks, supporting the hypothesis that a longer preoperative (consolidation) period might be beneficial regarding response rates. CTC °III–IV toxicity was slightly higher than in our cohort (45%).
CR rates were within the same range of longer-lasting consolidation protocols, such as the OPRA trial [5]. We reported higher toxicity compared to less-intense, shorter protocols (such as ARO-12 [1]), but saw higher response rates. It seems that longer-lasting neoadjuvant protocols, comprising simultaneous conventional fractionated CRT and TNT for at least 4 months, have the potential to maximize tumor regression. The tolerability of these protocols and our TNT regime were comparable, but increased long-term therapy-related side effects, such as polyneuropathy, have to be considered and closely monitored. However, to maintain an adequate tolerability in our cohort, dose reductions had to be performed often and only a small percentage of patients received the scheduled dose. The small sample size does not allow us to conclude whether the high CR rates were attributable to a high cumulative chemotherapy dose or the long neoadjuvant period. This aspect needs to be further investigated in order to evaluate the possibility and safety of de-escalating the chemotherapy in order to reduce toxicity, which is probably mostly related to high cumulative oxaliplatin doses. We saw a high rate of polyneuropathy, as patients in this cohort often received cumulative oxaliplatin doses of more than 750 mg/sqm, which is associated with a higher prevalence of polyneuropathy [17,18].
If adjuvant CTx would be indicated instead of consolidating CTx, comparable or even higher cumulative CTx doses would be administered. Consequently, higher toxicity was reported by trials reporting on adjuvant therapy as the standard of care [11,12,19]. These findings emphasize the importance of risk stratification upfront in TNT to avoid overtherapy as well as close monitoring of therapy-related side effects for consecutive individual dose adaptions [20].
The promising early results reported here need to be validated through additional data to obtain a statistically significant cohort. We are currently performing a local pilot study, applying this TNT regime (PRIMO Trial, NCT 05524012) [21]. Multimodal diagnostic, comprising, e.g., multiparametric magnetic resonance imaging (MRI) and liquid biopsy, will be used to quantify tumor response longitudinally in the PRIMO trial. Furthermore, long-term results are necessary to assess the effectiveness of this treatment intensification regarding PFS and OS.
5. Conclusions
The long-term TNT protocol, consisting of conventional fractionated long-course CRT and 4 months of consolidation CTx, seems to maximize tumor regression and response rates.
We experienced comparable tolerability of this regime and promising response rates compared to other TNT protocols. Nevertheless, the application of nine cycles of FOLFOX4 is a demanding therapy, leading to relevant hematological toxicity or neuropathy in a subset of patients. This requires a frequent evaluation of side effects with treatment adaptation. Long-term outcomes and a greater cohort size are needed to validate these early data.
Author Contributions
All authors contributed substantially to of this as further specified: Conceptualization, G.W.W. and A.W.; methodology, G.W.W.; data curation & formal analysis, G.W.W.; resources & investigation, T.E., C.S., H.M., H.H., Y.L. and M.H.; writing—original draft preparation, G.W.W., S.K. and A.W.; writing—review and editing, T.E., C.S., H.H., H.M., Y.L. and M.H.; visualization, G.W.W.; project administration, A.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. G.W.W. receives funding from the “Clinician Scientist”-program of the Interdisciplinary Center for Clinical Research, Jena University Hospital (grant-No.: CSP-11) outside of this work. The APC was funded by the German Research Foundation Projekt-Nr. 512648189 and the Open Access Publication Fund of the Thueringer Universitaets- und Landesbibliothek Jena.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the institutional Ethics Committee of JENA UNIVERSITY HOSPITAL (2022-2786-Daten).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of this analysis. For Figure 2, we obtained the consent of this patient for publication use prior to submission.
Data Availability Statement
The datasets generated and analyzed during the current study will be not publicly available due to local data privacy restrictions but anonymized data will be available from the corresponding author after completion of recruitment on reasonable request.
Acknowledgments
We would like to thank Hannah K. Schöberl for language editing.
Conflicts of Interest
The authors declare no conflict of interest regarding this work. Funding of GW outside of this work had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
| ACO | Arbeitsgemeinschaft Chirurgische Onkologie of DKG (Surgical Oncology working group of German Cancer Society) |
| AIO | Arbeitsgemeinschaft Internistische Onkologie of DKG (Medical Oncology working group of German Cancer Society) |
| ALAT | alanine transaminase |
| AP | alkaline phosphatase |
| ARO | Arbeitsgemeinschaft Radiologische Onkologie of DKG (Radiation Oncology working group of German Cancer Society) |
| ASAT | aspartate transaminase |
| BMI | body mass index |
| cCR | clinical complete response |
| COPD | chronic obstructive pulmonary disease |
| CRM+ | infiltration of mesorectal plane |
| CRT | chemoradiotherapy |
| CT | computed tomography |
| CTC | common toxicity criteria |
| CTx | chemotherapy |
| d | days (since beginning of Radiotherapy/Chemotherapy) |
| DFS | Disease-free survival |
| EMVI+ | extramural vascular invasion |
| gGT | gamma-glutamyl transferase |
| GFR | glomerular filtration rate |
| GOLD | Global Initiative for Chronic Obstructive Lung Disease |
| KPS | Karnofsky’s Performance Status |
| LARC | locally advanced rectal cancer |
| MRI | magnetic resonance imaging |
| N | positive lymph nodes |
| NOM | Non-operative management (“watch and wait”) |
| NYHA | New York Heart Association |
| OS | overall survival |
| pCR | pathological complete response |
| QoL | quality of life |
| q2w | once every 2 weeks |
| PFS | Progression-free survival |
| RT | radiotherapy |
| SD | standard deviation |
| sqm | square meter (reference on body surface area) |
| TME | total mesorectal excision |
| TNT | total neoadjuvant therapy |
| UICC | Union Internationale Contre Le Cancer |
| ULN | upper limit of normal range |
Appendix A
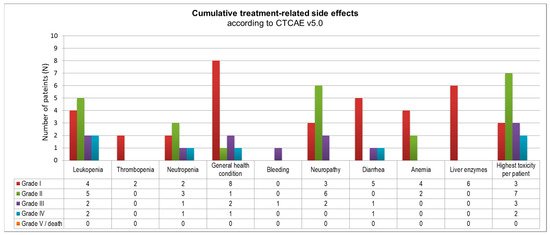
Figure A1.
Overview of reported cumulative toxicities according to EORTC CTCAE v5.0. Overall, Grade 3 or 4 toxicity was reported in 5/15 patients (last column).
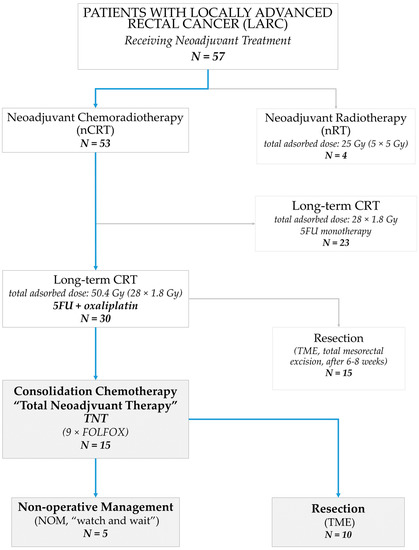
Figure A2.
Study profile showing all patients who received a neoadjuvant treatment for locally advanced rectal cancer (LARC) at our institution between March 2020 and April 2022. The subgroup comprising all patients who underwent consolidation chemotherapy, i.e., total neoadjuvant therapy (TNT), is described in this work.
References
- Fokas, E.; Allgäuer, M.; Polat, B.; Klautke, G.; Grabenbauer, G.G.; Fietkau, R.; Kuhnt, T.; Staib, L.; Brunner, T.; Grosu, A.-L.; et al. Randomized Phase II Trial of Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: CAO/ARO/AIO-12. J. Clin. Oncol. 2019, 37, 3212–3222. [Google Scholar] [CrossRef] [PubMed]
- Gani, C.; Polat, B.; Ott, O.; Germer, E.; Königsrainer, A.; Kirschniak, A.; Clasen, S.; Fokas, E.; Zips, D.; Rödel, C. Totale Neoadjuvante Therapie als Organerhaltende Therapie des Rektumkarzinoms: Die CAO/ARO/AIO-16 Phase II Studie. Strahlenther Onkol 2022, 198 (Suppl. S1), 8. [Google Scholar] [CrossRef]
- Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.-K.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Bosset, J.-F.; Etienne, P.-L.; Rio, E.; François, É.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouché, O.; Gargot, D.; et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Aguilar, J.; Patil, S.; Gollub, M.J.; Kim, J.K.; Yuval, J.B.; Thompson, H.M.; Verheij, F.S.; Omer, D.M.; Lee, M.; Dunne, R.F.; et al. Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy. J. Clin. Oncol. 2022, 40, 2546–2556. [Google Scholar] [CrossRef] [PubMed]
- Gani, C.; Kirschniak, A.; Zips, D. Watchful Waiting after Radiochemotherapy in Rectal Cancer: When Is It Feasible? Visc. Med. 2019, 35, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; Schlenska-Lange, A.; Polat, B.; Klautke, G.; Grabenbauer, G.G.; Fietkau, R.; Kuhnt, T.; Staib, L.; Brunner, T.; Grosu, A.L.; et al. Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Patients With Locally Advanced Rectal Cancer: Long-term Results of the CAO/ARO/AIO-12 Randomized Clinical Trial. JAMA Oncol. 2022, 8, e215445. [Google Scholar] [CrossRef] [PubMed]
- Hupkens, B.J.P.; Martens, M.H.; Stoot, J.H.; Berbee, M.; Melenhorst, J.; Beets-Tan, R.G.; Beets, G.L.; Breukink, S.O. Quality of Life in Rectal Cancer Patients After Chemoradiation: Watch-and-Wait Policy Versus Standard Resection—A Matched-Controlled Study. Dis. Colon Rectum 2017, 60, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Rödel, C.; Graeven, U.; Fietkau, R.; Hohenberger, W.; Hothorn, T.; Arnold, D.; Hofheinz, R.D.; Ghadimi, M.; Wolff, H.A.; Lang-Welzenbach, M.; et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): Final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015, 16, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Hofheinz, R.D.; Arnold, D.; Fokas, E.; Kaufmann, M.; Hothorn, T.; Folprecht, G.; Fietkau, R.; Hohenberger, W.; Ghadimi, M.; Liersch, T.; et al. Impact of age on the efficacy of oxaliplatin in the preoperative chemoradiotherapy and adjuvant chemotherapy of rectal cancer: A post hoc analysis of the CAO/ARO/AIO-04 phase III trial. Ann. Oncol. 2018, 29, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.S.; Kim, S.Y.; Lee, J.S.; Nam, B.-H.; Kim, K.-p.; Kim, J.E.; Park, Y.S.; Park, J.O.; Baek, J.Y.; Kim, T.-Y.; et al. Oxaliplatin-Based Adjuvant Chemotherapy for Rectal Cancer After Preoperative Chemoradiotherapy (ADORE): Long-Term Results of a Randomized Controlled Trial. J. Clin. Oncol. 2019, 37, 3111–3123. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.H.; Harling, H.; Kirkeby, L.T.; Wille-Jørgensen, P.; Mocellin, S. Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database Syst. Rev. 2012, 2012, Cd004078. [Google Scholar] [CrossRef] [PubMed]
- Bosset, J.F.; Calais, G.; Mineur, L.; Maingon, P.; Stojanovic-Rundic, S.; Bensadoun, R.J.; Bardet, E.; Beny, A.; Ollier, J.C.; Bolla, M.; et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: Long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014, 15, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Kim, Y.; Kong, M. Adjuvant chemotherapy in rectal cancer patients who achieved a pathological complete response after preoperative chemoradiotherapy: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 10008. [Google Scholar] [CrossRef] [PubMed]
- ACO; AIO; ARO. Konsentierte Stellungnahme der AIO, der ACO und der ARO zur Neoadjuvanten Therapie Beim Rektumkarzinom. 2020. Available online: www.aio-portal.de (accessed on 20 February 2023).
- ACO; AIO; ARO. Konsentierte Stellungnahme der ACO, der AIO und der ARO zum „Watch and Wait“-Konzept Mit intendiertem Organerhalt bei Rektumkarzinomen des mittleren und unteren Drittels. 2020. Available online: https://www.aio-portal.de/stellungnahmen.html (accessed on 20 February 2023).
- Saif, M.W.; Reardon, J. Management of oxaliplatin-induced peripheral neuropathy. Ther. Clin. Risk Manag. 2005, 1, 249–258. [Google Scholar] [PubMed]
- de Gramont, A.; Figer, A.; Seymour, M.; Homerin, M.; Hmissi, A.; Cassidy, J.; Boni, C.; Cortes-Funes, H.; Cervantes, A.; Freyer, G.; et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J. Clin. Oncol. 2000, 18, 2938–2947. [Google Scholar] [CrossRef] [PubMed]
- de Torres-Olombrada, M.V.; Juez-Martel, I.; Rodríguez-Caravaca, G.; Duran-Poveda, M. Adjuvant chemotherapy in locally advanced rectal cancer: Deciding on the optimal strategy. Rev. Investig. Clin. 2020, 72, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, C.L.; Lacchetti, C.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Hertz, D.L.; Kelley, M.R.; Lavino, A.; Lustberg, M.B.; Paice, J.A.; et al. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: ASCO Guideline Update. J. Clin. Oncol. 2020, 38, 3325–3348. [Google Scholar] [CrossRef] [PubMed]
- Wurschi, G.W.; Güllmar, D.; Gaßler, N.; Clement, J.; Kesselmeier, M.; Müller-Wurschi, J.J.; Settmacher, U.; Mothes, H.; Helfritzsch, H.; Liebe, Y.; et al. Planning adaptive treatment by longitudinal response assessment implementing MR imaging, liquid biopsy and analysis of microenvironment during neoadjuvant treatment of rectal cancer (PRIMO). Medicine 2023, 102, e33575. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).