Impact of Fixed-Duration Oral Targeted Therapies on the Economic Burden of Chronic Lymphocytic Leukemia in Canada
Abstract
1. Introduction
2. Materials and Methods
2.1. Model Structure
2.2. Patient Characteristics
| Parameters | Model | Reference |
|---|---|---|
| Probabilities, % (unless otherwise stated) | ||
| Probability of WW at diagnosis | 85.00 | Assumption; Chen [18] |
| Median time to first treatment (years) | 4.8 | Parikh [2] |
| Transition probability from WW to first treatment by cycle | 1.65 | Model calibration |
| Proportion of patients in IV therapy | 100.00 | When both formulas available |
| Prevalence of del(17)p | 7.00 | Hallek [19] |
| Proportion of mutated IGHV | 40.00 | Assumption, confirmed by KOL |
| Proportion of non-mutated IGHV | 60.00 | Assumption, confirmed by KOL |
| Median age at diagnosis (years) | 71 | LLSC [1] |
| Probability of age at diagnosis | Statistics Canada [17] | |
| Age < 65 years | 33.75 * | |
| Age 65–70 years | 14.34 * | |
| Age > 70 years | 51.91 * | |
| Probability of fitness | Assumption, confirmed by KOL | |
| Age < 65 years | 90.00 | |
| Age 65–70 years | 50.00 | |
| Age > 70 years | 15.0 * | |
| Probability of discontinuing OTT for each 4-week cycle | Burger [20] Model calibration | |
| In first-line treatment | 0.70 | |
| For relapsed patients | 1.40 | |
| Probability of death by cycle of 28 days according to the repartition of CLL age Category | 0.695 * | Statistics Canada [21] Model calibration |
| Costs, C$ | ||
| Follow-up and laboratory monitoring costs | ||
| Electrolyte panel | 18.08 * | Code L226, 204, 053, 165, 194, 061, 700 [22] |
| Renal panel | 13.32 * | Code L251, 067, 700 [22] |
| Liver function test | 21.15 * | Code L223, 222, 191, 029, 030, 031, 005, 208, 700 [22] |
| CBC panel | 14.74 * | Code L393, 700 [22] |
| Coagulation parameters | 13.42 * | Code L445, 700 [22] |
| Serology | 21.01 * | Code L319, 700 [22] |
| Chemotherapy infusion, administration, and management | 105.15 | Schedule of benefits. Code G359 [23] |
| Professional fees | ||
| Consultation, Hematology | 157.00 | Schedule of Benefits. Code A615 [23] |
| Partial assessment, Hematology | 38.05 | Schedule of Benefits. Code A618 [23] |
| Nurse average wage (C$/min) | 0.63 * | Statistic Canada [24]; Job Bank Canada, NOC 3012 [25] |
| Pharmacist average wage (C$/min) | 0.87 * | Statistic Canada [24]; Job Bank Canada, NOC 3131 [25] |
| Adverse events | ||
| Anemia | 759.71 * | OCC, code D649 [26]. Assuming 2% managed inpatient |
| Neutropenia | 523.23 * | OCC, code D700 [26]. Assuming 100% managed outpatient |
| Febrile neutropenia | 10,918 * | OCC, code R508 [26]. Assuming 100% managed inpatient |
| Thrombocytopenia | 441.86 * | OCC, code D696 [26]. Assuming 100% managed outpatient |
| Infection | 1831 * | OCC, code A499/B349 [26]. Assuming 25% managed inpatient |
| Atrial fibrillation | 1413 * | OCC, code I4890 [26]. Assuming 10% managed inpatient |
| Palliative care | 9326 * | CIHI, Code 810. (Average all adult patients, Canada) |
2.3. Simulated Clinical Pathway
2.4. Treatment Algorithms
2.5. Cost Data
2.6. Model Outcomes
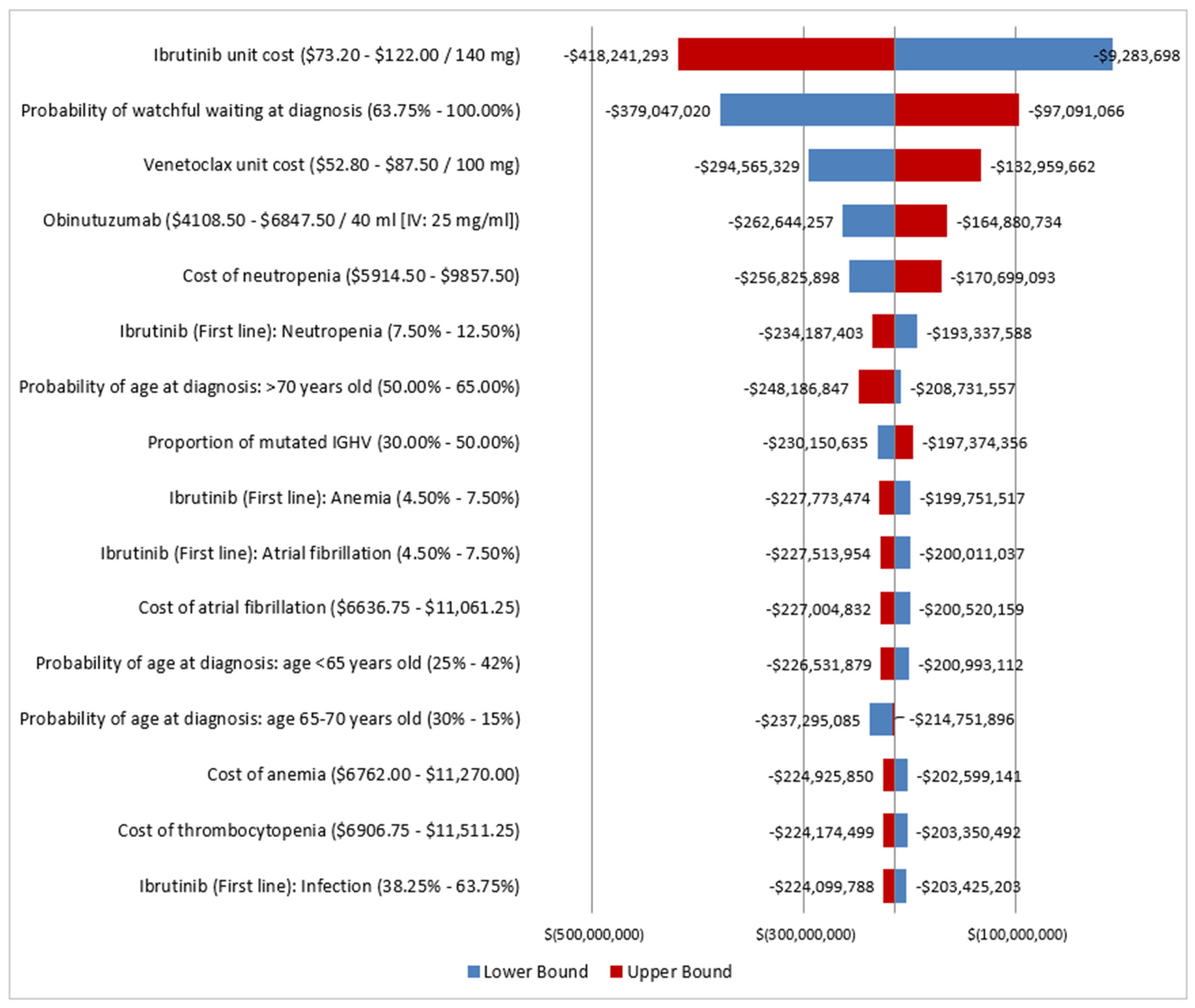
2.7. Sensitivity Analysis
3. Results
3.1. Disease Burden
3.2. Cost Burden
3.2.1. Total Annual Cost of CLL
3.2.2. Cost of First-Line Therapy for CLL
3.2.3. Cost of Second-Line Therapy for CLL
3.3. Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leukemia and Lymphoma Society of Canada. Blood Cancer in Canada-Facts and Stats. Available online: https://www.llscanada.org/disease-information/facts-and-statistics#Leukemia (accessed on 1 October 2020).
- Parikh, S.A.; Rabe, K.G.; Kay, N.E.; Call, T.G.; Ding, W.; Schwager, S.M.; Bowen, D.A.; Conte, M.; Jelinek, D.F.; Slager, S.L.; et al. Chronic lymphocytic leukemia in young (≤55 years) patients: A comprehensive analysis of prognostic factors and outcomes. Haematologica 2014, 99, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Hallek, M.J.; Pagel, J.M. Chemoimmunotherapy Versus Targeted Treatment in Chronic Lymphocytic Leukemia: When, How Long, How Much, and in Which Combination? Am. Soc. Clin. Oncol. Educ. Book 2016, 35, e387–e398. [Google Scholar] [CrossRef] [PubMed]
- Canadian Agency for Drugs and Technologies in Health (CADTH)-pCODR. Provincial Funding Summary: Ibrutinib (Imbruvica) for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (pCODR 10043). Available online: https://www.cadth.ca/sites/default/files/pcodr/pcodr-provfund_ibrutinib_imbruvica-CLL.pdf (accessed on 5 November 2020).
- Canadian Agency for Drugs and Technologies in Health (CADTH)-pCODR. Provincial Funding Summary: Ibrutinib (Imbruvica) for Chronic Lymphocytic Leukemia/Small Lymphocytic Leukemia (Previously Untreated) (pCODR 10085). Available online: https://www.cadth.ca/sites/default/files/pcodr/pcodr-provfund_ibrutinib_imbruvica-cll-sll-preun.pdf (accessed on 7 November 2020).
- Product Monograph Including Patient Medication Information: Calquence (Acalabrutinib Capsules); AstraZeneca Canada Inc.: Mississauga, ON, Canada, 2019.
- Pan-Canadian Oncology Drug Review (pCODR). pCODR Expert Review Committee (pERC) Final Recommendation: Acalabrutinib (CALQUENCE). Available online: https://www.cadth.ca/sites/default/files/pcodr/Reviews2020/10211AcalabrutinibCLL_fnRec_REDACT_EC_Post17Nov2020_final.pdf (accessed on 1 November 2020).
- Scheffold, A.; Stilgenbauer, S. Revolution of Chronic Lymphocytic Leukemia Therapy: The Chemo-Free Treatment Paradigm. Curr. Oncol. Rep. 2020, 22, 16. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Tedeschi, A.; Bairey, O.; Hillmen, P.; Coutre, S.E.; Devereux, S.; et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia 2020, 34, 787–798. [Google Scholar] [CrossRef]
- Munir, T.; Brown, J.R.; O’Brien, S.; Barrientos, J.C.; Barr, P.M.; Reddy, N.M.; Coutre, S.; Tam, C.S.; Mulligan, S.P.; Jaeger, U.; et al. Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am. J. Hematol. 2019, 94, 1353–1363. [Google Scholar] [CrossRef]
- Lachaine, J.; Beauchemin, C.; Guinan, K.; Thebault, P.; Aw, A.; Banerji, V.; Fleury, I.; Owen, C. Impact of Oral Targeted Therapy on the Economic Burden of Chronic Lymphocytic Leukemia in Canada. Curr. Oncol. 2021, 28, 332–345. [Google Scholar] [CrossRef]
- Canadian Agency for Drugs and Technologies in Health (CADTH)-pCODR. Provincial Funding Summary: Venetoclax (Venclexta) in Combo Rituximab for Chronic Lymphocytic Leukemia (pCODR 10162). Available online: https://www.cadth.ca/sites/default/files/pcodr/pcodr_provfund_10162_venetoclax_venclexta_and_rituximab.pdf (accessed on 7 November 2020).
- Pan-Canadian Oncology Drug Review (pCODR). pCODR Expert Review Committee (pERC) Final Recommendation: Venetoclax (Venclexta) in Combination with Obinutuzumab. Available online: https://www.cadth.ca/sites/default/files/pcodr/Reviews2020/10212VenetoclaxObinutuzumabCLL_fnRec_EC_Post17Nov2020_final.pdf (accessed on 1 November 2020).
- Al-Sawaf, O.; Zhang, C.; Tandon, M.; Sinha, A.; Fink, A.; Robrecht, S.; Samoylova, O.; Liberati, A.; Pinilla-Ibarz, J.; Opat, S.; et al. Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): Follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1188–1200. [Google Scholar] [CrossRef]
- Seymour, J.F.; Kipps, T.J.; Eichhorst, B.; Hillmen, P.; D’Rozario, J.; Assouline, S.; Owen, C.; Gerecitano, J.; Robak, T.; De la Serna, J.; et al. Venetoclax–Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2018, 378, 1107–1120. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Venclyxto-Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/venclyxto-epar-product-information_en.pdf (accessed on 1 May 2021).
- Statistics Canada. Number and Rates of New Cases of Primary Cancer, by Cancer Type, Age Group and Sex. Table: 13-10-0111-01. Available online: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310011101&pickMembers%5B0%5D=2.1&pickMembers%5B1%5D=3.1&pickMembers%5B2%5D=4.51 (accessed on 3 November 2020).
- Chen, Q.; Jain, N.; Ayer, T.; Wierda, W.G.; Flowers, C.R.; O’Brien, S.M.; Keating, M.J.; Kantarjian, H.M.; Chhatwal, J. Economic Burden of Chronic Lymphocytic Leukemia in the Era of Oral Targeted Therapies in the United States. J. Clin. Oncol. 2017, 35, 166–174. [Google Scholar] [CrossRef]
- Hallek, M.; Fischer, K.; Fingerle-Rowson, G.; Fink, A.M.; Busch, R.; Mayer, J.; Hensel, M.; Hopfinger, G.; Hess, G.; von Grunhagen, U.; et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: A randomised, open-label, phase 3 trial. Lancet 2010, 376, 1164–1174. [Google Scholar] [CrossRef]
- Burger, J.A.; Tedeschi, A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Bairey, O.; Hillmen, P.; Bartlett, N.L.; Li, J.; et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2015, 373, 2425–2437. [Google Scholar] [CrossRef] [PubMed]
- Statistics Canada. Life Expectancy and Other Elements of the Life table, Canada, All Provinces Except Prince Edward Island (Table: 13-10-0114-01). Available online: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310011401&pickMembers%5B0%5D=1.1&pickMembers%5B1%5D=3.1&pickMembers%5B2%5D=4.3&cubeTimeFrame.startYear=2013+%2F+2015&cubeTimeFrame.endYear=2017+%2F+2019&referencePeriods=20130101%2C20170101 (accessed on 3 November 2020).
- Ministry of Health and Long Term Care Ontario Health Insurance Plan. Schedule of Benefits for Laboratory Services. 2020. Available online: https://health.gov.on.ca/en/pro/programs/ohip/sob/physserv/sob_master.pdf (accessed on 3 November 2020).
- Ministry of Health and Long Term Care Ontario Health Insurance Plan. Schedule of Benefits-Physician Services under the Health Insurance Act. 2020. Available online: https://health.gov.on.ca/en/pro/programs/ohip/sob/lab/lab_mn2020.pdf (accessed on 3 November 2020).
- Statistics Canada. Average Usual Hours and Wages by Selected Characteristics, Monthly, Unadjusted for Seasonality (Table: 14-10-0320-02). Available online: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1410032002 (accessed on 1 October 2020).
- Government of Canada. Job Bank-Wage Report. Available online: https://www.jobbank.gc.ca/wagereport/location/geo9219 (accessed on 3 October 2020).
- Ontario Ministry of Health and Long Term Care. Ontario Care Costing Analysis Tool. Available online: https://hsimi.ca/occp/occpreports/ (accessed on 2 October 2020).
- Goede, V.; Fischer, K.; Dyer, M.J.S.; Müller, L.; Smolej, L.; Di Bernardo, M.C.; Knapp, A.; Nielsen, T.; Hallek, M. Overall survival benefit of obinutuzumab over rituximab when combined with chlorambucil in patients with chronic lymphocytic leukemia and comorbidities: Final survival analaysis of CLL 11 study. EMA 2018, 215923, S151. [Google Scholar]
- Goede, V.; Fischer, K.; Busch, R.; Engelke, A.; Eichhorst, B.; Wendtner, C.M.; Chagorova, T.; de la Serna, J.; Dilhuydy, M.S.; Illmer, T.; et al. Obinutuzumab plus Chlorambucil in Patients with CLL and Coexisting Conditions. N. Engl. J. Med. 2014, 370, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Bahlo, J.; Fink, A.M.; Goede, V.; Herling, C.D.; Cramer, P.; Langerbeins, P.; von Tresckow, J.; Engelke, A.; Maurer, C.; et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: Updated results of the CLL8 trial. Blood 2016, 127, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Woyach, J.A.; Ruppert, A.S.; Heerema, N.A.; Peterson, B.L.; Gribben, J.G.; Morrison, V.A.; Rai, K.R.; Larson, R.A.; Byrd, J.C. Chemoimmunotherapy with fludarabine and rituximab produces extended overall survival and progression-free survival in chronic lymphocytic leukemia: Long-term follow-up of CALGB study 9712. J. Clin. Oncol. 2011, 29, 1349–1355. [Google Scholar] [CrossRef]
- Byrd, J.C.; Petersonm, B.L.; Morrison, V.A.; Park, K.; Jacobson, R.; Hoke, E.; Vardiman, J.; Rai, K.; Schiffer, C.A.; Larson, R.A. Randomized phase 2 study of fludarabine with concurrent versus sequentialtreatment with rituximab in symptomatic, untreated patients with B-cellchronic lymphocytic leukemia: Results from Cancer and LeukemiaGroup B 9712 (CALGB 9712). Blood 2003, 101, 6–14. [Google Scholar] [CrossRef]
- Eichhorst, B.F.; Busch, R.; Stilgenbauer, S.; Stauch, M.; Bergmann, M.A.; Ritgen, M.; Kranzhofer, N.; Rohrberg, R.; Soling, U.; Burkhard, O.; et al. First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood 2009, 114, 3382–3391. [Google Scholar] [CrossRef]
- Woyach, J.A.; Ruppert, A.S.; Heerema, N.A.; Zhao, W.; Booth, A.W.; Ding, W.; Bartlett, N.L.; Brander, D.M.; Barr, P.M.; Rogers, K.A.; et al. Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. N. Engl. J. Med. 2018, 379, 2517–2528. [Google Scholar] [CrossRef]
- Fischer, K.; Cramer, P.; Busch, R.; Bottcher, S.; Bahlo, J.; Schubert, J.; Pfluger, K.H.; Schott, S.; Goede, V.; Isfort, S.; et al. Bendamustine in Combination With Rituximab for Previously Untreated Patients with Chronic Lymphocytic Leukemia: A Multicenter Phase II Trial of the German Chronic Lymphocytic Leukemia Study Group. J. Clin. Oncol. 2012, 30, 3209–3216. [Google Scholar] [CrossRef]
- Sharman, J.P.; Egyed, M.; Jurczak, W.; Skarbnik, A.; Pagel, J.M.; Flinn, I.W.; Kamdar, M.; Munir, T.; Walewska, R.; Corbett, G.; et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzumab for treatment-naive chronic lymphocytic leukaemia (ELEVATE-TN): A randomised, controlled, phase 3 trial. Lancet 2020, 395, 1278–1291. [Google Scholar] [CrossRef]
- Fischer, K.; Al-Sawaf, O.; Bahlo, J.; Fink, A.M.; Tandon, M.; Dixon, M.; Robrecht, S.; Warburton, S.; Humphrey, K.; Samoylova, O.; et al. Venetoclax and Obinutuzumab in Patients with CLL and Coexisting Conditions. N. Engl. J. Med. 2019, 380, 2225–2236. [Google Scholar] [CrossRef] [PubMed]
- Niederle, N.; Megdenberg, D.; Balleisen, L.; Heit, W.; Knauf, W.; Weiß, J.; Freier, W.; Hinke, A.; Ibach, S.; Eimermacher, H. Bendamustine compared to fludarabine as second-line treatment in chronic lymphocytic leukemia. Ann. Hematol. 2013, 92, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Wierda, W.G.; O’Brien, S.; Wen, S.; Faderl, S.; Garcia-Manero, G.; Thomas, D.; Do, K.A.; Cortes, J.; Koller, C.; Beran, M.; et al. Chemoimmunotherapy With Fludarabine, Cyclophosphamide, and Rituximab for Relapsed and Refractory Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2005, 23, 4070–4078. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.C.; Furman, R.R.; Coutre, S.E.; Burger, J.A.; Blum, K.A.; Coleman, M.; Wierda, W.G.; Jones, J.A.; Zhao, W.; Heerema, N.A.; et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood 2015, 125, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Kater, A.P.; Kipps, T.J.; Eichhorst, B.; Hillmen, P.; D’Rozario, J.; Owen, C.; Assouline, S.E.; Lamanna, N.; Robak, T.J.; la Serna, J.; et al. Five-Year Analysis of Murano Study Demonstrates Enduring Undetectable Minimal Residual Disease (uMRD) in a Subset of Relapsed/Refractory Chronic Lymphocytic Leukemia (R/R CLL) Patients (Pts) Following Fixed-Duration Venetoclax-Rituximab (VenR) Therapy (Tx). Available online: https://ash.confex.com/ash/2020/webprogram/Paper136109.html (accessed on 5 December 2020).
- Ghia, P.; Pluta, A.; Wach, M.; Lysak, D.; Kozak, T.; Simkovic, M.; Kaplan, P.; Kraychok, I.; Illes, A.; de la Serna, J.; et al. ASCEND: Phase III, Randomized Trial of Acalabrutinib Versus Idelalisib Plus Rituximab or Bendamustine Plus Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. JCO Clin. Cancer Inform. 2020, 38, 2849–2861. [Google Scholar] [CrossRef]
- Byrd, J.C.; Wierda, W.G.; Schuh, A.; Devereux, S.; Chaves, J.M.; Brown, J.R.; Hillmen, P.; Martin, P.; Awan, F.T.; Stephens, D.M.; et al. Acalabrutinib monotherapy in patients with relapsed/refractory chronic lymphocytic leukemia: Updated phase 2 results. Blood 2020, 135, 1204–1213. [Google Scholar] [CrossRef]
- Jones, J.A.; Mato, A.R.; Wierda, W.G.; Davids, M.S.; Choi, M.; Cheson, B.D.; Furman, R.R.; Lamanna, N.; Barr, P.M.; Zhou, L.; et al. Venetoclax for Chronic Lymphocytic Leukaemia Progressing after Ibrutinib: A Multicentre, Open-Label Phase 2 Trial. Lancet Oncol. 2018, 19, 65–67. [Google Scholar] [CrossRef]
- Stilgenbauer, S.; Eichhorst, B.; Schetelig, J.; Hillmen, P.; Seymour, J.F.; Coutre, S.; Jurczak, W.; Mulligan, S.P.; Schuh, A.; Assouline, S.; et al. Venetoclax for patients with chronic lymphocytic leukemia with 17p deletion: Results from the full population of a phase II pivotal trial. J. Clin. Oncol. 2018, 36, 1973–1980. [Google Scholar] [CrossRef]
- Alberta Health Services. Clinical Practice Guideline LYHE0007-Chronic Lymphocytic Leukemia (Version 6). Available online: https://www.albertahealthservices.ca/assets/info/hp/cancer/if-hp-cancer-guide-lyhe007-cll.pdf (accessed on 5 November 2020).
- Cancer Care Ontario. Available online: https://www.cancercareontario.ca/en/drugformulary/regimens (accessed on 1 November 2020).
- IQVIA Delta PA. November 2020.
- Canadian Institute for Health Information (CIHI). Patient Cost Estimator. Available online: https://www.cihi.ca/en/patient-cost-estimator (accessed on 1 November 2020).
- Cho, S.K.; Samp, J.C.; Keim, H.; Masaquel, A.S.; Johnson, S.J.; Parise, H. PCN84-total cost of care and budget impact for patients with cll treated with venetoclax. Value Health 2018, 21, S28. [Google Scholar] [CrossRef]
- Cho, S.K.; Manzoor, B.S.; Sail, K.R.; Parise, H.; Ravelo, A.; Shapouri, S.; Kapustyan, T.; Sharmokh, S.; Virabhak, S.; Davids, M.S.; et al. Budget Impact of 12-Month Fixed Treatment Duration Venetoclax in Combination with Obinutuzumab in Previously Untreated Chronic Lymphocytic Leukemia Patients in the United States. Pharmacoeconomics 2020, 38, 941–951. [Google Scholar] [CrossRef]
- Fang, H.; Ravonimbola, H.; Hazra, N.C.; Zhou, Z.; Manzoor, B.S.; Levy, V.; Ysebaert, L.; Kabore, N.; Sail, K. Economic Impact of Treatment Sequences for Chronic Lymphocytic Leukemia (CLL) and Budget Impact Analysis of Venetoclax Plus Obinutuzumab Sequences for CLL Patients Not Eligible for Full Dose Fludarabine in France. Blood 2020, 136, 28–29. [Google Scholar] [CrossRef]
- Zenz, T.; Gribben, J.G.; Hallek, M.; Dohner, H.; Keating, M.J.; Stilgenbauer, S. Risk categories and refractory CLL in the era of chemoimmunotherapy. Blood 2012, 119, 4101–4107. [Google Scholar] [CrossRef] [PubMed]
- Banerji, V.; Abdel-Samad, N.; Aw, A.; Bernard, M.P.; Desjardins, S.; Johnson, N.; Lembo, P.; Pelizon, C.; Peters, A.; Tremblay, S.; et al. The Management and use of Healthcare Resources in Patients with Chronic Lymphocyctic Leukemia (CLL) Initiating Venetoclax in Routine Clinical Practice (DEVOTE) Across Canada: Interim Analysis. Eur. Hematol. Assoc. 2020, 81, Abstract EP722. [Google Scholar]
- Mato, A.R.; Thompson, M.; Allan, J.N.; Brander, D.M.; Pagel, J.M.; Ujjani, C.S.; Hill, B.T.; Lamanna, N.; Lansigan, F.; Jacobs, R.; et al. Real-world outcomes and management strategies for venetoclax-treated chronic lymphocytic leukemia patients in the United States. Haematologica 2018, 103, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Roeker, L.E.; Fox, C.P.; Eyre, T.A.; Brander, D.M.; Allan, J.N.; Schuster, S.J.; Nabhan, C.; Hill, B.T.; Shah, N.N.; Lansigan, F.; et al. Tumor Lysis, Adverse Events, and Dose Adjustments in 297 Venetoclax-Treated CLL Patients in Routine Clinical Practice. Clin. Cancer Res. 2019, 25, 4264–4270. [Google Scholar] [CrossRef]
- Parikh, S.A.; Achenbach, S.J.; Call, T.G.; Rabe, K.G.; Ding, W.; Leis, J.F.; Kenderian, S.S.; Chanan-Khan, A.A.; Koehler, A.B.; Schwager, S.M.; et al. The impact of dose modification and temporary interruption of ibrutinib on outcomes of chronic lymphocytic leukemia patients in routine clinical practice. Cancer Med. 2020, 9, 3390–3399. [Google Scholar] [CrossRef]
- Mato, A.R.; Timlin, C.; Ujjani, C.; Skarbnik, A.; Howlett, C.; Banerjee, R.; Nabhan, C.; Schuster, S.J. Comparable outcomes in chronic lymphocytic leukaemia (CLL) patients treated with reduced-dose ibrutinib: Results from a multi-centre study. Br. J. Haematol. 2018, 181, 259–261. [Google Scholar] [CrossRef]
- Hardy-Abeloos, C.; Pinotti, R.; Gabrilove, J. Ibrutinib dose modifications in the management of CLL. J. Hematol. Oncol. 2020, 13, 66. [Google Scholar] [CrossRef]
- Uminski, K.; Brown, K.; Bucher, O.; Hibbert, I.; Dhaliwal, D.H.; Johnston, J.B.; Geirnaert, M.; Dawe, D.E.; Banerji, V. Descriptive analysis of dosing and outcomes for patients with ibrutinib-treated relapsed or refractory chronic lymphocytic leukemia in a Canadian centre. Curr. Oncol. 2019, 26, e610–e617. [Google Scholar] [CrossRef]
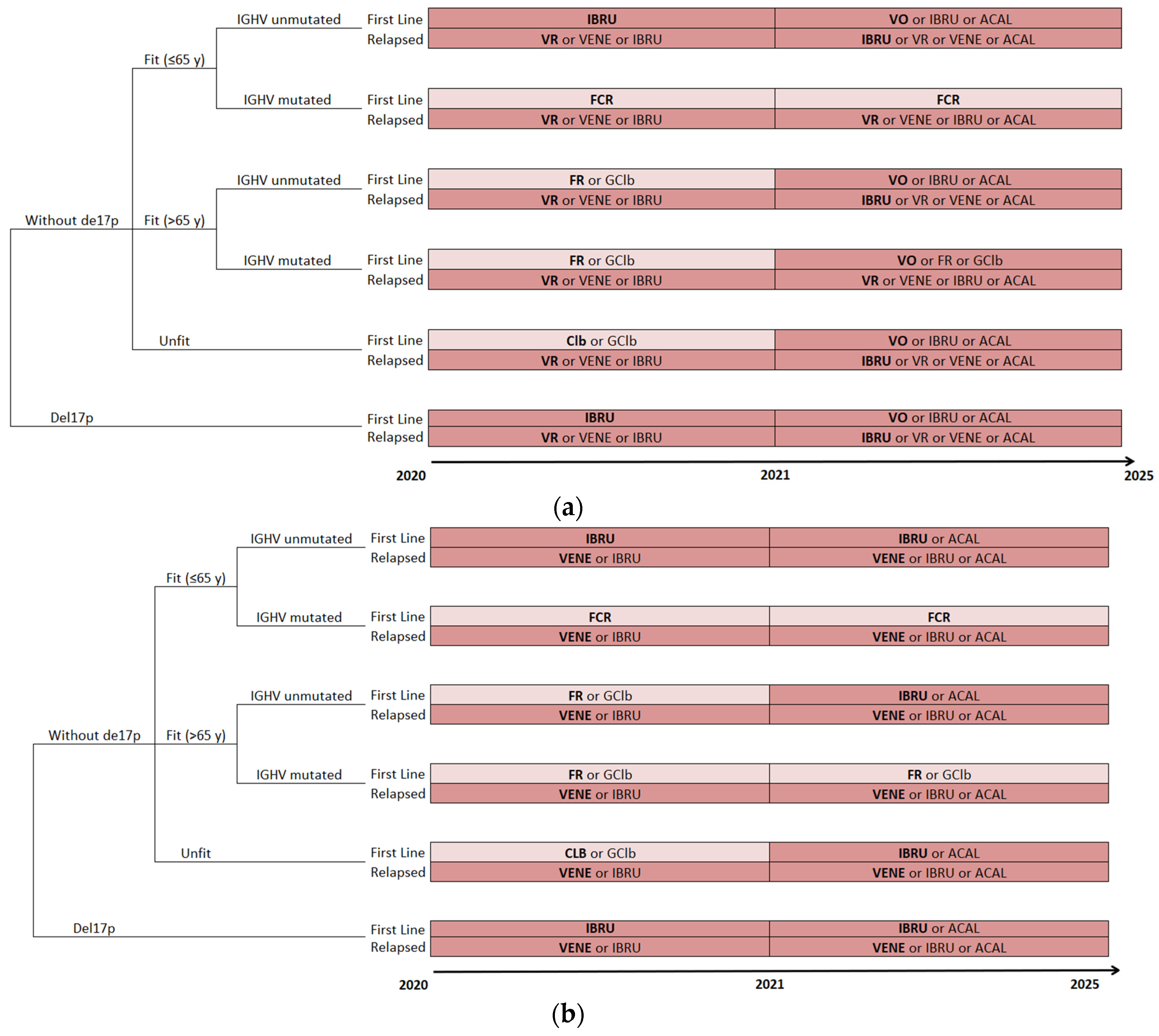

| Treatments | PFS and OS | Grade 3 or 4 Adverse Events (%) | Drug Cost a (C$/Cycle) | References |
|---|---|---|---|---|
| First-line setting | ||||
| GClb | Median PFS, 29.8 months | Anemia, 4 Neutropenia, 33 Thrombocytopenia, 10 Infection, 12 | Cycle 1: 16,497 Cycles 2–6: 5541 | Goede [27] Goede, 2014 [28] |
| FCR | <65 years, 5-year PFS, 48% ≥65 years, 5-year PFS, 43% IGHV mutated, 5-year PFS, 67% IGHV unmutated, 5-year PFS, 33% | Anemia, 6 Neutropenia, 30 Thrombocytopenia, 9 Infection, 24 | Cycle 1: 3067 Cycles 2–6: 3769 | Fischer [29] Hallek [19] |
| FR | Median PFS, 42.0 months | Anemia, 40 Neutropenia, 76 Thrombocytopenia, 20 Infection, 20 | Cycle 1: 3194 Cycles 2–6: 3896 | Woyach [30] Byrd [31] |
| F | Median PFS, 19.0 months | Anemia, 15 Neutropenia, 12 Thrombocytopenia, 15 Infection, 80 | 1089 | Eichhorst [32] |
| BR | >70 years, median PFS, 43.0 months | Anemia, 31 Neutropenia, 31 Thrombocytopenia, 35 Infection, 12 | Cycle 1: 6357 Cycles 2–6: 7059 | Woyach [33] Fischer [34] |
| Clb | Median PFS, 15.0 months | Anemia, 27 Neutropenia, 12 Thrombocytopenia, 20 Infection, 4 | Cycle 1: 264.30 Cycles 2–6: 176.20 | Burger [9] Eichhorst [32] |
| Ibrutinib | 5-year PFS, 70% TP53 mutation, 5-year PFS, 56% IGHV mutated, 5-year PFS, 81% IGHV unmutated, 5-year PFS, 67% | Anemia, 6 Neutropenia, 10 Thrombocytopenia, 2 Infection, 6 Atrial fibrillation, 6 | 8198 | Burger [9,20] |
| ACAL | 24-month PFS, 87% | Anemia, 7 Neutropenia, 10 Thrombocytopenia, 5 Infection, 5 | 7615 | Sharman [35] |
| VO | 3-year PFS, 82% Del(17p), 3-year PFS, 49% IGHV mutated, 3-year PFS, 87% IGHV unmutated, 3-year PFS, 81% | Anemia, 8 Neutropenia, 53 Febrile neutropenia, 5 Thrombocytopenia, 14 Infection, 18 | Cycle 1: 16,532 Cycle 2: 9153 Cycles 3–6: 13,318 Cycles 7–13: 7840 | Al-Sawaf [14] Fischer [36] |
| Second-line setting | ||||
| F | Median PFS, 14.8 months Median OS, 41.0 months | Anemia, 80 Neutropenia, 17 Thrombocytopenia, 60 Infection, 15 | 1089 | Niederle [37] |
| FCR | Median PFS, 28.0 months Median OS, 42.0 months | Anemia, 24 Neutropenia, 81 Thrombocytopenia, 34 Infection, 16 | Cycle 1: 3067 Cycles 2–6: 3769 | Wierda [38] |
| Ibrutinib | Median PFS, 44.1 months Del (17p) median PFS, 40.6 months IGHV mutated, median PFS, 48.4 months IGHV unmutated, median PFS, 49.7 months Median OS, 67.7 months Del(17p) median OS, 61.8 months | Anemia, 0 Neutropenia, 18 Thrombocytopenia, 10 Infection, 51 Atrial fibrillation, 6 | 8198 | Munir [10] Byrd [39] |
| BR | 2-year PFS, excluding del(17p), 17% 5-year OS, 62% | Anemia, 14 Neutropenia, 39 Thrombocytopenia, 10 Infection, 22 | Cycle 1: 6357 Cycles 2–6: 7059 | Kater [40] Seymour [15] |
| ACAL | 12-month PFS, 88% 12-month OS, 94% | Anemia, 7 Neutropenia, 14 Febrile neutropenia, 2 Thrombocytopenia, 2 Infection, 1 Atrial fibrillation, 2 | 7615 | Ghia [41] Byrd [42] |
| Venetoclax | Median PFS, 24.7 months Del (17p), 24-month PFS, 54% 12-month OS, 91% Del (17p), 24-month OS, 73% | Anemia, 29 Neutropenia, 51 Febrile neutropenia, 13 Thrombocytopenia, 29 Infection, 11 | Cycle 1: 1813 Cycles 2+: 7840 | Jones [43] Stilgenbauer [44] |
| VR | Median PFS, 53.6 months Without del(17p), median PFS, 56.6 months Del(17p), median PFS, 45.3 months <65 years, median PFS, 49.0 months ≥65 years, median PFS, 57.0 months 5-year OS, 82% | Anemia, 11 Neutropenia, 58 Febrile neutropenia, 4 Thrombocytopenia, 6 Infection, 18 | Ramp-up: 3773 Cycle 1: 9945 Cycles 2–6: 10,647 Cycles 7+: 7840 | EMA [16] Seymour [15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lachaine, J.; Guinan, K.; Aw, A.; Banerji, V.; Fleury, I.; Owen, C. Impact of Fixed-Duration Oral Targeted Therapies on the Economic Burden of Chronic Lymphocytic Leukemia in Canada. Curr. Oncol. 2023, 30, 4483-4498. https://doi.org/10.3390/curroncol30050339
Lachaine J, Guinan K, Aw A, Banerji V, Fleury I, Owen C. Impact of Fixed-Duration Oral Targeted Therapies on the Economic Burden of Chronic Lymphocytic Leukemia in Canada. Current Oncology. 2023; 30(5):4483-4498. https://doi.org/10.3390/curroncol30050339
Chicago/Turabian StyleLachaine, Jean, Kimberly Guinan, Andrew Aw, Versha Banerji, Isabelle Fleury, and Carolyn Owen. 2023. "Impact of Fixed-Duration Oral Targeted Therapies on the Economic Burden of Chronic Lymphocytic Leukemia in Canada" Current Oncology 30, no. 5: 4483-4498. https://doi.org/10.3390/curroncol30050339
APA StyleLachaine, J., Guinan, K., Aw, A., Banerji, V., Fleury, I., & Owen, C. (2023). Impact of Fixed-Duration Oral Targeted Therapies on the Economic Burden of Chronic Lymphocytic Leukemia in Canada. Current Oncology, 30(5), 4483-4498. https://doi.org/10.3390/curroncol30050339





