Capturing the True Cost of Breast Cancer Treatment: Molecular Subtype and Stage-Specific per-Case Activity-Based Costing
Abstract
:1. Introduction
2. Methods
2.1. Costing Sources
2.2. Diagnostic Imaging
2.3. Pathology
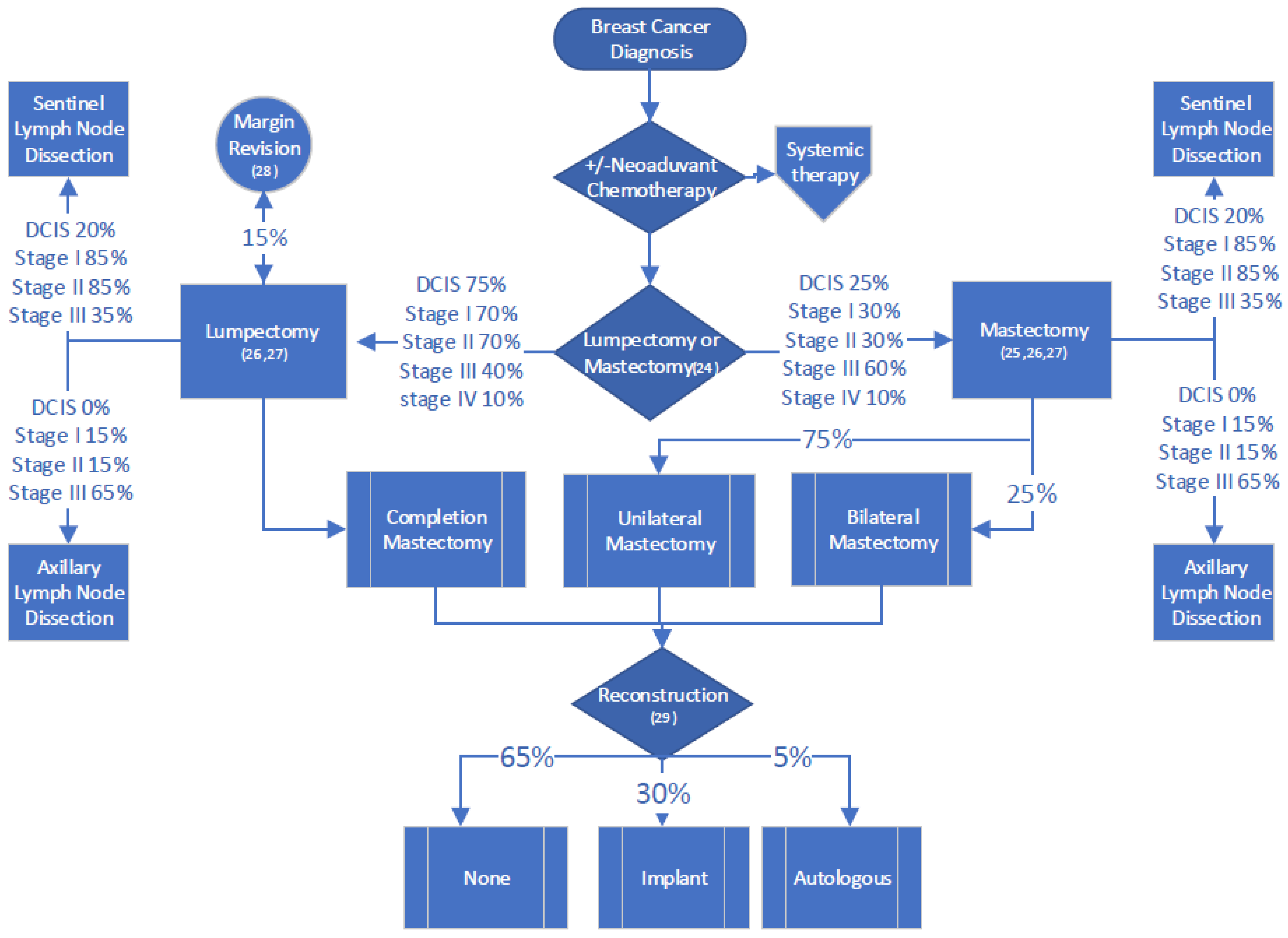
2.4. Surgery
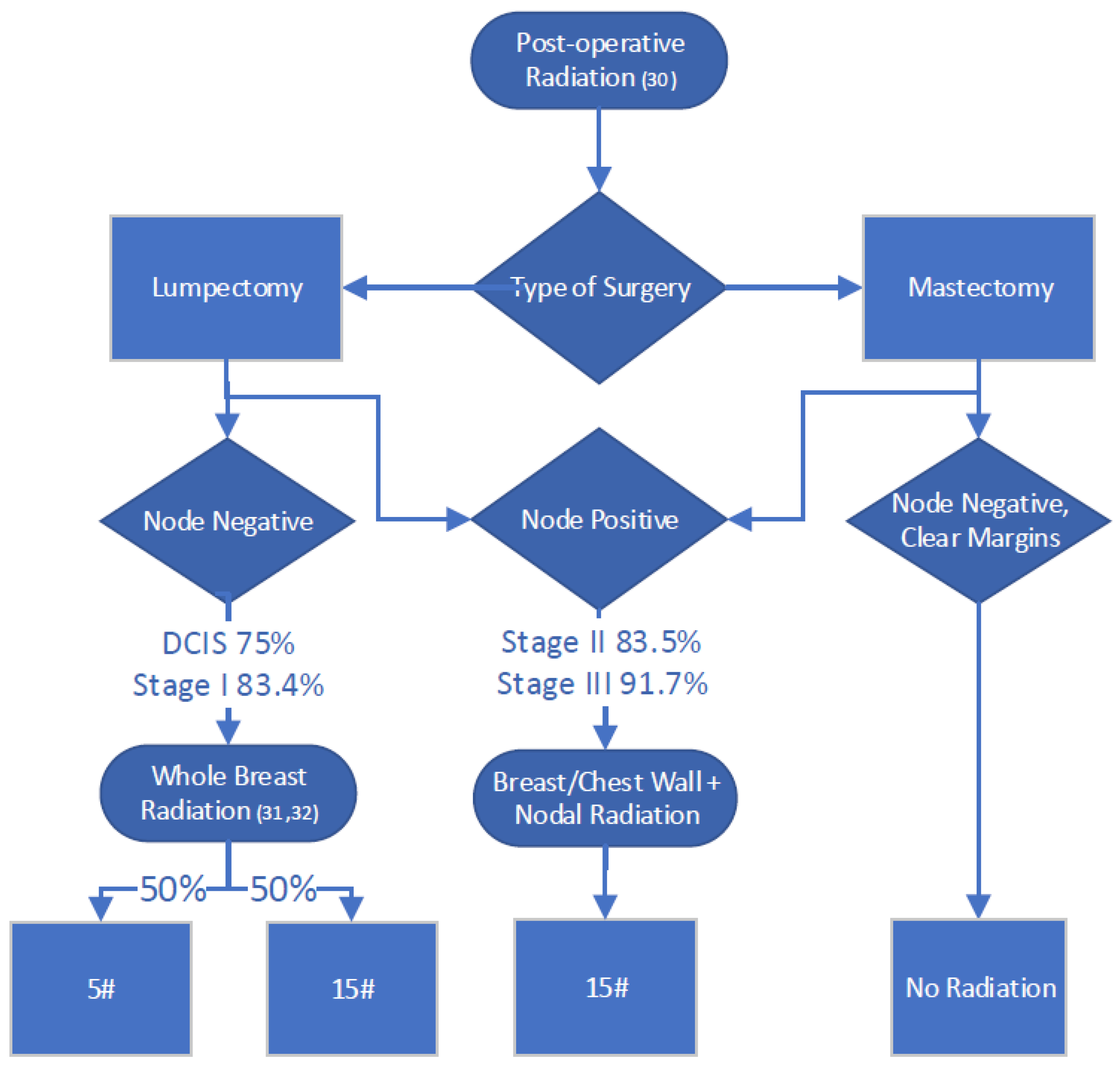
2.5. Radiation Therapy
2.6. Systemic Therapy
2.6.1. HR+
2.6.2. HER2+ and HR+/HER2+
2.6.3. Triple Negative
2.7. Inpatient, Emergency Room and Home Care Costs
2.8. Derivation of Per-Case Cost
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brenner, D.R.; Poirier, A.; Woods, R.R.; Ellison, L.F.; Billette, J.M.; Demers, A.A.; Zhang, S.X.; Yao, C.; Finley, C.; Fitzgerald, N.; et al. Projected estimates of cancer in Canada in 2022. CMAJ 2022, 194, E601–E607. [Google Scholar] [CrossRef]
- Lee, S.; Breast Cancer Statistics. Canadian Cancer Society. Available online: https://cancer.ca/en/cancer-information/cancer-types/breast/statistics (accessed on 23 June 2023).
- Wilkinson, A.N. Demystifying Breast Cancer. Can. Fam. Physician 2023, 69, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Mogyorósy, Z.; Smith, P. The Main Methodological Issues in Costing Health Care Services—A Literature Review; Mogyorosy, Working Papers 007cherp; Centre for Health Economics, University of York: York, UK, 2005. [Google Scholar]
- De Oliveira, C.; Bremner, K.E.; Pataky, R.; Gunraj, N.; Chan, K.; Peacock, S.; Krahn, M.D. Understanding the Costs of Cancer Care before and after Diagnosis for the 21 Most Common Cancers in Ontario: A Population-Based Descriptive Study. CMAJ Open 2013, 1, E1–E8. [Google Scholar] [CrossRef]
- Brezden-Masley, C.; Fathers, K.E.; Coombes, M.E.; Pourmirza, B.; Xue, C.; Jerzak, K.J. A Population-Based Comparison of Treatment Patterns, Resource Utilization, and Costs by Cancer Stage for Ontario Patients with Triple-Negative Breast Cancer. Breast Cancer Res. Treat. 2021, 185, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Arnaboldi, M.; Lapsley, I. Activity Based Costing in Healthcare: A UK Case Study. Res. Healthc. Financ. Manag. 2005, 10, 61–75. [Google Scholar]
- Da Silva Etges, A.P.B.; Cruz, L.N.; Notti, R.K.; Neyeloff, J.L.; Schlatter, R.P.; Astigarraga, C.C.; Falavigna, M.; Polanczyk, C.A. An 8-Step Framework for Implementing Time-Driven Activity-Based Costing in Healthcare Studies. Eur. J. Health Econ. 2019, 20, 1133–1145. [Google Scholar] [CrossRef]
- Xu, X.; Grossetta Nardini, H.K.; Ruger, J.P. Micro-Costing Studies in the Health and Medical Literature: Protocol for a Systematic Review. Syst. Rev. 2014, 47, 1–7. [Google Scholar] [CrossRef]
- Yong, J.H.; Nadeau, C.; Flanagan, W.M.; Coldman, A.J.; Asakawa, K.; Garner, R.; Fitzgerald, N.; Yaffe, M.J.; Miller, A.B. The OncoSim-Breast Cancer Microsimulation Model. Curr. Oncol. 2022, 29, 1619–1633. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Edge, S.B.; Hortobagyi, G.N. Eighth Edition of the AJCC Cancer Staging Manual: Breast Cancer. Ann. Surg. Oncol. 2018, 25, 1783–1785. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular Portraits of Human Breast Tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Government of Canada SC. Canadian Cancer Registry (CCR). Statistics Canada. 2023. Available online: https://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=3207 (accessed on 9 June 2023).
- OHIP-bulletins-health care professionals-OHLTC. Government of Ontario, Ministry of Health and Long-Term Care. 2023. Available online: https://www.health.gov.on.ca/en/pro/programs/ohip/sob/ (accessed on 9 June 2023).
- Ontario Ministry of Health and Long-Term Care. Formulary Search. 2021. Available online: https://www.formulary.health.gov.on.ca/formulary/ (accessed on 9 June 2023).
- Cancer Care Ontario. New Drug Funding Program. 2023. Available online: https://www.cancercareontario.ca/en/Funding/New_Drug_Funding_Program (accessed on 9 June 2023).
- Government of Ontario, Ministry of Health and Long-Term Care. Health System Funding Reform Quality-Based Procedures. 2022. Available online: https://www.health.gov.on.ca/en/pro/programs/ecfa/funding/hs_funding_qbp.aspx (accessed on 9 June 2023).
- Canadian Agency for Drugs and Technologies in Health. Homepage. 2023. Available online: https://www.C$th.ca/ (accessed on 9 June 2023).
- Arnaout, A.; Varela, N.P.; Allarakhia, M.; Grimard, L.; Hey, A.; Lau, J.; Thain, L.; Eisen, A. Baseline Staging Imaging for Distant Metastasis in Women with Stages I, II, and III Breast Cancer. Curr. Oncol. 2020, 27, 123–145. [Google Scholar] [CrossRef]
- Lin, N.U.; Thomssen, C.; Cardoso, F.; Cameron, D.; Cufer, T.; Fallowfield, L.; Francis, P.A.; Kyriakides, S.; Pagani, O.; Senkus, E.; et al. International Guidelines for Management of Metastatic Breast Cancer (MBC) from the European School of Oncology (ESO)–MBC Task Force: Surveillance, Staging, and Evaluation of Patients with Early-Stage and Metastatic Breast Cancer. Breast 2013, 22, 203–210. [Google Scholar] [CrossRef]
- Geiersbach, K.B.; Sill, D.R.; Meyer, R.G.; Yuhas, J.A.; Sukov, W.R.; Mounajjed, T.; Carter, J.M.; Jenkins, R.B.; Chen, B. HER2 Testing for Breast Cancer in the Genomics Laboratory: A Sea Change for Fluorescence in Situ Hybridization. Arch. Pathol. Lab. Med. 2021, 145, 883–886. [Google Scholar] [CrossRef]
- Sparano, J.A.; Gray, R.J.; Makower, D.F.; Pritchard, K.I.; Albain, K.S.; Hayes, D.F.; Geyer, C.E.; Dees, E.C.; Perez, E.A.; Olson, J.A.; et al. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N. Engl. J. Med. 2015, 373, 2005–2014. [Google Scholar] [CrossRef]
- Kalinsky, K.; Barlow, W.E.; Gralow, J.R.; Meric-Bernstam, F.; Albain, K.S.; Hayes, D.F.; Lin, N.U.; Perez, E.A.; Goldstein, L.J.; Chia, S.K.L.; et al. 21-Gene Assay to Inform Chemotherapy Benefit in Node-Positive Breast Cancer. N. Engl. J. Med. 2021, 385, 2336–2347. [Google Scholar] [CrossRef] [PubMed]
- Kapur, H.; Chen, L.; Warburton, R.; Pao, J.-S.; Dingee, C.; Urve, K.; Bazzarelli, A.; McKevitt, E. De-Escalating Breast Cancer Surgery: Should We Apply Quality Indicators from Other Jurisdictions in Canada? Curr. Oncol. 2022, 29, 144–154. [Google Scholar] [CrossRef]
- Lim, D.W.; Metcalfe, K.A.; Narod, S.A. Bilateral Mastectomy in Women with Unilateral Breast Cancer. JAMA Surgery 2021, 156, 569–576. [Google Scholar] [CrossRef]
- Findlay-Shirras, L.; Lima, I.; Smith, G.; Clemons, M.; Arnaout, A. Canada Follows the US in the Rise of Bilateral Mastectomies for Unilateral Breast Cancer: A 23-Year Population Cohort Study. Breast Cancer Res. Treat. 2021, 185, 517–525. [Google Scholar] [CrossRef]
- Keilty, D.; Namini, S.N.; Swain, M.; Maganti, M.; Cil, T.D.; McCready, D.R.; Cescon, D.W.; Amir, E.; Fleming, R.; Mulligan, A.M.; et al. Patterns of Recurrence and Predictors of Survival in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy, Surgery, and Radiation. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.; Hamm, J.; McGahan, C.; Baliski, C. Surgeon Volume, Patient Age, and Tumor-Related Factors Influence the Need for Re-Excision after Breast-Conserving Surgery. Ann. Surg. Oncol. 2016, 23, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Doherty, C.; Pearce, S.; Baxter, N.; Knowles, S.; Ross, D.; McClure, J.A.; Brackstone, M. Trends in Immediate Breast Reconstruction and Radiation after Mastectomy: A Population Study. Breast J. 2020, 26, 446–453. [Google Scholar] [CrossRef]
- Mittmann, N.; Liu, N.; Cheng, S.Y.; Seung, S.J.; Saxena, F.E.; Hong, N.J.L.; Earle, C.C.; Cheung, M.C.; Leighl, N.B.; Coburn, N.G.; et al. Health System Costs for Cancer Medications and Radiation Treatment in Ontario for the 4 Most Common Cancers: A Retrospective Cohort Study. Can. Med. Assoc. Open Access J. 2020, 8, E191–E198. [Google Scholar] [CrossRef] [PubMed]
- Whelan, T.J.; Pignol, J.-P.; Levine, M.N.; Julian, J.A.; MacKenzie, R.; Parpia, S.; Shelley, W.; Grimard, L.; Bowen, J.; Lukka, H.; et al. Long-Term Results of Hypofractionated Radiation Therapy for Breast Cancer. N. Engl. J. Med. 2010, 362, 513–520. [Google Scholar] [CrossRef]
- Brunt, A.M.; Haviland, J.S.; Wheatley, D.A.; Sydenham, M.A.; Alhasso, A.; Bloomfield, D.J.; Chan, C.; Churn, M.; Cleator, S.; Coles, C.E.; et al. Hypofractionated Breast Radiotherapy for 1 Week versus 3 Weeks (FAST-Forward): 5-Year Efficacy and Late Normal Tissue Effects Results from a Multicentre, Non-Inferiority, Randomised, Phase 3 Trial. Lancet 2020, 395, 1613–1626. [Google Scholar] [CrossRef] [PubMed]
- Steinauer, K.; Gross, M.W.; Huang, D.J.; Eppenberger-Castori, S.; Güth, U. Radiotherapy in Patients with Distant Metastatic Breast Cancer. Radiat. Oncol. 2014, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cancer Care Ontario. Guidelines & Advice. Government of Ontario. 2017. Available online: https://www.cancercareontario.ca/en/guidelines-advice (accessed on 22 June 2023).
- Breast Cancer. 2023. Available online: https://old-prod.asco.org/practice-patients/guidelines/breast-cancer (accessed on 23 June 2023).
- C$th Provisional Funding Algorithms. Available online: https://www.C$th.ca/C$th-provisional-funding-algorithms (accessed on 23 June 2023).
- Burstein, H.J.; Lacchetti, C.; Anderson, H.; Buchholz, T.A.; Davidson, N.E.; Gelmon, K.A.; Giordano, S.H.; Hudis, C.A.; Solky, A.J.; Stearns, V.; et al. Adjuvant Endocrine Therapy for Women with Hormone Receptor–Positive Breast Cancer: ASCO Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2019, 37, 423–438. [Google Scholar] [CrossRef]
- Gnant, M.; Fitzal, F.; Rinnerthaler, G.; Steger, G.G.; Greil-Ressler, S.; Balic, M.; Heck, D.; Jakesz, R.; Thaler, J.; Egle, D.; et al. Duration of Adjuvant Aromatase-Inhibitor Therapy in Postmenopausal Breast Cancer. N. Engl. J. Med. 2021, 385, 395–405. [Google Scholar] [CrossRef]
- Denduluri, N.; Somerfield, M.R.; Chavez-MacGregor, M.; Comander, A.H.; Dayao, Z.; Eisen, A.; Freedman, R.A.; Gopalakrishnan, R.; Graff, S.L.; Hassett, M.J.; et al. Selection of Optimal Adjuvant Chemotherapy and Targeted Therapy for Early Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 685–693. [Google Scholar] [CrossRef]
- Bonilla, L.; Ben-Aharon, I.; Vidal, L.; Gafter-Gvili, A.; Leibovici, L.; Stemmer, S.M. Dose-Dense Chemotherapy in Nonmetastatic Breast Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Natl. Cancer Inst. 2010, 102, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Lacchetti, C.; Anderson, H.; Buchholz, T.A.; Davidson, N.E.; Gelmon, K.E.; Giordano, S.H.; Hudis, C.A.; Solky, A.J.; Stearns, V.; et al. Adjuvant Endocrine Therapy for Women with Hormone Receptor–Positive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update on Ovarian Suppression. J. Clin. Oncol. 2016, 34, 1689–1701. [Google Scholar] [CrossRef]
- Johnston, S.R.; Harbeck, N.; Hegg, R.; Toi, M.; Martin, M.; Shao, Z.M.; Zhang, Q.Y.; Rodriguez, J.L.; Campone, M.; Hamilton, E.; et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2−, node-positive, high-risk, early breast cancer (monarchE). J. Clin. Oncol. 2020, 38, 3987. [Google Scholar] [CrossRef] [PubMed]
- Eisen, A.; Somerfield, M.R.; Accordino, M.K.; Blanchette, P.S.; Clemons, M.J.; Dhesy-Thind, S.; Dillmon, M.S.; D’Oronzo, S.; Fletcher, G.G.; Frank, E.S.; et al. Use of Adjuvant Bisphosphonates and Other Bone-Modifying Agents in Breast Cancer: ASCO-OH (CCO) Guideline Update. J. Clin. Oncol. 2022, 40, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Somerfield, M.R.; Barton, D.L.; Dorris, A.; Fallowfield, L.J.; Jain, D.; Johnston, S.R.D.; Korde, L.A.; Litton, J.K.; Macrae, E.R.; et al. Endocrine Treatment and Targeted Therapy for Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 3959–3977. [Google Scholar] [CrossRef] [PubMed]
- Nathan, M.R.; Schmid, P. A Review of Fulvestrant in Breast Cancer. Oncol. Ther. 2017, 5, 17–29. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Barry, W.T.; Dang, C.T.; Yardley, D.A.; Moy, B.; Marcom, P.K.; Albain, K.S.; Rugo, H.S.; Ellis, M.; Shapira, I.; et al. Adjuvant Paclitaxel and Trastuzumab for Node-Negative, HER2-Positive Breast Cancer. N. Engl. J. Med. 2015, 372, 134–141. [Google Scholar] [CrossRef]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1485. [Google Scholar] [CrossRef]
- Giordano, S.H.; Franzoi, M.A.B.; Temin, S.; Anders, C.K.; Chandarlapaty, S.; Crews, J.R.; Kirshner, J.J.; Krop, I.E.; Lin, N.U.; Morikawa, A.; et al. Systemic Therapy for Advanced Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2022, 40, 2612–2635. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef]
- Cortes, J.; Rugo, H.S.; Cescon, D.W.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Perez-Garcia, J.; Iwata, H.; et al. Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 387, 217–226. [Google Scholar] [CrossRef]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Mittmann, N.; Porter, J.M.; Rangrej, J.; Seung, S.J.; Liu, N.; Saskin, R.; Cheung, M.C.; Leighl, N.B.; Hoch, J.S.; Trudeau, M.; et al. Health System Costs for Stage-Specific Breast Cancer: A Population-Based Approach. Curr. Oncol. 2014, 21, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Inflation Calculator. Available online: https://www.bankofcanada.ca/rates/related/inflation-calculator/ (accessed on 23 June 2023).
- Brezden-Masley, C.; Fathers, K.E.; Coombes, M.E.; Pourmirza, B.; Xue, C.; Jerzak, K.J. A Population-Based Comparison of Treatment Patterns, Resource Utilization, and Costs by Cancer Stage for Ontario Patients with Triple-Negative Breast Cancer. Cancer Med. 2020, 9, 7548–7557. [Google Scholar] [CrossRef] [PubMed]
- Grady, I.; Grady, S.; Chanisheva, N. Long-Term Cost of Breast Cancer Treatment to the United States Medicare Program by Stage at Diagnosis. Eur. J. Health Econ. 2021, 22, 1365–1370. [Google Scholar] [CrossRef]
- Schneider, P.; Ramaekers, B.; Pouwels, X.; Geurts, S.M.E.; Khava, I.E. Ibragimova; Maaike de Boer; B.E.P.J. Vriens; van; Marien den Boer; Pepels, M.J.; Vivianne, C.G. Tjan-Heijnen; Joore, M.A. Direct Medical Costs of Advanced Breast Cancer Treatment: A Real-World Study in the Southeast of the Netherlands. Value Health 2021, 24, 668–675. [Google Scholar] [CrossRef]
- Nagra, N.; Tsangaris, E.; Means, J.; Hassett, M.; Dominici, L.; Bellon, J.; Broyles, J.; Kaplan, R.; Feeley, T.; Pusic, A. Time-driven activity-based costing in breast cancer care delivery. Ann. Surg. Oncol. 2022, 29, 510–521. [Google Scholar] [CrossRef]
- Mittmann, N.; Stout, N.K.; Lee, P.; Tosteson, A.N.; Trentham-Dietz, A.; Alagoz, O.; Yaffe, M.J. Total Cost-Effectiveness of Mammography Screening Strategies. Health Rep. 2015, 26, 16. [Google Scholar]
- Wilkinson, A.N.; Billette, J.-M.; Ellison, L.F.; Killip, M.A.; Islam, N.; Seely, J.M. The Impact of Organised Screening Programs on Breast Cancer Stage at Diagnosis for Canadian Women Aged 40–49 and 50–59. Curr. Oncol. 2022, 29, 5627–5643. [Google Scholar] [CrossRef]
- Garaszczuk, R.; Yong, J.H.E.; Sun, Z.; de Oliveira, C. The Economic Burden of Cancer in Canada from a Societal Perspective. Curr. Oncol. 2022, 29, 2735–2748. [Google Scholar] [CrossRef]

| Subtype | Stage | ||||
|---|---|---|---|---|---|
| DCIS | I | II | III | IV | |
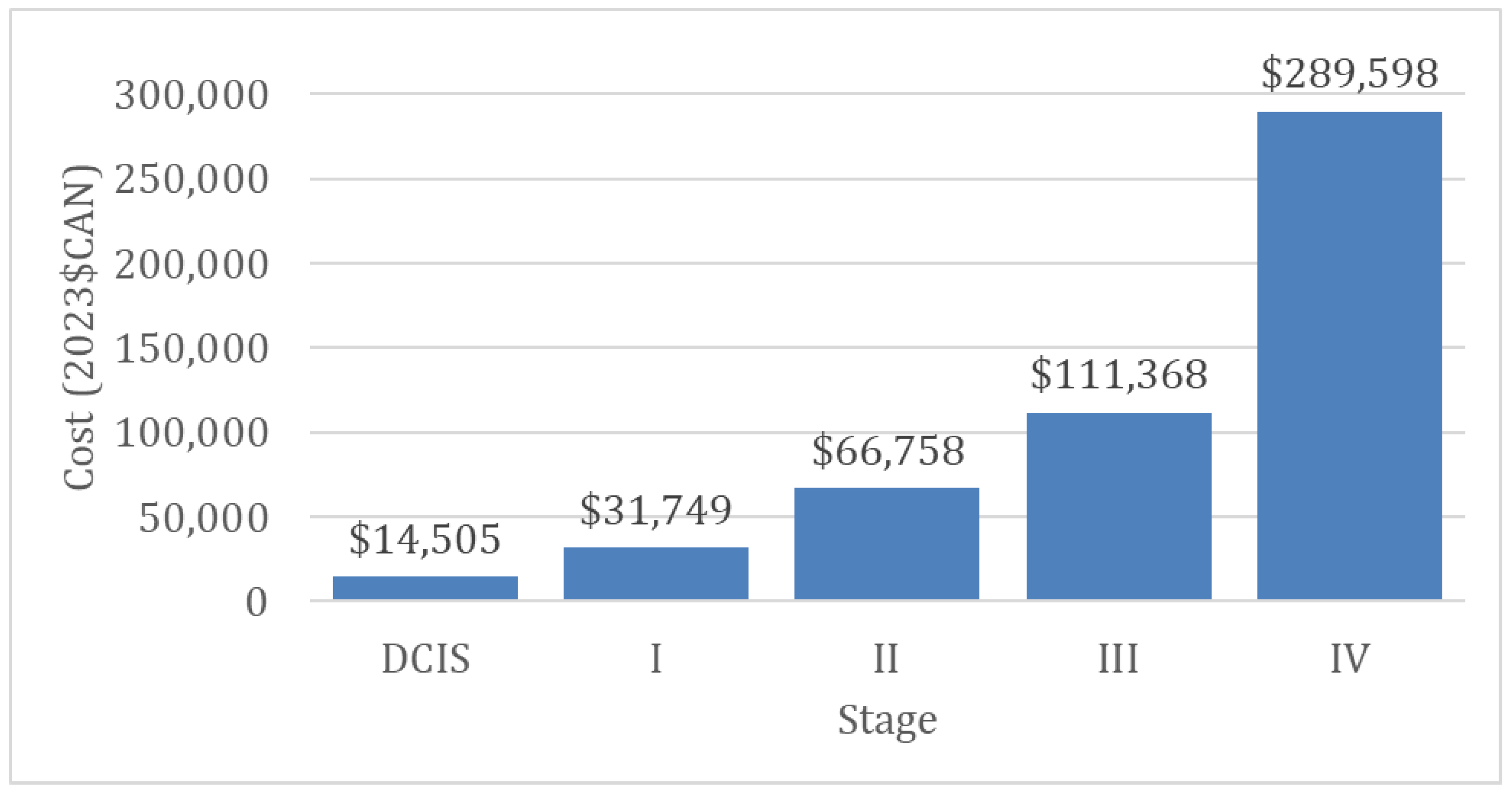
| HR+ | 14,505 | 28,201 | 60,289 | 117,269 | 256,693 |
| HR+/HER2+ | C$ 56,401 | 76,547 | 86,653 | 516,415 | |
| HER2+ | 47,201 | 67,136 | 75,954 | 514,992 | |
| TN | 25,247 | 101,811 | C110,798 | 193,490 | |
| Mean | 14,505 | 39,263 | 76,446 | 97,668 | 370,398 |
| Subtype | Stage Comparison | ||||||
|---|---|---|---|---|---|---|---|
| DCIS vs. I | DCIS vs. II | DCIS vs. IV | III vs. I | III vs. II | IV vs. I | IV vs. II | |
| HR+ | 0.5 | 0.2 | 0.1 | 4.2 | 1.9 | 9.1 | 4.3 |
| HR+/HER2+ | 0.3 | 0.2 | 0.0 | 1.5 | 1.1 | 9.2 | 6.7 |
| HER2+ | 0.3 | 0.2 | 0.0 | 1.6 | 1.1 | 10.9 | 7.7 |
| TN | 0.6 | 0.1 | 0.1 | 4.4 | 1.1 | 7.7 | 1.9 |
| Mean | 0.4 | 0.2 | 0.0 | 3.0 | 1.4 | 9.2 | 5.0 |
| Subtype | Treatment Modality | Stage | ||||
|---|---|---|---|---|---|---|
| DCIS | I | II | III | IV | ||
| HR+ | Diagnostic Imaging | C$ 0 | C$ 169 | C$ 169 | C$ 424 | C$ 6614 |
| Pathology | C$ 1813 | C$ 4113 | C$ 5755 | C$ 2305 | C$ 2116 | |
| Surgery | C$ 6798 | C$ 7808 | C$ 7938 | C$ 9436 | C$ 1438 | |
| Radiation Therapy | C$ 3682 | C$ 3682 | C$ 5337 | C$ 4986 | C$ 1711 | |
| Systemic Therapy | C$ 2212 | C$ 4307 | C$ 27,665 | C$ 80,162 | C$ 209,228 | |
| ER/Inpatient/Home care | C$ 0 | C$ 8122 | C$ 13,425 | C$ 19,956 | C$ 35,587 | |
| HR+/HER2+ | Diagnostic Imaging | C$ 169 | C$ 169 | C$ 424 | C$ 7788 | |
| Pathology | C$ 1190 | C$ 1190 | C$ 2252 | C$ 2116 | ||
| Surgery | C$ 7808 | C$ 7938 | C$ 9532 | C$ 1438 | ||
| Radiation Therapy | C$ 3687 | C$ 5401 | C$ 4986 | C$ 1856 | ||
| Systemic Therapy | C$ 35,425 | C$ 48,423 | C$ 49,503 | C$ 467,631 | ||
| ER/Inpatient/Home care | C$ 8122 | C$ 13,425 | C$ 19,956 | C$ 35,587 | ||
| HER2+ | Diagnostic Imaging | C$ 169 | C$ 169 | C$ 255 | C$ 7788 | |
| Pathology | C$ 1253 | C$ 1253 | C$ 2314 | C$ 2116 | ||
| Surgery | C$ 7808 | C$ 7938 | C$ 9532 | C$ 1438 | ||
| Radiation Therapy | C$ 3684 | C$ 5401 | C$ 4986 | C$ 1914 | ||
| Systemic Therapy | C$ 26,166 | C$ 38,949 | C$ 38,910 | C$ 466,150 | ||
| ER/Inpatient/Home care | C$ 8122 | C$ 13,425 | C$ 19,956 | C$ 35,587 | ||
| TN | Diagnostic Imaging | C$ 169 | C$ 169 | C$ 424 | C$ 3202 | |
| Pathology | C$ 1561 | C$ 1561 | C$ 2622 | C$ 2572 | ||
| Surgery | C$ 7808 | C$ 7938 | C$ 9532 | C$ 1438 | ||
| Radiation Therapy | C$ 3687 | C$ 5401 | C$ 4986 | C$ 1923 | ||
| Systemic Therapy | C$ 3901 | C$ 73,317 | C$ 73,278 | C$ 148,769 | ||
| ER/Inpatient/Home care | C$ 8122 | C$ 13,425 | C$ 19,956 | C$ 35,587 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilkinson, A.N.; Seely, J.M.; Rushton, M.; Williams, P.; Cordeiro, E.; Allard-Coutu, A.; Look Hong, N.J.; Moideen, N.; Robinson, J.; Renaud, J.; et al. Capturing the True Cost of Breast Cancer Treatment: Molecular Subtype and Stage-Specific per-Case Activity-Based Costing. Curr. Oncol. 2023, 30, 7860-7873. https://doi.org/10.3390/curroncol30090571
Wilkinson AN, Seely JM, Rushton M, Williams P, Cordeiro E, Allard-Coutu A, Look Hong NJ, Moideen N, Robinson J, Renaud J, et al. Capturing the True Cost of Breast Cancer Treatment: Molecular Subtype and Stage-Specific per-Case Activity-Based Costing. Current Oncology. 2023; 30(9):7860-7873. https://doi.org/10.3390/curroncol30090571
Chicago/Turabian StyleWilkinson, Anna N., Jean M. Seely, Moira Rushton, Phillip Williams, Erin Cordeiro, Alexandra Allard-Coutu, Nicole J. Look Hong, Nikitha Moideen, Jessica Robinson, Julie Renaud, and et al. 2023. "Capturing the True Cost of Breast Cancer Treatment: Molecular Subtype and Stage-Specific per-Case Activity-Based Costing" Current Oncology 30, no. 9: 7860-7873. https://doi.org/10.3390/curroncol30090571
APA StyleWilkinson, A. N., Seely, J. M., Rushton, M., Williams, P., Cordeiro, E., Allard-Coutu, A., Look Hong, N. J., Moideen, N., Robinson, J., Renaud, J., Mainprize, J. G., & Yaffe, M. J. (2023). Capturing the True Cost of Breast Cancer Treatment: Molecular Subtype and Stage-Specific per-Case Activity-Based Costing. Current Oncology, 30(9), 7860-7873. https://doi.org/10.3390/curroncol30090571







