Comparison of Prognostic Factors for Merkel Cell Carcinoma, Mucosal Melanoma and Cutaneous Malignant Melanoma: Insights into Their Etiologies
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Statistical Methods
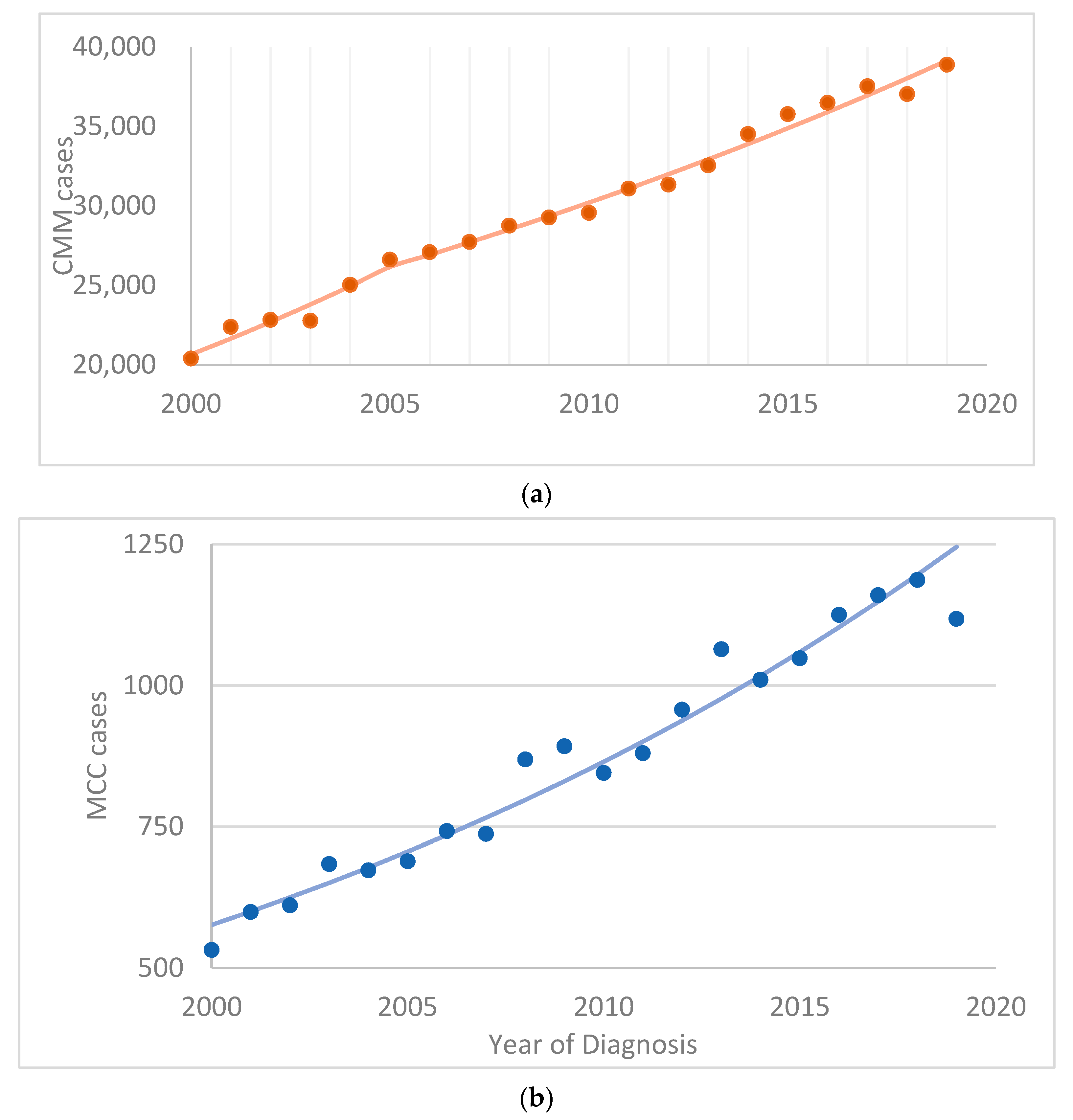
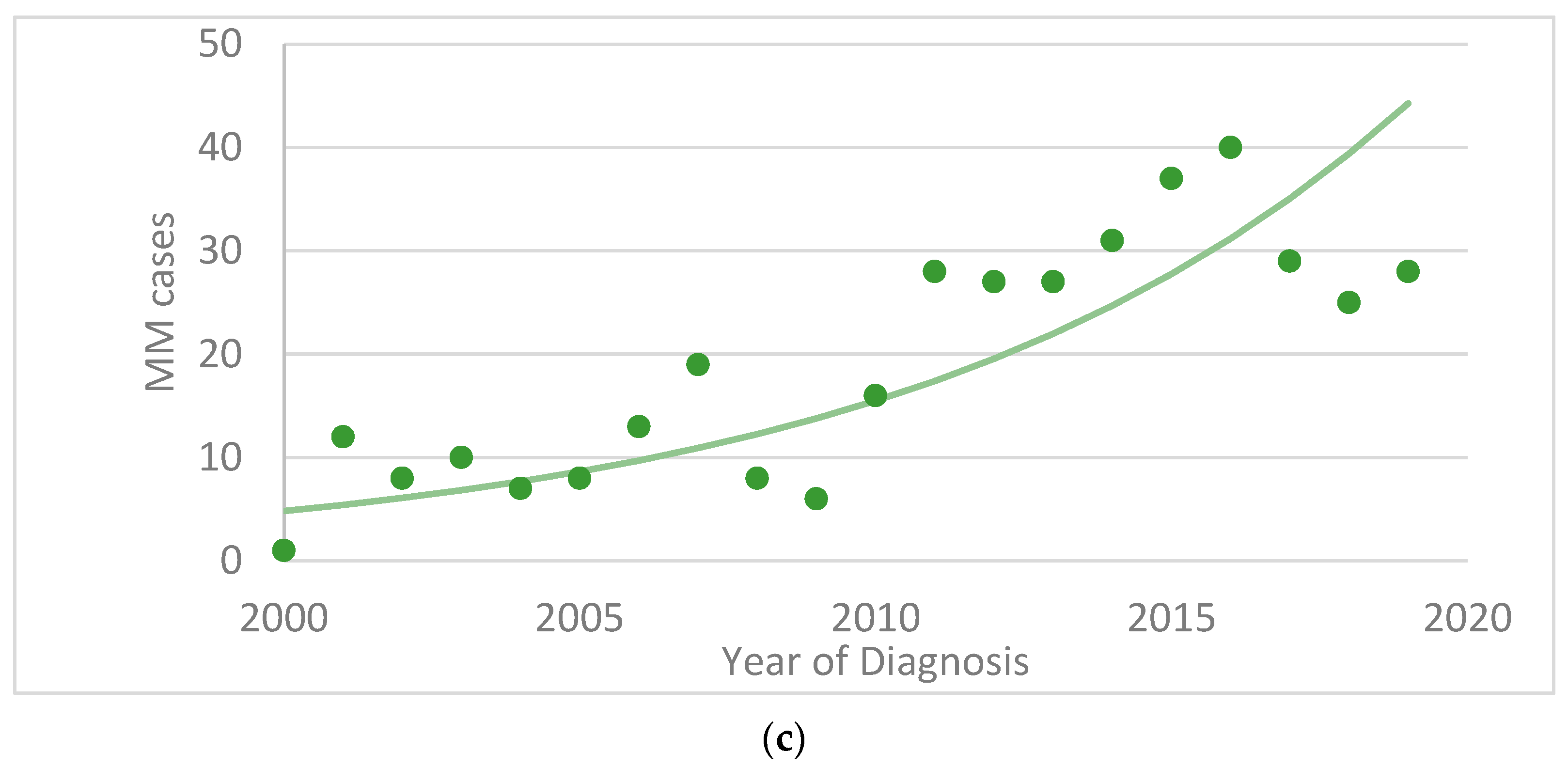
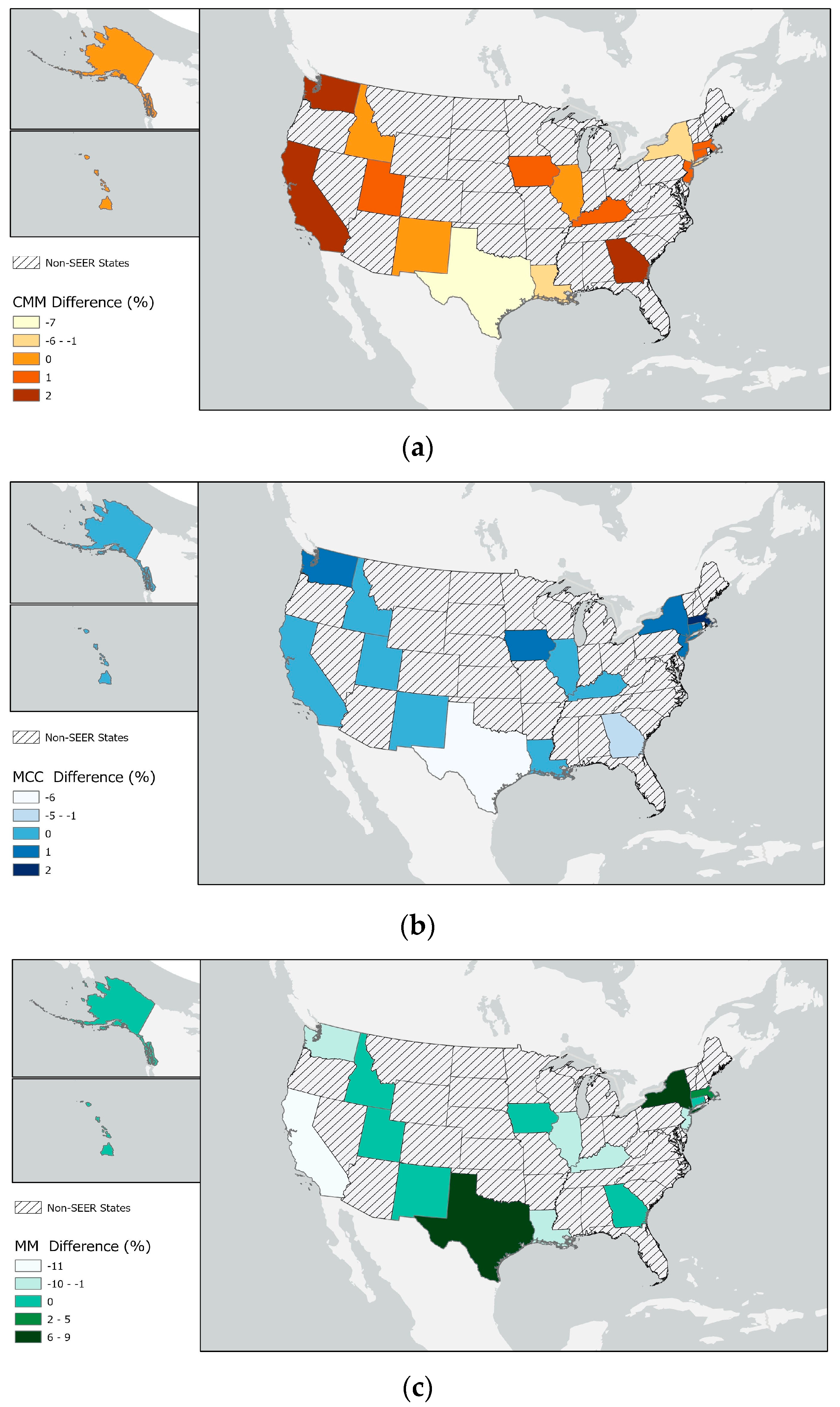
3. Results
4. Discussion
4.1. Cutaneous Malignant Melanoma (CMM)
4.2. Merkel Cell Carcinoma (MCC)
4.3. Mucosal Melanoma (MM)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sunshine, J.C.; Jahchan, N.S.; Sage, J.; Choi, J. Are there multiple cells of origin of Merkel cell carcinoma? Oncogene 2018, 37, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Moshiri, A.S.; Nghiem, P. Milestones in the staging, classification, and biology of Merkel cell carcinoma. J. Natl. Compr. Cancer Netw. 2014, 12, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Talsma, C.E.; Wang, M.; Andea, A.A.; Fullen, D.R.; Harms, P.W.; Chan, M.P. Expression of p16 in Merkel cell carcinoma. J. Cutan. Pathol. 2021, 48, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Tothill, R.; Estall, V.; Rischin, D. Merkel cell carcinoma: Emerging biology, current approaches, and future directions. Am. Soc. Clin. Oncol. Educ. Book 2015, 35, e519–e526. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.E.; Brewer, J.D. Merkel cell carcinoma in immunosuppressed patients. Cancers 2014, 6, 1328–1350. [Google Scholar] [CrossRef]
- Rockville Merkel Cell Carcinoma Group. Merkel cell carcinoma: Recent progress and current priorities on etiology, pathogenesis, and clinical management. J. Clin. Oncol. 2009, 27, 4021–4026. [Google Scholar] [CrossRef]
- Carbo-Bague, A.; Rubio-Casadevall, J.; Puigdemont, M.; Sanvisens, A.; Oliveras, G.; Coll, M.; Del Olmo, B.; Perez-Bueno, F.; Marcos-Gragera, R. Epidemiology and Molecular Profile of Mucosal Melanoma: A Population-Based Study in Southern Europe. Cancers 2022, 14, 780. [Google Scholar] [CrossRef]
- Sohal, R.J.; Sohal, S.; Wazir, A.; Benjamin, S. Mucosal Melanoma: A Rare Entity and Review of the Literature. Cureus 2020, 12, e9483. [Google Scholar] [CrossRef]
- Tyrrell, H.; Payne, M. Combatting mucosal melanoma: Recent advances and future perspectives. Melanoma Manag. 2018, 5, MMT11. [Google Scholar] [CrossRef]
- Gandini, S.; Sera, F.; Cattaruzza, M.S.; Pasquini, P.; Zanetti, R.; Masini, C.; Boyle, P.; Melchi, C.F. Meta-analysis of risk factors for cutaneous melanoma: III. Family history, actinic damage and phenotypic factors. Eur. J. Cancer 2005, 41, 2040–2059. [Google Scholar] [CrossRef]
- Elwood, J.M.; Jopson, J. Melanoma and sun exposure: An overview of published studies. Int. J. Cancer 1997, 73, 198–203. [Google Scholar] [CrossRef]
- Gandini, S.; Sera, F.; Cattaruzza, M.S.; Pasquini, P.; Picconi, O.; Boyle, P.; Melchi, C.F. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur. J. Cancer 2005, 41, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Boniol, M.; Autier, P.; Boyle, P.; Gandini, S. Cutaneous melanoma attributable to sunbed use: Systematic review and meta-analysis. BMJ 2012, 345, e4757. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.C.; Stang, A.; DeCaprio, J.A.; Cerroni, L.; Lebbe, C.; Veness, M.; Nghiem, P. Merkel cell carcinoma. Nat. Rev. Dis. Prim. 2017, 3, 17077. [Google Scholar] [CrossRef] [PubMed]
- Llombart, B.; Requena, C.; Cruz, J. Update on Merkel Cell Carcinoma: Epidemiology, Etiopathogenesis, Clinical Features, Diagnosis, and Staging. Actas Dermosifiliogr. 2017, 108, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Toker, C. Trabecular carcinoma of the skin. Arch. Dermatol. 1972, 105, 107–110. [Google Scholar] [CrossRef]
- Tello, T.L.; Coggshall, K.; Yom, S.S.; Yu, S.S. Merkel cell carcinoma: An update and review: Current and future therapy. J. Am. Acad. Dermatol. 2018, 78, 445–454. [Google Scholar] [CrossRef]
- Duprat, J.P.; Landman, G.; Salvajoli, J.V.; Brechtbuhl, E.R. A review of the epidemiology and treatment of Merkel cell carcinoma. Clinics 2011, 66, 1817–1823. [Google Scholar] [CrossRef]
- Goepfert, H.; Remmler, D.; Silva, E.; Wheeler, B. Merkel cell carcinoma (endocrine carcinoma of the skin) of the head and neck. Arch. Otolaryngol. 1984, 110, 707–712. [Google Scholar] [CrossRef]
- Starrett, G.J.; Thakuria, M.; Chen, T.; Marcelus, C.; Cheng, J.; Nomburg, J.; Thorner, A.R.; Slevin, M.K.; Powers, W.; Burns, R.T.; et al. Clinical and molecular characterization of virus-positive and virus-negative Merkel cell carcinoma. Genome Med. 2020, 12, 30. [Google Scholar] [CrossRef]
- Mihajlovic, M.; Vlajkovic, S.; Jovanovic, P.; Stefanovic, V. Primary mucosal melanomas: A comprehensive review. Int. J. Clin. Exp. Pathol. 2012, 5, 739–753. [Google Scholar] [PubMed]
- Lombardo, N.; Della Corte, M.; Pelaia, C.; Piazzetta, G.; Lobello, N.; Del Duca, E.; Bennardo, L.; Nistico, S.P. Primary Mucosal Melanoma Presenting with a Unilateral Nasal Obstruction of the Left Inferior Turbinate. Medicina 2021, 57, 359. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Nemoto, K.; Ogawa, Y.; Hareyama, M.; Yoshida, H.; Takamura, A.; Ohmori, K.; Hamamoto, Y.; Sugita, T.; Saitoh, M.; et al. A multi-institutional retrospective analysis of external radiotherapy for mucosal melanoma of the head and neck in Northern Japan. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 495–500. [Google Scholar] [CrossRef]
- Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat Database: Incidence—SEER Research Data, 17 Registries & 22 Registries, Nov 2021 Sub (1975–2019)—Linked To County Attributes—Time Dependent (1990–2019) Income/Rurality, 1969–2020 Counties. National Cancer Institute, DCCPS, Surveillance Research Program, released April 2022, Based on the November 2021 Submission. Available online: www.seer.cancer.gov (accessed on 2 February 2023).
- Fritz, A.G. International Classification of Diseases for Oncology: ICD-O, 3rd ed.; World Health Organization: Geneva, Switzerland, 2000; p. vii. [Google Scholar]
- Statistical Research and Applications Branch, National Cancer Institute. Joinpoint Regression Program, Version 4.2.0.1.; National Cancer Institute: Bethesda, MD, USA, 2015. [Google Scholar]
- Kim, H.J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef]
- National Cancer Institute. About Rare Cancers. Available online: https://www.cancer.gov/pediatric-adult-rare-tumor/rare-tumors/about-rare-cancers (accessed on 2 February 2023).
- Enninga, E.A.L.; Moser, J.C.; Weaver, A.L.; Markovic, S.N.; Brewer, J.D.; Leontovich, A.A.; Hieken, T.J.; Shuster, L.; Kottschade, L.A.; Olariu, A.; et al. Survival of cutaneous melanoma based on sex, age, and stage in the United States, 1992–2011. Cancer Med. 2017, 6, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Dennis, L.K. Increasing risk of melanoma with increasing age. JAMA 1999, 282, 1037–1038. [Google Scholar] [CrossRef]
- Schulman, J.M.; Fisher, D.E. Indoor ultraviolet tanning and skin cancer: Health risks and opportunities. Curr. Opin. Oncol. 2009, 21, 144–149. [Google Scholar] [CrossRef]
- Collins, L.; Quinn, A.; Stasko, T. Skin Cancer and Immunosuppression. Dermatol. Clin. 2019, 37, 83–94. [Google Scholar] [CrossRef]
- Eftekhari, A.; Ahmadian, E.; Salatin, S.; Sharifi, S.; Dizaj, S.M.; Khalilov, R.; Hasanzadeh, M. Current analytical approaches in diagnosis of melanoma. Trends Anal. Chem. 2019, 116, 122–135. [Google Scholar] [CrossRef]
- Saranga-Perry, V.; Ambe, C.; Zager, J.S.; Kudchadkar, R.R. Recent developments in the medical and surgical treatment of melanoma. CA Cancer J. Clin. 2014, 64, 171–185. [Google Scholar] [CrossRef]
- Howard, R.A.; Dores, G.M.; Curtis, R.E.; Anderson, W.F.; Travis, L.B. Merkel cell carcinoma and multiple primary cancers. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Touze, A.; Gaitan, J.; Arnold, F.; Cazal, R.; Fleury, M.J.; Combelas, N.; Sizaret, P.Y.; Guyetant, S.; Maruani, A.; Baay, M.; et al. Generation of Merkel cell polyomavirus (MCV)-like particles and their application to detection of MCV antibodies. J. Clin. Microbiol. 2010, 48, 1767–1770. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Hussain, K. Merkel cell carcinoma. Clin. Exp. Dermatol. 2021, 46, 814–819. [Google Scholar] [CrossRef]
- Iyer, J.G.; Storer, B.E.; Paulson, K.G.; Lemos, B.; Phillips, J.L.; Bichakjian, C.K.; Zeitouni, N.; Gershenwald, J.E.; Sondak, V.; Otley, C.C.; et al. Relationships among primary tumor size, number of involved nodes, and survival for 8044 cases of Merkel cell carcinoma. J. Am. Acad. Dermatol. 2014, 70, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Keeling, E.; O’Leary, E.; Deady, S.; Neill, J.P.O.; Conlon, P.J.; Moloney, F.J. Gender and immunosuppression impact on Merkel cell carcinoma diagnosis and prognosis. A population based cohort study. Ski. Health Dis. 2022, 2, e80. [Google Scholar] [CrossRef]
- Becker, J.C.; Houben, R.; Ugurel, S.; Trefzer, U.; Pfohler, C.; Schrama, D. MC polyomavirus is frequently present in Merkel cell carcinoma of European patients. J. Investig. Dermatol. 2009, 129, 248–250. [Google Scholar] [CrossRef] [PubMed]
- DeCaprio, J.A. Merkel cell polyomavirus and Merkel cell carcinoma. Philos. Trans. R Soc. Lond. B Biol. Sci. 2017, 372, 20160276. [Google Scholar] [CrossRef]
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef]
- Santos-Juanes, J.; Fernandez-Vega, I.; Fuentes, N.; Galache, C.; Coto-Segura, P.; Vivanco, B.; Astudillo, A.; Martinez-Camblor, P. Merkel cell carcinoma and Merkel cell polyomavirus: A systematic review and meta-analysis. Br. J. Dermatol. 2015, 173, 42–49. [Google Scholar] [CrossRef]
- Zwijnenburg, E.M.; Lubeek, S.F.K.; Werner, J.E.M.; Amir, A.L.; Weijs, W.L.J.; Takes, R.P.; Pegge, S.A.H.; van Herpen, C.M.L.; Adema, G.J.; Kaanders, J. Merkel Cell Carcinoma: New Trends. Cancers 2021, 13, 1614. [Google Scholar] [CrossRef]
- Grundhoff, A.; Fischer, N. Merkel cell polyomavirus, a highly prevalent virus with tumorigenic potential. Curr. Opin. Virol. 2015, 14, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; MacDonald, M.; You, J. Merkel cell polyomavirus infection and Merkel cell carcinoma. Curr. Opin. Virol. 2016, 20, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Penn, I.; First, M.R. Merkel’s cell carcinoma in organ recipients: Report of 41 cases. Transplantation 1999, 68, 1717–1721. [Google Scholar] [CrossRef]
- An, K.P.; Ratner, D. Merkel cell carcinoma in the setting of HIV infection. J. Am. Acad. Dermatol. 2001, 45, 309–312. [Google Scholar] [CrossRef]
- Llombart, B.; Kindem, S.; Chust, M. Merkel Cell Carcinoma: An Update of Key Imaging Techniques, Prognostic Factors, Treatment, and Follow-up. Actas Dermosifiliogr. 2017, 108, 98–107. [Google Scholar] [CrossRef]
- Pink, R.; Ehrmann, J.; Molitor, M.; Tvrdy, P.; Michl, P.; Pazdera, J.; Hanuliak, J. Merkel cell carcinoma. A review. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2012, 156, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.D.; Su, J.C.; Chong, A.H. Skin Cancer Following Solid Organ Transplantation: A Review of Risk Factors and Models of Care. Am. J. Clin. Dermatol. 2018, 19, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Gianfreda, M.; Caiffi, S.; De Franceschi, T.; Dodero, C.; Durante, R.; Faletti, P.; Rebuttato, A.; Schivo, A.; Tassara, R. Merkel cell carcinoma of the skin in a patient with myasthenia gravis. Minerva Med. 2002, 93, 219–222. [Google Scholar] [PubMed]
- Gooptu, C.; Woollons, A.; Ross, J.; Price, M.; Wojnarowska, F.; Morris, P.J.; Wall, S.; Bunker, C.B. Merkel cell carcinoma arising after therapeutic immunosuppression. Br. J. Dermatol. 1997, 137, 637–641. [Google Scholar] [CrossRef]
- Lillis, J.; Ceilley, R.I.; Nelson, P. Merkel cell carcinoma in a patient with autoimmune hepatitis. J. Drugs Dermatol. 2005, 4, 357–359. [Google Scholar]
- Nemoto, I.; Sato-Matsumura, K.C.; Fujita, Y.; Natsuga, K.; Ujiie, H.; Tomita, Y.; Kato, N.; Kondo, M.; Ohnishi, K. Leukaemic dissemination of Merkel cell carcinoma in a patient with systemic lupus erythematosus. Clin. Exp. Dermatol. 2008, 33, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Roberto, M.; Botticelli, A.; Caggiati, A.; Chiriatti, A.; Della Rocca, C.; Ferraresi, V.; Musicco, F.; Pellacani, G.; Marchetti, P. A Regional Survey on Merkel Cell Carcinoma: A Plea for Uniform Patient Journey Modeling and Diagnostic-Therapeutic Pathway. Curr. Oncol. 2022, 29, 7229–7244. [Google Scholar] [CrossRef] [PubMed]
- Ziprin, P.; Smith, S.; Salerno, G.; Rosin, R.D. Two cases of merkel cell tumour arising in patients with chronic lymphocytic leukaemia. Br. J. Dermatol. 2000, 142, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, D.; Chen, X.; Sun, Z.; Zou, Y.; Chen, C.; Sun, S. The effect of anticancer treatment on cancer patients with COVID-19: A systematic review and meta-analysis. Cancer Med. 2021, 10, 1043–1056. [Google Scholar] [CrossRef]
- Jee, J.; Foote, M.B.; Lumish, M.; Stonestrom, A.J.; Wills, B.; Narendra, V.; Avutu, V.; Murciano-Goroff, Y.R.; Chan, J.E.; Derkach, A.; et al. Chemotherapy and COVID-19 Outcomes in Patients with Cancer. J. Clin. Oncol. 2020, 38, 3538–3546. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (N.C.C.N.). NCCN Clinical Practice Guidelines in Oncology. Merkel Cell Carcinoma. Available online: https://www.nccn.org/professionals/physician_gls/pdf/mcc.pdf (accessed on 16 January 2023).
- Becker, J.C.; Eigentler, T.; Frerich, B.; Gambichler, T.; Grabbe, S.; Holler, U.; Klumpp, B.; Loquai, C.; Krause-Bergmann, A.; Muller-Richter, U.; et al. S2k guidelines for Merkel cell carcinoma (MCC, neuroendocrine carcinoma of the skin)—Update 2018. J. Dtsch. Dermatol. Ges. 2019, 17, 562–576. [Google Scholar] [CrossRef]
- Chang, A.E.; Karnell, L.H.; Menck, H.R. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: A summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer 1998, 83, 1664–1678. [Google Scholar] [CrossRef]
- McLean, N.; Tighiouart, M.; Muller, S. Primary mucosal melanoma of the head and neck. Comparison of clinical presentation and histopathologic features of oral and sinonasal melanoma. Oral Oncol. 2008, 44, 1039–1046. [Google Scholar] [CrossRef]
- Marcus, D.M.; Marcus, R.P.; Prabhu, R.S.; Owonikoko, T.K.; Lawson, D.H.; Switchenko, J.; Beitler, J.J. Rising incidence of mucosal melanoma of the head and neck in the United States. J. Ski. Cancer 2012, 2012, 231693. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Weirather, J.L.; Manos, M.; Quattrochi, B.J.; Sholl, L.M.; Brennick, R.C.; Bowling, P.; Bailey, N.; Magarace, L.; Ott, P.A.; et al. Characterization of genetics in patients with mucosal melanoma treated with immune checkpoint blockade. Cancer Med. 2021, 10, 2627–2635. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, N.; Gong, L.; Lan, L.; Yu, Z.; Pan, Z. Clinicopathological Features, Staging Classification, and Clinical Outcomes of Esophageal Melanoma: Evaluation of a Pooled Case Series. Front. Oncol. 2022, 12, 858145. [Google Scholar] [CrossRef]
- Amit, M.; Na’ara, S.; Hanna, E.Y. Contemporary Treatment Approaches to Sinonasal Mucosal Melanoma. Curr. Oncol. Rep. 2018, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Bachar, G.; Loh, K.S.; O’Sullivan, B.; Goldstein, D.; Wood, S.; Brown, D.; Irish, J. Mucosal melanomas of the head and neck: Experience of the Princess Margaret Hospital. Head Neck 2008, 30, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Gal, T.J.; Silver, N.; Huang, B. Demographics and treatment trends in sinonasal mucosal melanoma. Laryngoscope 2011, 121, 2026–2033. [Google Scholar] [CrossRef] [PubMed]
- Thariat, J.; Poissonnet, G.; Marcy, P.Y.; Lattes, L.; Butori, C.; Guevara, N.; Dassonville, O.; Santini, J.; Bensadoun, R.J.; Castillo, L. Effect of surgical modality and hypofractionated split-course radiotherapy on local control and survival from sinonasal mucosal melanoma. Clin. Oncol. 2011, 23, 579–586. [Google Scholar] [CrossRef]
- Chi, Z.; Li, S.; Sheng, X.; Si, L.; Cui, C.; Han, M.; Guo, J. Clinical presentation, histology, and prognoses of malignant melanoma in ethnic Chinese: A study of 522 consecutive cases. BMC Cancer 2011, 11, 85. [Google Scholar] [CrossRef]
- Chang, Y.; Moore, P.S. Merkel cell carcinoma: A virus-induced human cancer. Annu. Rev. Pathol. 2012, 7, 123–144. [Google Scholar] [CrossRef]
- Cui, C.; Lian, B.; Zhou, L.; Song, X.; Zhang, X.; Wu, D.; Chi, Z.; Si, L.; Sheng, X.; Kong, Y.; et al. Multifactorial Analysis of Prognostic Factors and Survival Rates among 706 Mucosal Melanoma Patients. Ann. Surg. Oncol. 2018, 25, 2184–2192. [Google Scholar] [CrossRef]

| Body Location | CMM | MCC | MM | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | All | Male | Female | All | Male | Female | All | |
| All Skin surfaces * | 347,752 | 249,989 | 597,741 | 10,686 | 6387 | 17,073 | 25 | 28 | 53 |
| Melanoma of the skin | 347,752 | 249,989 | 597,741 | 0 | 0 | 0 | 25 | 28 | 53 |
| Other non-epithelial skin | 0 | 0 | 0 | 10,686 | 6387 | 17,073 | 0 | 0 | 0 |
| All Sites | 347,752 | 249,989 | 597,741 | 10,918 | 6504 | 17,422 | 138 | 242 | 380 |
| Sites other than skin | 0 | 0 | 0 | 232 | 117 | 349 | 113 | 214 | 327 |
| Oral cavity and pharynx | 0 | 0 | 0 | 111 | 35 | 146 | 32 | 31 | 63 |
| Digestive system | 0 | 0 | 0 | 3 | 2 | 5 | 15 | 27 | 42 |
| Respiratory system | 0 | 0 | 0 | 12 | 22 | 34 | 49 | 65 | 114 |
| Bones and joints | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Soft tissue, including heart | 0 | 0 | 0 | 29 | 19 | 48 | 0 | 0 | 0 |
| Breasts | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 |
| Female genital system | 0 | 0 | 0 | 0 | 12 | 12 | 0 | 82 | 82 |
| Male genital system | 0 | 0 | 0 | 3 | 0 | 3 | 3 | 0 | 3 |
| Urinary system | 0 | 0 | 0 | 1 | 0 | 1 | 3 | 3 | 6 |
| Eye and eye orbit | 0 | 0 | 0 | 4 | 4 | 8 | 10 | 6 | 16 |
| Brain and other nervous systems | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Endocrine system | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Blood * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Miscellaneous | 0 | 0 | 0 | 66 | 23 | 89 | 1 | 0 | 1 |
| SEER Registry | 2020 | % of Total | CMM | MCC | MM | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Census | (Expected) | Male | Female | All | Male | Female | All | Male | Female | All | |
| Alaska Natives | 111,575 | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 1% | 0% | 0% |
| California (4 SEER sites) | 39,538,223 | 25% | 28% | 25% | 27% | 26% | 25% | 25% | 12% | 14% | 14% |
| Connecticut | 3,605,944 | 2% | 3% | 3% | 3% | 3% | 4% | 3% | 2% | 2% | 2% |
| Georgia (3 SEER sites) | 10,711,908 | 6% | 8% | 8% | 8% | 5% | 4% | 5% | 3% | 7% | 6% |
| Hawaii | 1,455,271 | 1% | 1% | 1% | 1% | 1% | 1% | 1% | 2% | 2% | 2% |
| Iowa | 3,190,369 | 2% | 3% | 3% | 3% | 3% | 3% | 3% | 1% | 2% | 2% |
| Idaho | 1,839,106 | 1% | 1% | 1% | 1% | 1% | 1% | 1% | 1% | 0% | 1% |
| Illinois | 12,812,508 | 8% | 8% | 8% | 8% | 7% | 8% | 8% | 7% | 5% | 6% |
| Kentucky | 4,505,836 | 3% | 4% | 4% | 4% | 3% | 3% | 3% | 2% | 1% | 2% |
| Louisiana | 4,657,757 | 3% | 3% | 2% | 2% | 3% | 3% | 3% | 1% | 2% | 2% |
| Massachusetts | 7,029,917 | 4% | 5% | 6% | 5% | 6% | 7% | 6% | 6% | 11% | 9% |
| New Jersey | 9,288,994 | 6% | 7% | 7% | 7% | 7% | 8% | 7% | 4% | 5% | 5% |
| New Mexico | 2,117,522 | 1% | 1% | 1% | 1% | 1% | 1% | 1% | 2% | 2% | 2% |
| New York | 20,201,249 | 13% | 12% | 12% | 12% | 13% | 16% | 14% | 15% | 22% | 20% |
| Texas | 29,145,505 | 18% | 11% | 10% | 11% | 13% | 11% | 12% | 36% | 22% | 27% |
| Utah | 3,271,616 | 2% | 3% | 3% | 3% | 2% | 1% | 2% | 2% | 1% | 2% |
| Washington (Puget Sound) | 5,301,675 | 3% | 4% | 5% | 5% | 4% | 4% | 4% | 1% | 0% | 1% |
| Demographics | CMM | MCC | MM | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | All | Male | Female | All | Male | Female | All | |
| Overall Rate in 2000–2019 per 1,000,000 | 240.6 | 167.8 | 203.7 | 7.6 | 4.4 | 5.9 | 0.10 | 0.16 | 0.13 |
| Rate in 2000–2009 | 209.7 | 151 | 179.9 | 6.2 | 3.8 | 5 | 0.05 | 0.08 | 0.07 |
| Rate in 2010–2019 | 269.1 | 183.3 | 225.6 | 8.8 | 4.8 | 6.8 | 0.14 | 0.24 | 0.19 |
| % 1st primary malignant cancer | 76% | 83% | 79% | 68% | 75% | 71% | 79% | 80% | 80% |
| Age | |||||||||
| 15–44 years | 59.9 | 94.1 | 76.8 | 0.2 | 0.1 | 0.2 | 0.0 | 0.0 | 0.0 |
| 45–54 years | 247.0 | 225.5 | 236.1 | 2.1 | 1.2 | 1.7 | 0.1 | 0.1 | 0.1 |
| 55–64 years | 504.8 | 299.2 | 398.2 | 8.8 | 4.8 | 6.7 | 0.2 | 0.4 | 0.3 |
| 65–74 years | 933.3 | 404.9 | 648.3 | 31.0 | 13.7 | 21.7 | 0.4 | 0.5 | 0.4 |
| 75+ years | 1466.2 | 504.3 | 879.1 | 94.0 | 38.0 | 59.8 | 0.6 | 0.8 | 0.7 |
| Race/Ethnicity * | |||||||||
| Non-Hispanic White | 399.4 | 268.5 | 332.9 | 12.3 | 6.7 | 9.5 | 0.1 | 0.2 | 0.2 |
| Non-Hispanic Black | 7.7 | 9.0 | 8.4 | 0.8 | 0.7 | 0.7 | 0.1 | 0.0 | 0.1 |
| Non-Hispanic American Indian/Alaska Native | 62.0 | 55.0 | 58.5 | 3.1 | 2.1 | 2.6 | 0.2 | 0.0 | 0.1 |
| Non-Hispanic Asian or Pacific Islander | 13.3 | 12.2 | 12.8 | 1.4 | 1.1 | 1.2 | 0.1 | 0.1 | 0.1 |
| Hispanic (All Races) | 25.0 | 31.7 | 28.3 | 1.5 | 1.7 | 1.6 | 0.0 | 0.1 | 0.1 |
| Non-Hispanic Unknown Race | ~ | ~ | ~ | ~ | ~ | ~ | ~ | ~ | ~ |
| Percentage at each Stage | |||||||||
| Localized | 56% | 58% | 57% | 39% | 43% | 40% | 26% | 37% | 33% |
| Regional | 7% | 6% | 7% | 19% | 16% | 18% | 30% | 28% | 28% |
| Distant | 4% | 3% | 3% | 8% | 6% | 7% | 14% | 10% | 11% |
| Unstaged | 6% | 6% | 6% | 8% | 8% | 8% | 6% | 5% | 6% |
| Blank(s) | 27% | 28% | 27% | 27% | 28% | 27% | 25% | 21% | 22% |
| 5-Year Survival Percentage | CMM | MCC | MM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |||||||
| Obs. | Rel. | Obs. | Rel. | Obs. | Rel. | Obs. | Rel. | Obs. | Rel. | Obs. | Rel. | |
| Overall | 79.4 | 90.4 | 87.3 | 94.4 | 43.5 | 57.0 | 54.5 | 70.5 | 47.3 | 50.2 | 41.2 | 45.3 |
| Males & Females | ||||||||||||
| Age | Obs. | Rel. | ||||||||||
| 15–44 years | 90.7 | 91.7 | 96.4 | 96.9 | 71.7 | 72.5 | 73.1 | 73.7 | 76.2 | 76.7 | ||
| 45–54 years | 88.5 | 91.2 | 94.0 | 95.7 | 65.0 | 66.9 | 84.9 | 85.9 | 50.7 | 51.7 | ||
| 55–64 years | 85.5 | 90.9 | 91.6 | 95.0 | 59.5 | 63.4 | 79.6 | 82.8 | 48.0 | 49.7 | ||
| 65–74 years | 79.5 | 90.9 | 85.9 | 94.0 | 57.2 | 65.6 | 68.0 | 74.8 | 33.3 | 36.3 | ||
| 75+ years | 54.3 | 86.8 | 59.1 | 87.3 | 28.4 | 47.3 | 40.3 | 63.3 | 20.2 | 27.4 | ||
| Stage | ||||||||||||
| Localized | 86.3 | 98.1 | 92.1 | 99.3 | 49.0 | 66.5 | 60.5 | 78.9 | 65.6 | 72.1 | ||
| Regional | 54.8 | 62.3 | 62.9 | 69.9 | 45.5 | 57.3 | 51.9 | 65.7 | 26.9 | 28.8 | ||
| Distant | 19.1 | 21.6 | 22.5 | 24.8 | 14.1 | 18.4 | 28.0 | 33.4 | 9.1 | 9.4 | ||
| Unstaged | 69.9 | 81.2 | 78.3 | 86.0 | 38.1 | 49.6 | 43.8 | 62.7 | 83.3 | 86.4 | ||
| Breslow Thickness * | Male | Female | Male | Female | Male | Female | ||||||
| ≤0.1 mm | 2.9% | 2.7% | 0.0% | 0.0% | 0.0% | 1.0% | ||||||
| 0.2–9.7 mm | 49.1% | 48.5% | 0.0% | 0.0% | 8.5% | 17.3% | ||||||
| 9.8+ mm | 0.9% | 0.7% | 0.0% | 0.0% | 0.0% | 3.1% | ||||||
| Unknown | 47.2% | 48.1% | 100.0% | 100.0% | 91.5% | 78.6% | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dennis, L.K.; Brown, H.E.; Arrington, A.K. Comparison of Prognostic Factors for Merkel Cell Carcinoma, Mucosal Melanoma and Cutaneous Malignant Melanoma: Insights into Their Etiologies. Curr. Oncol. 2023, 30, 3974-3988. https://doi.org/10.3390/curroncol30040301
Dennis LK, Brown HE, Arrington AK. Comparison of Prognostic Factors for Merkel Cell Carcinoma, Mucosal Melanoma and Cutaneous Malignant Melanoma: Insights into Their Etiologies. Current Oncology. 2023; 30(4):3974-3988. https://doi.org/10.3390/curroncol30040301
Chicago/Turabian StyleDennis, Leslie K., Heidi E. Brown, and Amanda K. Arrington. 2023. "Comparison of Prognostic Factors for Merkel Cell Carcinoma, Mucosal Melanoma and Cutaneous Malignant Melanoma: Insights into Their Etiologies" Current Oncology 30, no. 4: 3974-3988. https://doi.org/10.3390/curroncol30040301
APA StyleDennis, L. K., Brown, H. E., & Arrington, A. K. (2023). Comparison of Prognostic Factors for Merkel Cell Carcinoma, Mucosal Melanoma and Cutaneous Malignant Melanoma: Insights into Their Etiologies. Current Oncology, 30(4), 3974-3988. https://doi.org/10.3390/curroncol30040301






