A Systematic Review of Surgical Management Strategies in the Treatment of Peritoneal Carcinomatosis of Neuroendocrine Origin
Abstract
1. Introduction
2. Materials and Methods
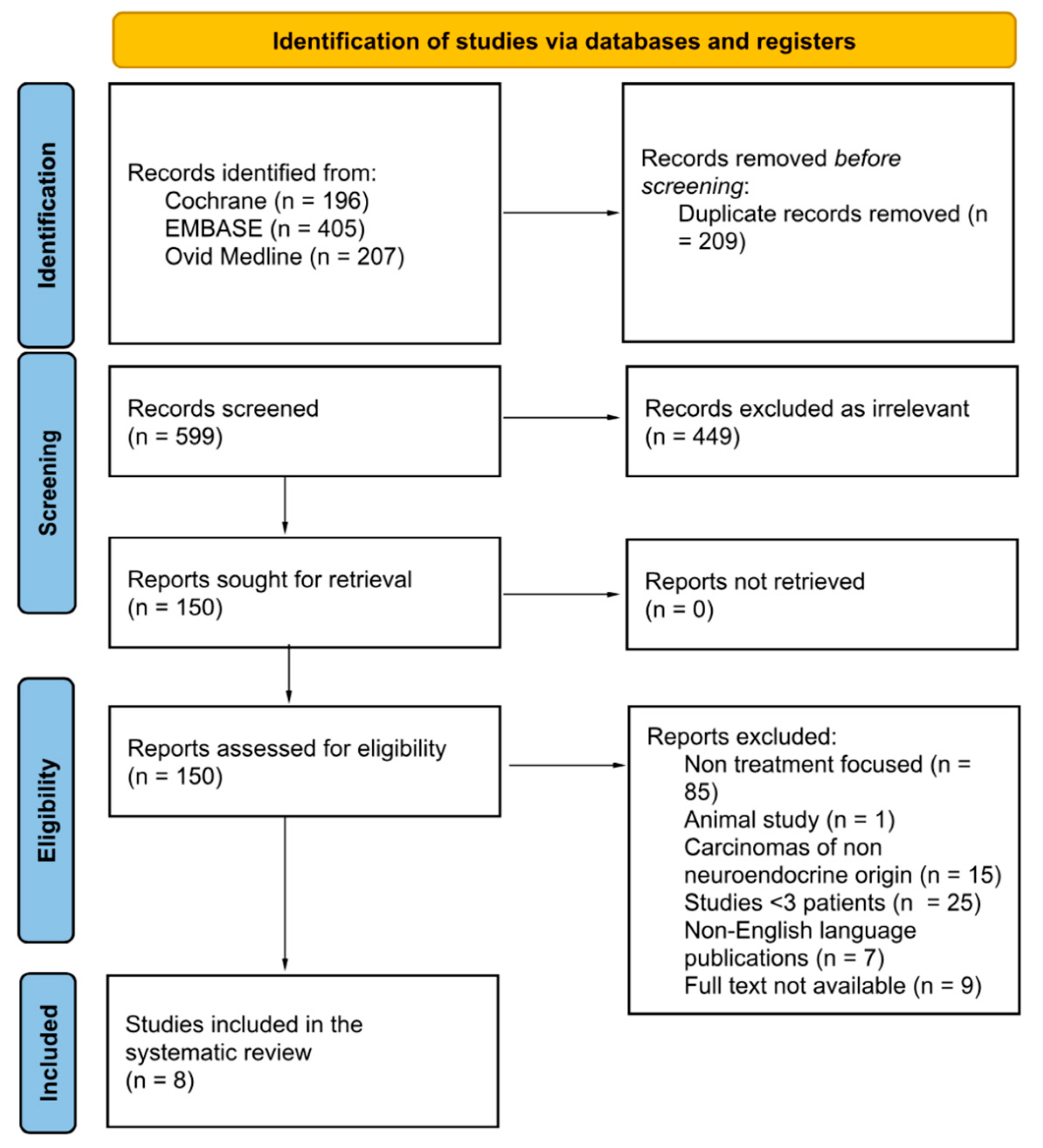
2.1. Search Strategy and Study Selection Process
2.2. Data Extraction
2.3. Risk of Bias Assessment
3. Results
3.1. Literature Search and Study Characteristics
3.2. Study Population
3.3. Treatment Associated Morbidity/Mortality
3.4. Survival Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Coccolini, F. Peritoneal carcinomatosis. World J. Gastroenterol. 2013, 19, 6979. [Google Scholar] [CrossRef] [PubMed]
- Lee, S. Treatments for Primary Peritoneal Carcinoma, Canadian Cancer Society. Available online: https://cancer.ca/en/cancer-information/cancer-types/ovarian/treatment/extra-ovarian-primary-peritoneal-carcinoma (accessed on 22 March 2023).
- Qu, Z.-B. Management of Pseudomyxoma peritonei. World J. Gastroenterol. 2006, 12, 6124. [Google Scholar] [CrossRef] [PubMed]
- Leiting, J.L.; Grotz, T.E. Optimising outcomes for patients with gastric cancer peritoneal carcinomatosis. World J. Gastrointest. Oncol. 2018, 10, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.; Kurnit, K.C.; Kim, J.S. Ovarian cancer peritoneal carcinomatosis: A narrative review. Dig. Med. Res. 2022, 5, 43. [Google Scholar] [CrossRef]
- Ramage, J.K.; Ahmed, A.; Ardill, J.; Bax, N.; Breen, D.J.; E Caplin, M.; Corrie, P.; Davar, J.; Davies, A.H.; Lewington, V.; et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (nets). Gut 2012, 61, 6–32. Available online: https://gut.bmj.com/content/61/1/6 (accessed on 22 March 2023). [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Hernán, M.A.; McAleenan, A.; Reeves, B.C.; Higgins, J.P.T. Chapter 25: Assessing risk of bias in a non-randomised study. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: Singapore, 2022. [Google Scholar]
- Benhaim, L.; Faron, M.; Hadoux, J.; Gelli, M.; Sourrouille, I.; Burtin, P.; Honoré, C.; Malka, D.; Leboulleux, S.; Ducreux, M.; et al. Long-Term Results after Surgical Resection of Peritoneal Metastasis from Neuroendocrine Tumours. Neuroendocrinology 2021, 111, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.L.; Dixon, M.; Law, C.H.L.; Koujanian, S.; Beyfuss, K.A.; Singh, S.; Myrehaug, S.; Hallet, J. Outcomes of Cytoreductive Surgery for Metastatic Low-Grade Neuroendocrine Tumours in the Setting of Extrahepatic Metastases. Ann. Surg. Oncol. 2018, 25, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Madsen, A.H.; Ladekarl, M.; Villadsen, G.E.; Grønbæk, H.; Sørensen, M.M.; Stribolt, K.; Verwaal, V.J.; Iversen, L.H. Effects of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in the Treatment of Goblet Cell Carcinoma: A Prospective Cohort Study. Ann. Surg. Oncol. 2018, 25, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Brandl, A.; Zielinski, C.B.; Raue, W.; Pratschke, J.; Rau, B. Peritoneal metastases of rare carcinomas treated with cytoreductive surgery and HIPEC—A single centre case series. Ann. Med. Surg. 2017, 22, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Goéré, D.; Passot, G.; Gelli, M.; Levine, E.A.; Bartlett, D.L.; Sugarbaker, P.H.; Glehen, O. Complete cytoreductive surgery plus HIPEC for peritoneal metastases from unusual cancer sites of origin: Results from a worldwide analysis issue of the Peritoneal Surface Oncology Group International (PSOGI). Int. J. Hyperth. 2017, 33, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Woltering, E.A.; Voros, B.A.; Beyer, D.T.; Wang, Y.-Z.; Thiagarajan, R.; Ryan, P.; Wright, A.; Ramirez, R.A.; Ricks, M.J.; Boudreaux, J.P. Aggressive Surgical Approach to the Management of Neuroendocrine Tumours: A Report of 1,000 Surgical Cytoreductions by a Single Institution. J. Am. Coll. Surg. 2017, 224, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; David, A.; Sourrouille, I.; Honoré, C.; Goéré, D.; Dumont, F.; Stoclin, A.; Baudin, E. Neuroendocrine carcinomas: Optimal surgery of peritoneal metastases (and associated intra-abdominal metastases). Surgery 2014, 155, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, R.; Mercier, F.; Passot, G.; Pasquer, A.; Gelli, M.; Levine, E.A.; Villeneuve, L.; Poncet, G.; Walter, T.; Glehen, O. Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for small bowel neuroendocrine tumours with peritoneal metastasis. Eur. J. Surg. Oncol. 2022, 48, 1626–1630. [Google Scholar] [CrossRef] [PubMed]
- Nanets. Available online: https://nanets.net/images/2020_Guidelines_Compendium.pdf (accessed on 29 January 2023).
| First Author | Year | Country | Study Design | Total Number of Study Patients (N) |
|---|---|---|---|---|
| Benhaim [9] | 2021 | France | Prospective | 88 |
| Chan [10] | 2018 | Canada | Prospective | 55 |
| Madsen [11] | 2018 | Denmark | Prospective Cohort | 48 |
| Brandl [12] | 2017 | Germany | Retrospective | 14 |
| Goere [13] | 2017 | France | Retrospective | 127 |
| Woltering [14] | 2017 | USA | Retrospective | 800 |
| Elias [15] | 2014 | France | Prospective/Retrospective | 41 |
| Hajjar [16] | 2022 | France | Prospective | 67 |
| First Author | Year | Method of PC Assessment and Staging Prior to CRS +/− HIPEC | Synchronous vs. Metachronous Peritoneal Disease | Synchronous Peritonectomy +/− HIPEC | PCI Criteria for Determination of Resectability |
|---|---|---|---|---|---|
| Benhaim [9] | 2021 |
|
|
|
|
| Chan [10] | 2018 |
|
|
|
|
| Madsen [11] | 2018 |
|
|
|
|
| Brandl [12] | 2017 |
|
|
|
|
| Goere [13] | 2017 |
|
|
|
|
| Woltering [14] | 2017 |
|
|
|
|
| Elias [15] | 2014 |
|
|
|
|
| Hajjar [16] | 2022 |
|
|
|
|
| Figure | Year | Treatment | Surgical Treatment | HIPEC Strategy | Post-Operative Adjuvant Therapy |
|---|---|---|---|---|---|
| Benhaim [9] | 2021 | CRS |
| N/A | N/A |
| Chan [10] | 2018 | CRS |
| N/A | N/A |
| Madsen [11] | 2018 | Group 1—Right-sided hemicolectomy—patients with GCC limited to non-perforated appendix Group 2—CRS + prophylactic HIPEC-patients with GCC and high risk of carcinomatosis (+perioperative chemotherapy if feasible) Group 3—CRS + HIPEC-patients with peritoneal carcinomatosis Group 4—Systemic palliative chemotherapy-patients with extensive carcinomatosis |
|
|
|
| Brandl [12] | 2017 | CRS, HIPEC |
|
| N/A |
| Goere [13] | 2017 | CRS + HIPEC |
|
| N/A |
| Woltering [14] | 2017 | CRS |
|
| N/A |
| Elias [15] | 2014 | CRS + immediate HIPEC (28) CRS alone (13) |
|
| N/A |
| Hajjar [16] | 2022 | CRS + HIPEC (36) CRS alone (31) |
| Not Specified | N/A |
| First Author | Year | Morbidity/Mortality | Survival Data |
|---|---|---|---|
| Benhaim [9] | 2021 |
| 5 year overall survival rates:
|
| Chan [10] | 2018 |
| 5 year overall survival rates:
|
| Madsen [11] | 2018 |
| 5 year survival rate:
|
| Brandl [12] | 2017 |
|
|
| Goere [13] | 2017 |
|
|
| Woltering [14] | 2017 |
|
|
| Elias [15] | 2014 |
|
|
| Hajjar [16] | 2022 |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fallows, M.; Samant, A.; Wilson, H.; Mirnezami, R. A Systematic Review of Surgical Management Strategies in the Treatment of Peritoneal Carcinomatosis of Neuroendocrine Origin. Curr. Oncol. 2023, 30, 6316-6329. https://doi.org/10.3390/curroncol30070466
Fallows M, Samant A, Wilson H, Mirnezami R. A Systematic Review of Surgical Management Strategies in the Treatment of Peritoneal Carcinomatosis of Neuroendocrine Origin. Current Oncology. 2023; 30(7):6316-6329. https://doi.org/10.3390/curroncol30070466
Chicago/Turabian StyleFallows, Megan, Ambareesh Samant, Harry Wilson, and Reza Mirnezami. 2023. "A Systematic Review of Surgical Management Strategies in the Treatment of Peritoneal Carcinomatosis of Neuroendocrine Origin" Current Oncology 30, no. 7: 6316-6329. https://doi.org/10.3390/curroncol30070466
APA StyleFallows, M., Samant, A., Wilson, H., & Mirnezami, R. (2023). A Systematic Review of Surgical Management Strategies in the Treatment of Peritoneal Carcinomatosis of Neuroendocrine Origin. Current Oncology, 30(7), 6316-6329. https://doi.org/10.3390/curroncol30070466





