Health Technology Reassessment: Addressing Uncertainty in Economic Evaluations of Oncology Drugs at Time of Reimbursement Using Long-Term Clinical Trial Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Modeling Approach
2.2. Clinical Inputs
2.3. Regimen and Dosing
2.4. Utility Values
2.5. Healthcare Resource Utilization
2.6. Costs
2.7. Statistical Analyses
3. Results
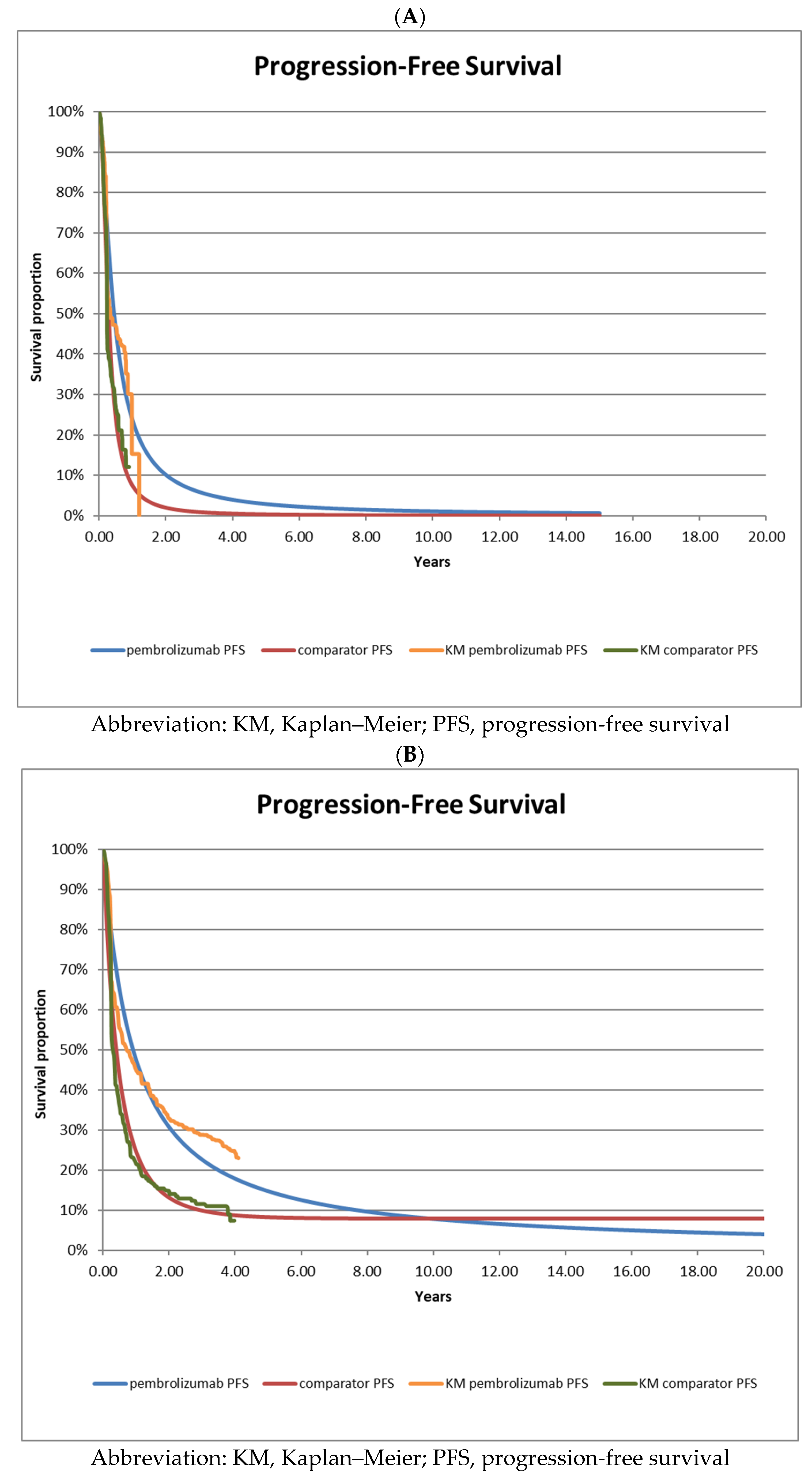
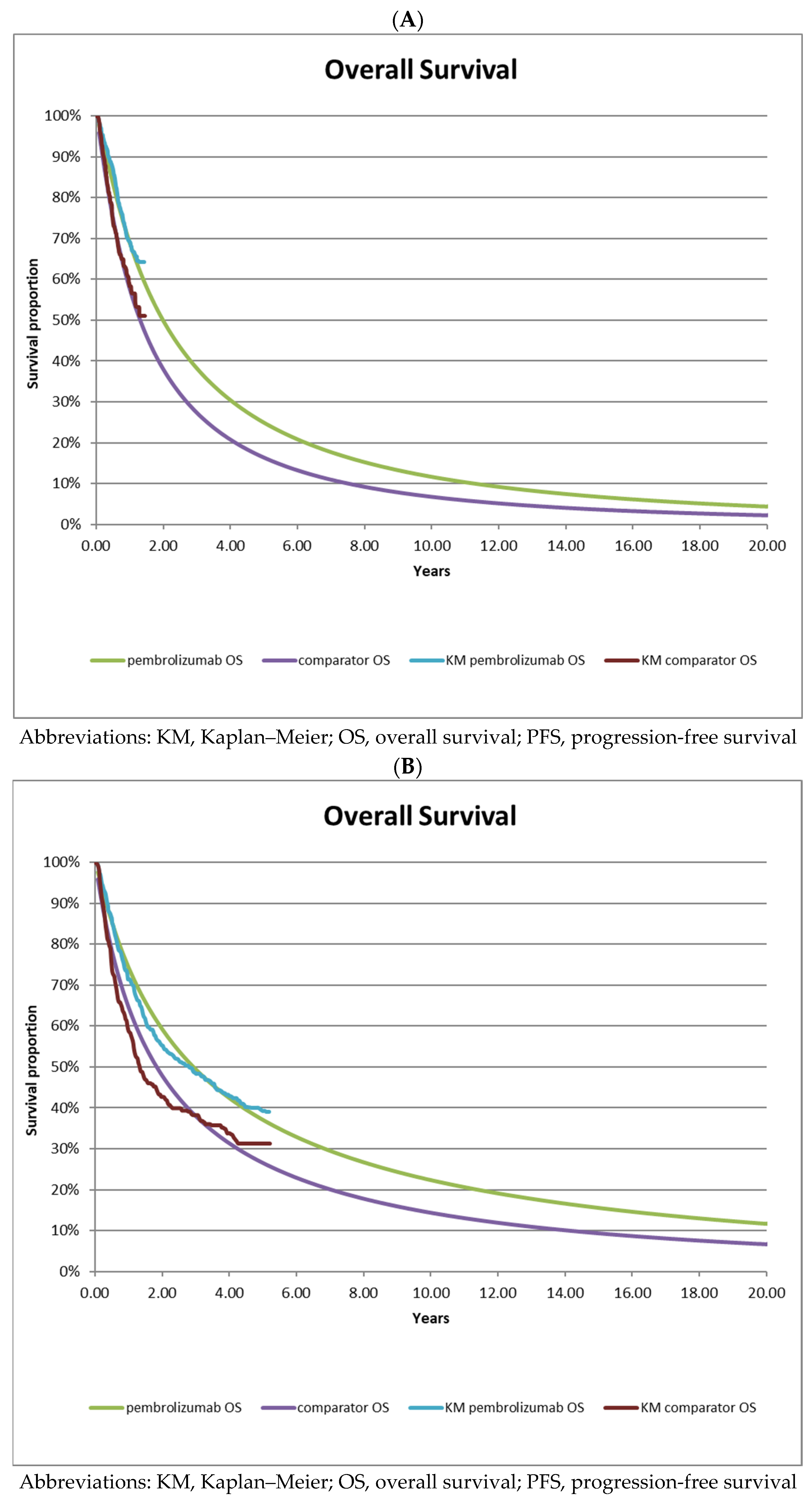
3.1. Survival Analysis of Interim Data [5] versus Long-Term Follow-Up Data [7]
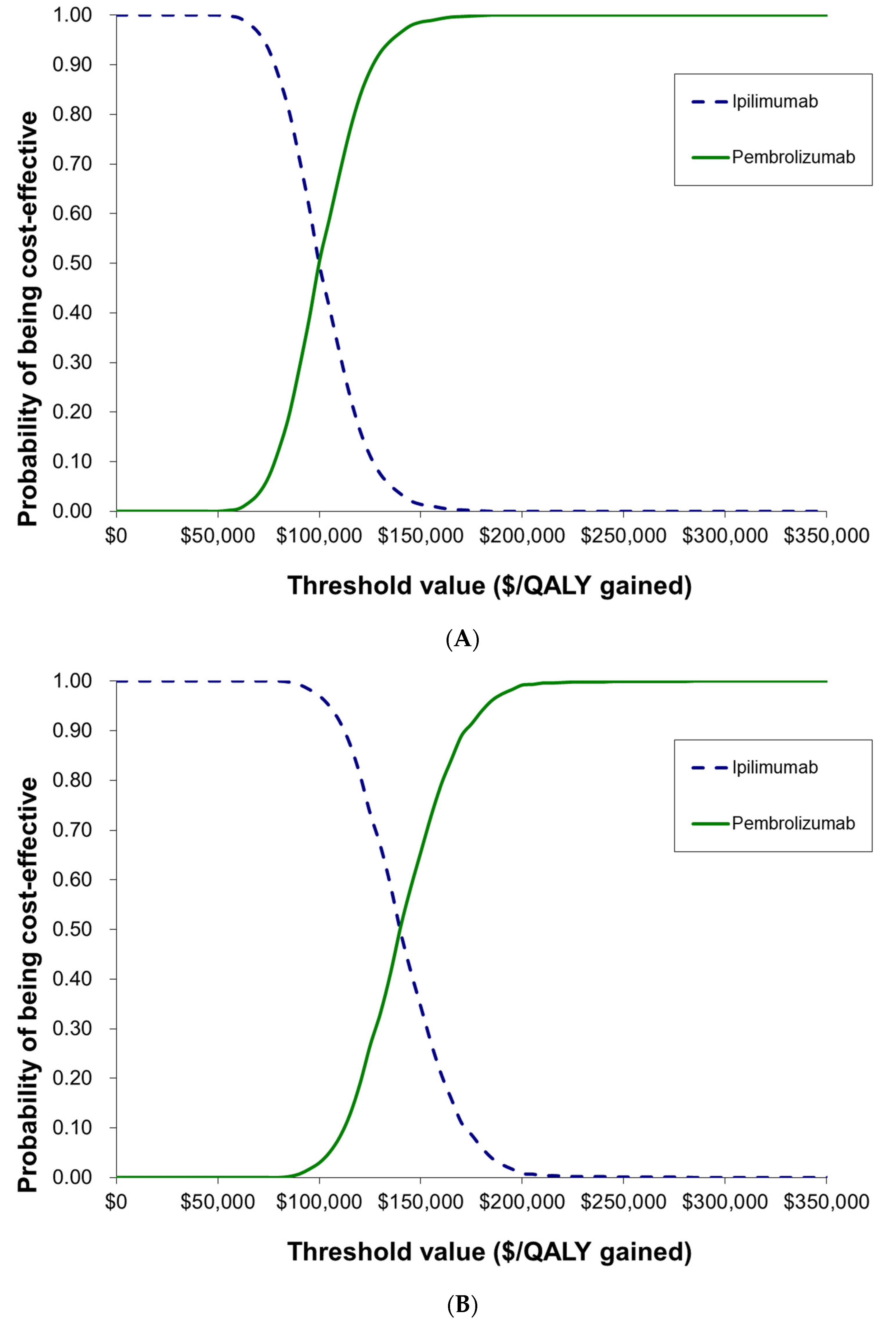
3.2. Cost-Effectiveness Analysis of Interim Data [5] versus Long-Term Follow-Up Data [7]
4. Discussion
4.1. Previous Studies
4.2. Strengths
4.3. Limitations
4.4. Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eckermann, S.; Karnon, J.; Willan, A.R. The Value of Value of Information. PharmacoEconomics 2010, 28, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.; Fojo, T.; Chamberlain, C.; Davis, C.; Sullivan, R. Do patient access schemes for high-cost cancer drugs deliver value to society?—Lessons from the NHS Cancer Drugs Fund. Ann. Oncol. 2017, 28, 1738–1750. [Google Scholar] [CrossRef] [PubMed]
- NHS England. National Cancer Drugs Fund List. 2023. Available online: https://www.england.nhs.uk/wp-content/uploads/2017/04/NationalCDF-List-ver1.265.pdf (accessed on 23 May 2023).
- National Institutute for Health and Care Excellence. NICE Strategy 2021 to 2026. 2021. Available online: https://static.nice.org.uk/NICE%20strategy%202021%20to%202026%20-%20Dynamic,%20Collaborative,%20Excellent.pdf (accessed on 6 January 2023).
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Schachter, J.; Ribas, A.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017, 390, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Ribas, A.; Schachter, J.; Arance, A.; Grob, J.-J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.M.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019, 20, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Merck Press Release. FDA Approves Expanded Indication for Merck’s KEYTRUDA® (Pembrolizumab) for the Treatment of Patients with Advanced Melanoma. 2015. Available online: https://www.merck.com/news/fda-approves-expanded-indication-for-mercks-keytruda-pembrolizumab-for-the-treatment-of-patients-with-advanced-melanoma/ (accessed on 4 November 2022).
- National Institute for Health and Care Excellence. Technology Appraisal Guidance—Pembrolizumab for Advanced Melanoma Not Previously Treated with Ipilimumab; National Institute for Health and Care Excellence: London, UK, 2015. [Google Scholar]
- pan-Canadian Oncology Drug Review Economic Guidance Panel. pCODR Final Economic Guidance Report—Pembrolizumab (Keytruda) for Metastatic Melanoma; Canadian Agency for Drugs and Technologies in Health: Toronto, ON, Canada, 2015.
- Schadendorf, D.; Hodi, F.S.; Robert, C.; Weber, J.S.; Margolin, K.; Hamid, O.; Patt, D.; Chen, T.-T.; Berman, D.M.; Wolchok, J.D. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J. Clin. Oncol. 2015, 33, 1889–1894. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chmielowski, B.; Pellissier, J.; Xu, R.; Stevinson, K.; Liu, F.X. Cost-Effectiveness of Pembrolizumab Versus Ipilimumab in Ipilimumab-Naïve Patients with Advanced Melanoma in the United States. J. Manag. Care Spec. Pharm. 2017, 23, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Tarhini, A.; Corman, S.L.; Rao, S.; Margolin, K.; Ji, X.; Mehta, S.; Botteman, M.F. Healthcare resource utilization and associated costs in patients with advanced melanoma receiving first-line ipilimumab. J. Cancer Ther. 2015, 6, 833. [Google Scholar] [CrossRef]
- Center for Medicare & Medicaid Services. FY 2015 Final Rule Tables (Table 1, Table 5 and Table 7); Center for Medicare & Medicaid Services: Baltimore, MD, USA, 2023.
- Center for Medicare & Medicaid Services. April 2023 Medicare Part B Drug and Biological Average Sales Price; Center for Medicare & Medicaid Services: Baltimore, MD, USA, 2023. Available online: https://www.cms.gov/files/zip/april-2023-asp-pricing-file.zip (accessed on 18 March 2023).
- Center for Medicare & Medicaid Services. Costs for Hospital Outpatient Services by HCPCS Code for CY 2023; Center for Medicare & Medicaid Services: Baltimore, MD, USA, 2023.
- U. S. Bureau of Labor Statistics. Consumer Price Index for All Urban Consumers (Not Seasonally Adjusted); U. S. Bureau of Labor Statistics: Washington, DC, USA, 2023. Available online: https://www.bls.gov/regions/mid-atlantic/data/consumerpriceindexhistorical_us_table.htm (accessed on 12 April 2023).
- Guyot, P.; Ades, A.E.; Ouwens, M.J.N.M.; Welton, N.J. Enhanced secondary analysis of survival data: Reconstructing the data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 2012, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Institute for Clinical and Economic Review. ICER’s Reference Case for Economic Evaluations: Principles and Rationale; Institute for Clinical and Economic Review: Boston, MA, USA, 2020. [Google Scholar]
- Briggs, A.C.K.; Sculpher, M. Decision Modelling for Health Economic Evaluation; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Balch, C.M.; Buzaid, A.C.; Soong, S.-J.; Atkins, M.B.; Cascinelli, N.; Coit, D.G.; Fleming, I.D.; Gershenwald, J.E.; Houghton, A.; Kirkwood, J.M.; et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J. Clin. Oncol. 2001, 19, 3635–3648. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, M.J.; Lambert, P.C.; Sweeting, M.J.; Pennington, R.; Crowther, M.J.; Abrams, K.R. NICE DSU Technical Support Document 21. Flexible Methods for Survival Analysis. 2020. Available online: http://www.nicedsu.org.uk (accessed on 26 February 2023).
- Lambert, P.C. Modeling of the Cure Fraction in Survival Studies. Stata J. 2007, 7, 351–375. [Google Scholar] [CrossRef]

| A—interim analysis data | ||||||
| Parametric Curve Fits | Weibull | Exponential | Log-Normal | Log-Logistic | Gamma | Gompertz |
| Ipilimumab OS-AIC | 933.1 | 932.7 | 921.7 | 927.6 | 932.0 | 934.6 |
| Ipilimumab PFS-AIC | 969.0 | 989.9 | 949.3 | 940.6 | 960.0 | 988.8 |
| Pembrolizumab OS-AIC | 818.4 | 821.0 | 813.6 | 816.1 | 817.6 | 822.2 |
| Pembrolizumab PFS-AIC | 964.9 | 965.1 | 947.8 | 947.4 | 962.4 | 965.6 |
| B—long-term follow-up data | ||||||
| Parametric Curve Fits | Weibull | Exponential | Log-Normal | Log-logistic | Gamma | Gompertz |
| Ipilimumab OS-AIC | 1583.2 | 1603.6 | 1542.8 | 1554.2 | 1591.6 | 1542.1 |
| Ipilimumab PFS-AIC | 1460.1 | 1471.7 | 1374.6 | 1370.4 | 1471.0 | 1404.0 |
| Pembrolizumab OS-AIC | 3189.3 | 3205.0 | 3140.5 | 3158.8 | 3196.2 | 3150.1 |
| Pembrolizumab PFS-AIC | 3277.5 | 3355.7 | 3178.0 | 3202.0 | 3304.0 | 3193.9 |
| A—interim analysis data | |||||
| Data Source | Ipilimumab | Pembrolizumab | HR (95% CI) | ||
| Median Survival | Events (N) | Median Survival | Events (N) | ||
| KEYNOTE-006 trial (OS) 2nd interim analysis | not reached | NR | not reached | NR | 0.69 (0.52–0.90) |
| Reconstructed (OS) | 15.7 | 112 | 24.0 | 90 | 0.67 (0.50–0.88) |
| KEYNOTE-006 trial (PFS) 2nd interim analysis | 2.8 | NR | 4.1 | NR | 0.58 (0.47–0.72) |
| Reconstructed (PFS) | 3.3 | 190 | 5.3 | 154 | 0.58 (0.47–0.72) |
| B—long-term follow-up data | |||||
| Data Source | Ipilimumab | Pembrolizumab | HR (95% CI) | ||
| Median Survival | Events (N) | Median Survival | Events (N) | ||
| KEYNOTE-006 trial (OS) long-term follow-up | 15.9 | 172 | 32.7 | 324 | 0.73 (0.61–0.88) |
| Reconstructed (OS) | 22.1 | 171 | 35.2 | 171 | 0.73 (0.59–0.90) |
| KEYNOTE-006 trial (PFS) long-term follow-up | 3.4 | 217 | 8.4 | 411 | 0.57 (0.48–0.67) |
| Reconstructed (PFS) | 4.8 | 222 | 10.9 | 402 | 0.55 (0.47–0.65) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ball, G.; Levine, M.A.H.; Thabane, L.; Tarride, J.-E. Health Technology Reassessment: Addressing Uncertainty in Economic Evaluations of Oncology Drugs at Time of Reimbursement Using Long-Term Clinical Trial Data. Curr. Oncol. 2023, 30, 6596-6608. https://doi.org/10.3390/curroncol30070484
Ball G, Levine MAH, Thabane L, Tarride J-E. Health Technology Reassessment: Addressing Uncertainty in Economic Evaluations of Oncology Drugs at Time of Reimbursement Using Long-Term Clinical Trial Data. Current Oncology. 2023; 30(7):6596-6608. https://doi.org/10.3390/curroncol30070484
Chicago/Turabian StyleBall, Graeme, Mitchell A. H. Levine, Lehana Thabane, and Jean-Eric Tarride. 2023. "Health Technology Reassessment: Addressing Uncertainty in Economic Evaluations of Oncology Drugs at Time of Reimbursement Using Long-Term Clinical Trial Data" Current Oncology 30, no. 7: 6596-6608. https://doi.org/10.3390/curroncol30070484
APA StyleBall, G., Levine, M. A. H., Thabane, L., & Tarride, J.-E. (2023). Health Technology Reassessment: Addressing Uncertainty in Economic Evaluations of Oncology Drugs at Time of Reimbursement Using Long-Term Clinical Trial Data. Current Oncology, 30(7), 6596-6608. https://doi.org/10.3390/curroncol30070484




