2023 Canadian Colposcopy Guideline: A Risk-Based Approach to Management and Surveillance of Cervical Dysplasia
Abstract
1. Introduction
2. Methods
3. Results
3.1. The Lower Anogenital Squamous Terminology (LAST) for HPV-Related Lesions of the Cervix
- Histopathology should be reported using the two-tiered terminology described by the LAST Project: LSIL for CIN1 and HSIL for CIN2/CIN3 (conditional, moderate).
- p16 immunohistochemistry may be used to upgrade CIN2 to CIN3. P16 should not be used to upgrade morphologically appearing CIN1 (strong, high).
3.2. Risk-Based Entry to Colposcopy
- People with a positive HPV screening test should undergo HPV genotyping and reflex cytology before referral to colposcopy (strong, high).
- People with HPV 16/18 should be referred to colposcopy (strong, high).
- People with HPV ‘other’ ASCUS or LSIL should have HPV testing repeated at 12 and 24 months, only referred to colposcopy if they meet other criteria or have persistent HPV “other” at 24 months (conditional, moderate).
- People with HPV-positive HSIL, ASC-H, AGC, AIS or cytology suspicious for invasive cancer should be referred directly to colposcopy, regardless of HPV genotype (strong, high).
- People with immunocompromise with any HR HPV should be referred to colposcopy (conditional, low).
3.3. The Initial Colposcopic Exam and Documentation
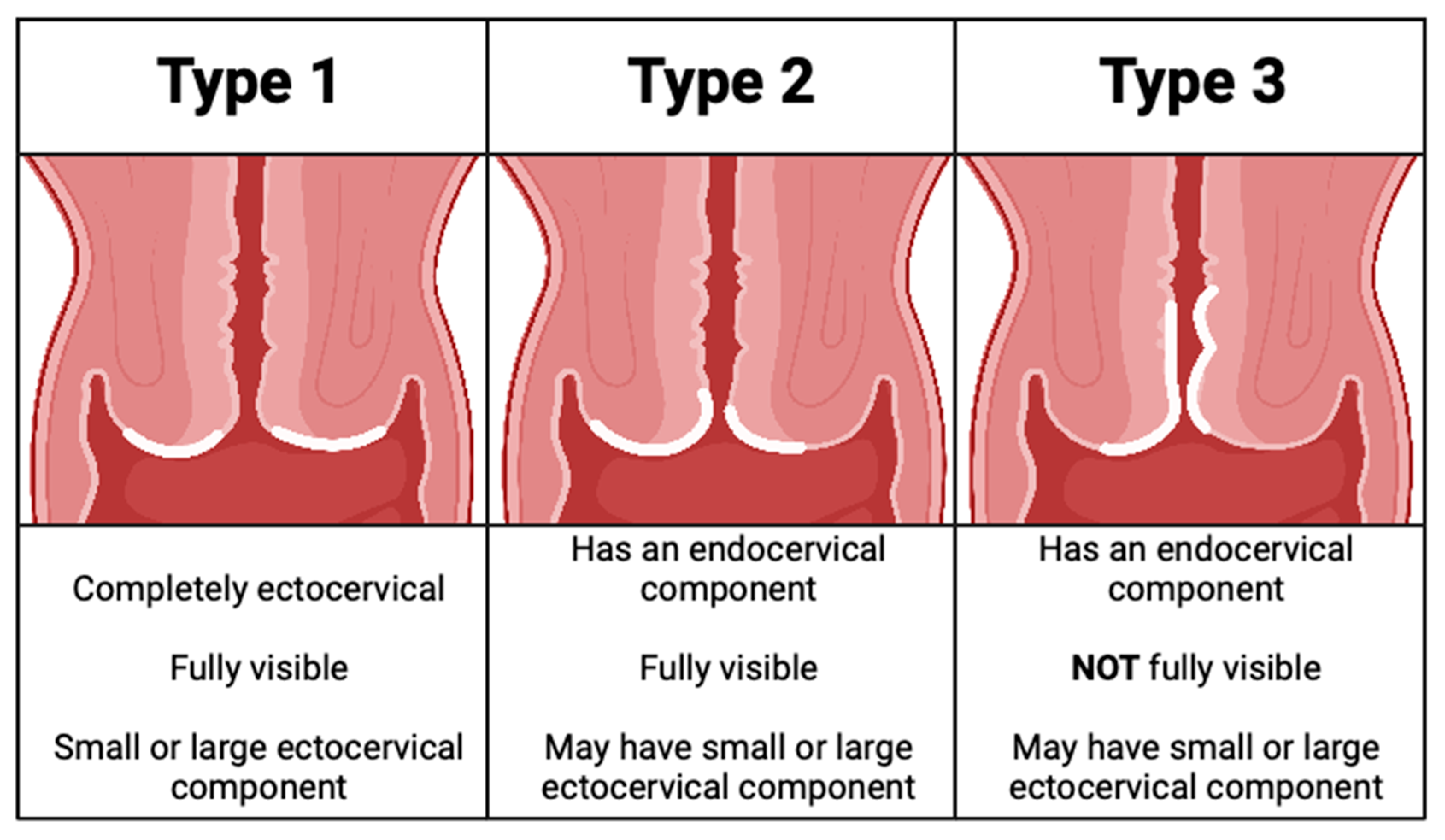
- The transformation zone should be assessed, and the type should be documented (strong, high).
- International Federation of Cervical Pathology and Colposcopy (IFCPC) terminology is recommended for documenting colposcopic findings (strong, high).
- Targeted biopsies of lesions are recommended. In the setting of HSIL, or positive HPV16/18, where colposcopic impression is normal, any area of acetowhitening, metaplasia or uncertainty should be biopsied (strong, high).
- Endocervical curettage and endometrial biopsies are contraindicated in pregnancy (strong, high).
- Endocervical curettage is recommended with: (i) a type 3 transformation zone, (ii) HSIL/ASC-H cytology when no lesion is identified, (iii) AGC/AIS cytology, (iv) when excisional treatment has positive margins and (v) in people over age 45 with HPV 16 (conditional, moderate).
- Endometrial sampling is recommended in those 35 years and older for all categories of AGC/AIS or atypical endometrial cells on cytology. Endometrial sampling is also indicated in those under 35 with increased risks of endometrial cancer (obesity, chronic anovulation or abnormal uterine bleeding) or atypical endometrial cells on cytology in those of any age (strong, moderate).
- For pain management for routine exam and cervical biopsies, thorough pre-procedure counseling and non-pharmacologic methods are recommended. Oral analgesics may be considered. Topical and injected analgesics are not recommended for routine exam and biopsies of the cervix (strong, low).
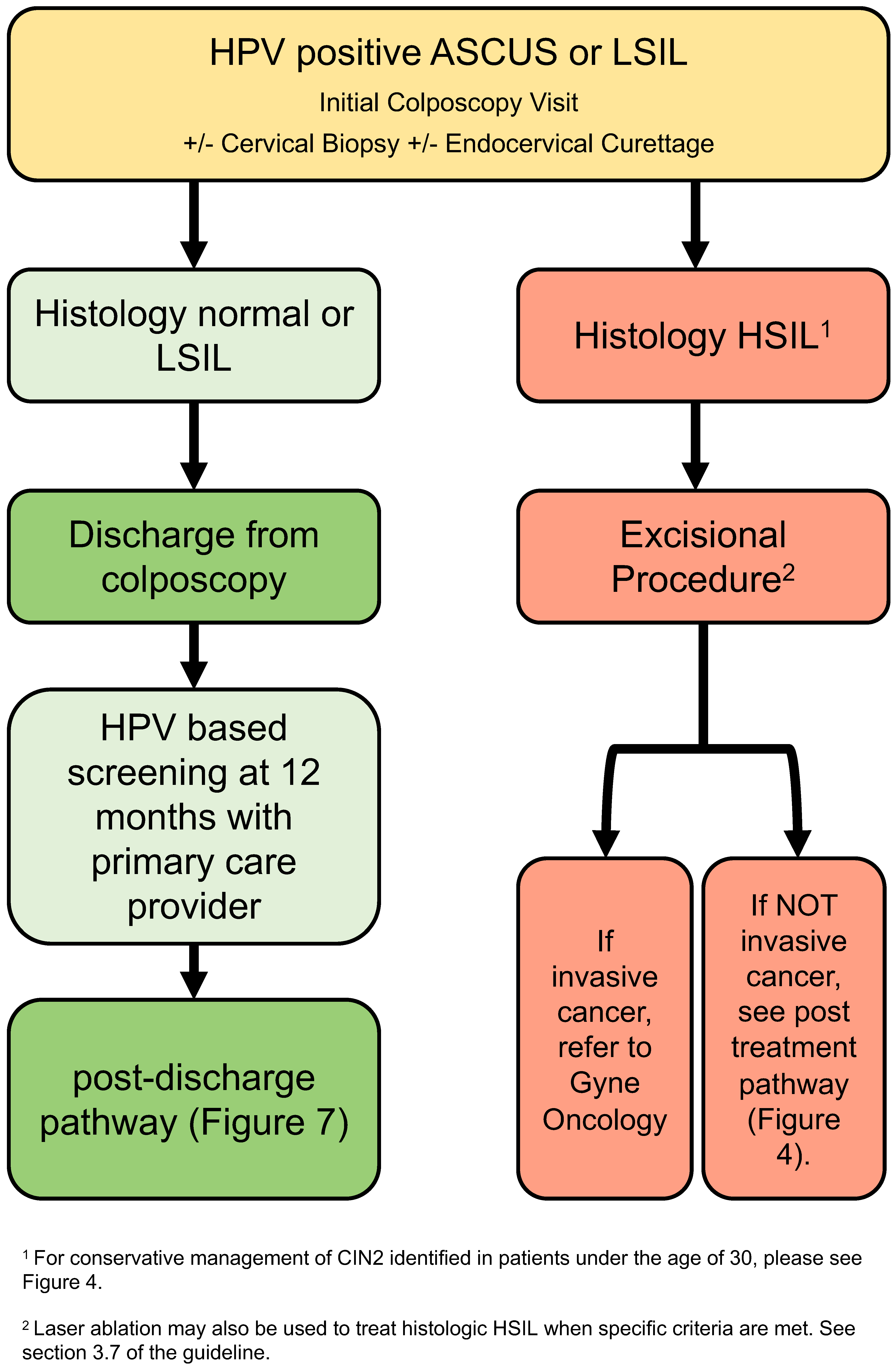
3.4. Low-Grade Referral Pathway (Figure 2)
- After initial colposcopy assessment, those with normal or LSIL histology can be discharged from colposcopy (strong, moderate).
- Where HSIL is identified on histology, excisional procedure is recommended (strong, high).
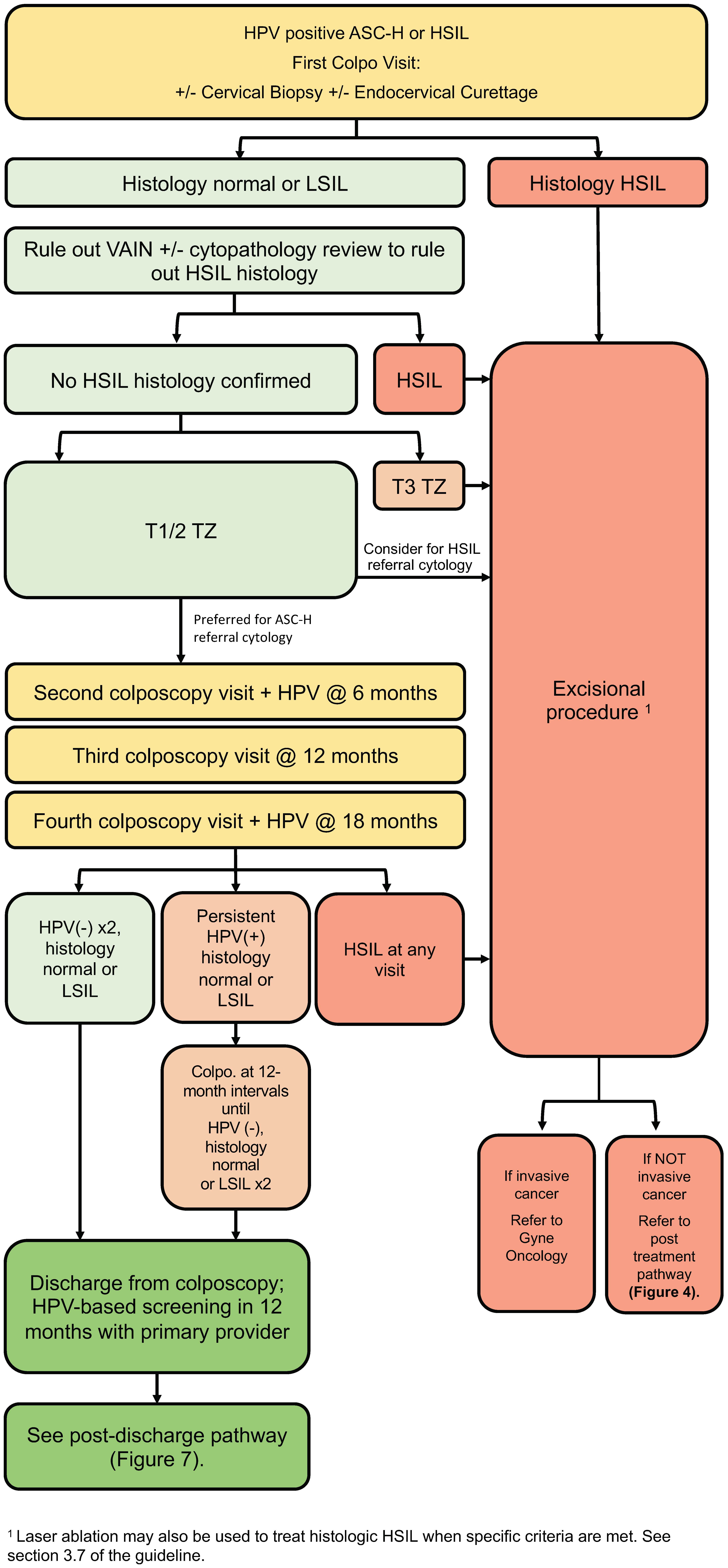
3.5. High-Grade Referral Pathway (Figure 3)
- People with evidence of histologic HSIL should undergo an excisional procedure (strong, high).
- In cases where no lesion is identified following referral for HPV-positive cytologic ASC-H/HSIL, review by an experienced cytopathologist should be considered (conditional, moderate).
- In cases where no cervical lesion is identified following referral for HPV-positive cytologic ASC-H/HSIL, VAIN must be ruled out by colposcopy (conditional, low).
- In cases of discordance, where no histologic HSIL is confirmed, management depends on transformation zone type and referral cytology (conditional, moderate).
- For a type 3 transformation zone, excisional procedure is recommended (conditional, moderate).
- For a type 1 or 2 transformation zone, the preferred management for ASC-H referral cytology is surveillance. For HSIL referral cytology, excisional procedure can be considered (conditional, moderate).
- If surveillance without treatment is undertaken, people should remain in colposcopy at 6-month intervals with HPV testing at annual intervals until HPV is negative on two consecutive tests and histology remains normal or LSIL (conditional, moderate).
- If HPV remains positive despite negative colposcopy, people should remain in colposcopy for surveillance at 12-month intervals until they meet the above criteria for discharge (conditional, moderate).
- If, during surveillance, there is evidence of cytologic or histologic HSIL, excisional procedure is recommended (strong, high).
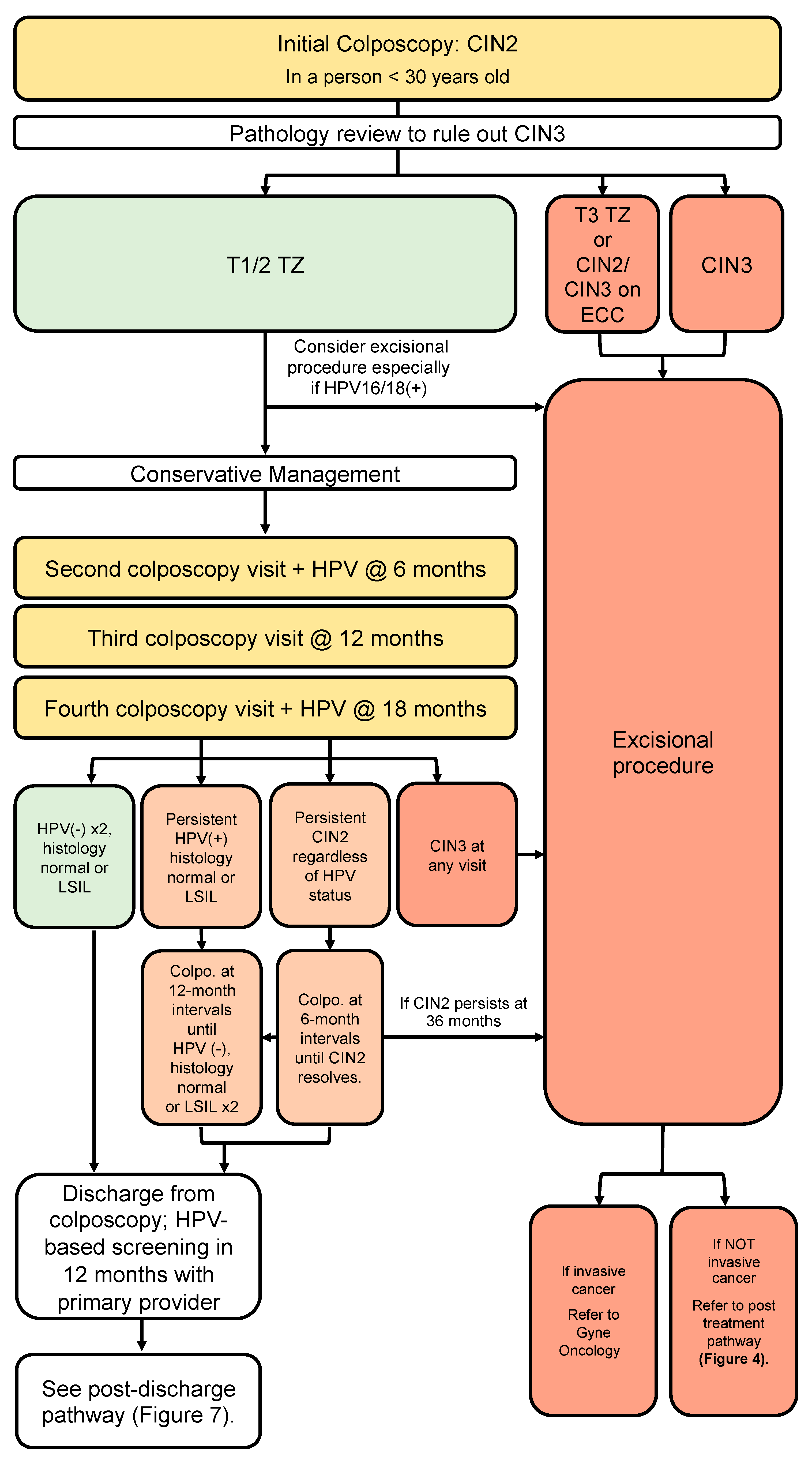
3.6. Conservative Management of CIN2 in People <30 Years Old Where Fertility Is a Concern (Figure 4)
- In people under the age of 30, where childbearing considerations outweigh the risk of pre-invasive or invasive disease, and where a pathologic distinction between CIN2 and CIN3 can be reliably made, a conservative approach may be undertaken (conditional, moderate).
- In these cases, review by an expert cytopathologist should rule out CIN3 (conditional, high).
- Findings of CIN2 in a young person with a type 3 transformation zone or CIN2 identified on endocervical curettage should undergo excisional procedure (conditional, moderate).
- Findings of CIN2 in a young person with a type 1 or 2 transformation zone may be managed conservatively for childbearing considerations; surveillance should include colposcopy at 6-month intervals and HPV testing at annual intervals for 3 years to allow these young people to resolve their HPV infections (conditional, moderate).
- If CIN2 persists, continue colposcopy at 6-month intervals with HPV testing annually. People under 30 with persistent CIN2 > 36 months or CIN3 at any colposcopy visit should have an excisional procedure (conditional, low).
- People under the age of 30 with initial CIN2 who are managed conservatively can be discharged from colposcopy once histology is normal or LSIL and HPV is negative on two consecutive annual follow-up visits (conditional, moderate).
- People under the age of 30 with initial CIN2 who remain HPV-positive at annual follow-up should remain in colposcopy with HPV tests at annual intervals (conditional, moderate).
3.7. Treatment for HSIL Histology
- Recommended treatment for histological HSIL is an excision procedure with a LEEP (strong, high).
- An ablative procedure with carbon dioxide laser is acceptable when used by trained and experienced colposcopists when specific criteria are met (conditional, low).
- Cryotherapy for HSIL is not recommended (strong, high).
- Treatment should be performed in the clinic setting with local anesthesia plus a vasopressor to the cervix (conditional, moderate).
3.8. Post-Treatment Pathway (Figure 5)
- All people undergoing an excisional procedure in colposcopy should have an HPV test of cure and cytology at 6 months post treatment, as well as colposcopic assessment and endocervical curettage if endocervical margin is positive on excision specimen (strong, high).
- People who are HPV-negative with normal, ASCUS or LSIL cytology and histology after treatment can be discharged from colposcopy (strong, moderate).
- People who remain persistently HPV-positive with cytology and/or histology that is normal, ASCUS or LSIL, regardless of genotype, should remain in colposcopy at 12-month intervals until HPV is negative (conditional, low).
- After discharge from colposcopy, people treated for HSIL should have 12-month HPV-based screening with their primary care provider. If HPV-negative, they can resume HPV-based screening at 3-year intervals indefinitely (conditional, low).

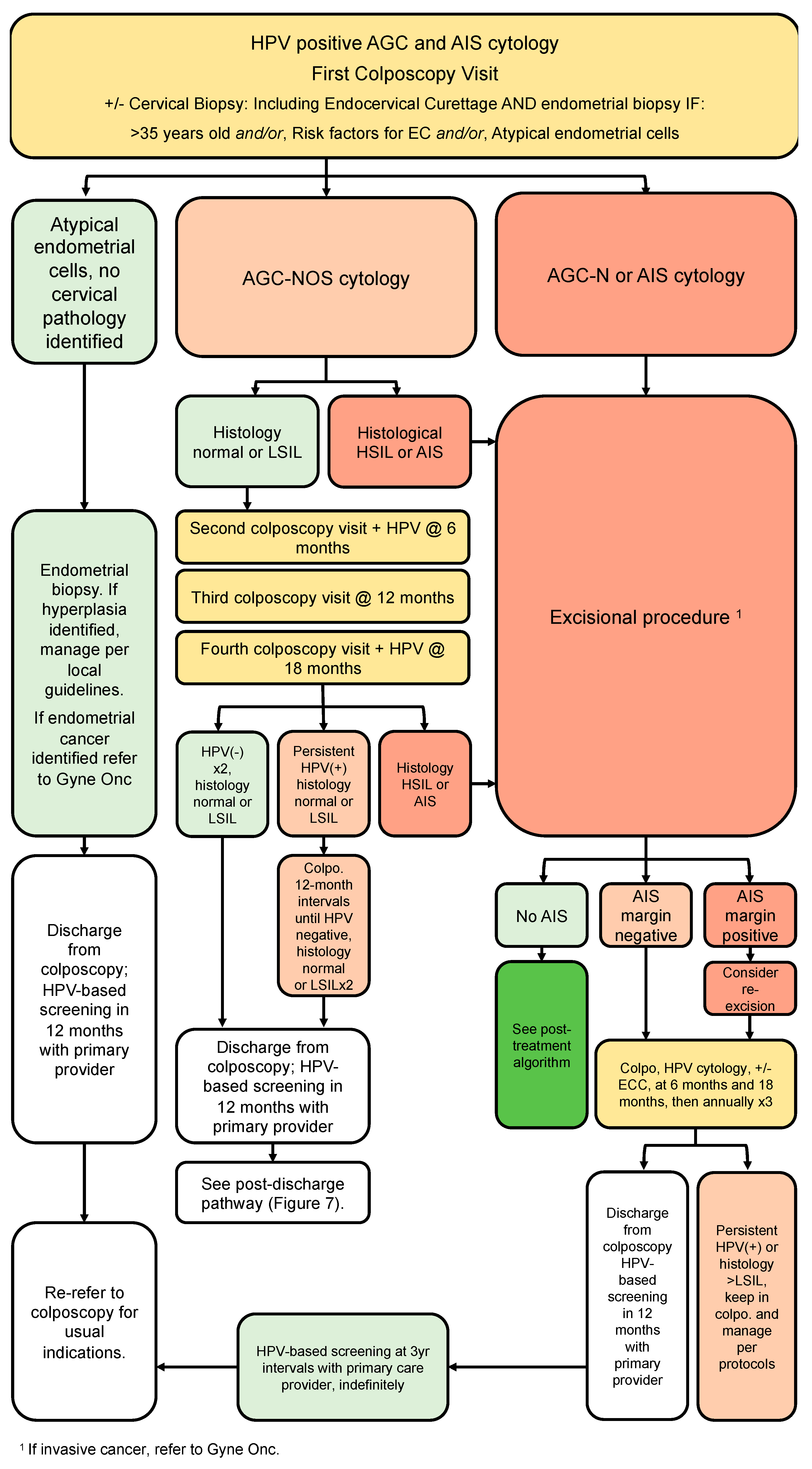
3.9. Glandular Pathway (Figure 6)
- All people with HPV-positive AGC or AIS cytology should be referred directly to colposcopy, regardless of HPV genotype (strong, high).
- At time of initial colposcopic assessment, all people with HPV-positive AGC or AIS should have endocervical curettage (strong, high).
- Endometrial biopsy is recommended for all people >35 years old AND/OR risk factors for endometrial cancer AND/OR atypical endometrial cells on cytology (strong, moderate).
- People referred with AGC-NOS cytology, in the absence of endometrial pathology and histologic HSIL or AIS, may be managed conservatively with colposcopy, cytology and HPV testing at annual intervals. If all negative on two consecutive visits, they can be discharged from colposcopy to HPV-based screening at 5-year intervals (conditional, low).
- All people AGC-N or AIS should undergo excisional procedure regardless of HPV status (strong, high).
- Following the excisional procedure for AIS/AGC-N, if margins are positive, consider re-excision (strong, high).
- Following excisional procedure for AIS/AGC-N, in the absence of cancer, surveillance with 6-monthly colposcopy, ECC and HPV testing is recommended; if surveillance is negative ×3 years in colposcopy (HPV-negative, ≤HSIL/AIS), people can be discharged to HPV-based screening at 3-year intervals. If HPV is persistently positive or histology shows HSIL or persistent glandular abnormalities, people should stay in colposcopy and be managed per algorithms (conditional, moderate).
- Hysterectomy can be considered when post-treatment margins/ECC are persistently positive for AIS and/or fertility is not desired (conditional, moderate).
3.9.1. Adenocarcinoma In Situ
3.9.2. Follow Up for Adenocarcinoma In Situ
3.10. Post-Discharge Follow-Up for People with Squamous Lesions Not Treated in Colposcopy (Figure 7)
- All people discharged from colposcopy should have HPV-based screening at 12 months with their primary care provider (conditional, very low).
- Subsequent management depends on referral cytology and HPV status.
- People referred with low-grade cytology who are HPV-negative on 12-month screening with their primary care provider may transition to routine HPV-based screening at 5-year intervals (conditional, moderate).
- People referred with low-grade cytology who are HPV-positive (regardless of genotype) at 12-month screening with their primary care provider should be re-referred to colposcopy for the usual indications (conditional, moderate).
- People referred with high-grade cytology (untreated) should have two negative annual HPV tests in colposcopy with colposcopic findings that are normal or LSIL before they can be discharged to 12-month HPV-based screening with their primary care provider (conditional, low). If HPV remains negative at 12-month post-colposcopy screening, they may transition to HPV-based screening at 3-year intervals indefinitely. If HPV is positive at 12-month post-colposcopy screening, they should be re-referred to colposcopy for the usual indications (conditional, high).
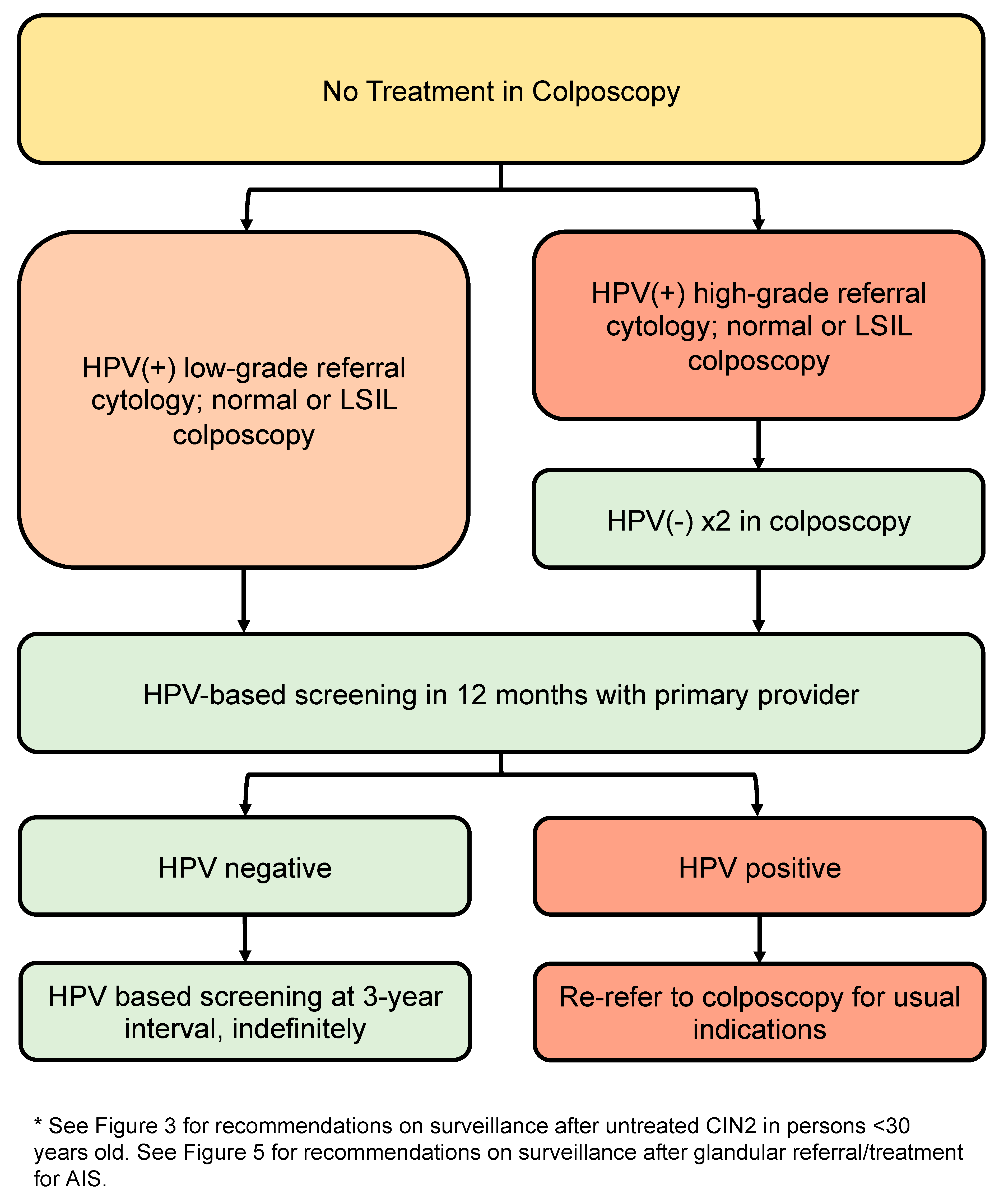
3.10.1. Post-Discharge Follow-Up of People with Low-Grade Referral Cytology (Untreated)
3.10.2. Post-Discharge Follow-Up of People with High-Grade Referral Cytology (Untreated)
3.11. Special Populations
3.11.1. People under the Age of 25
- Those under 25 should not have screening with HPV testing or cytology (strong, high).
- If screening occurs and high-grade cytologic abnormalities are identified, indications for colposcopy remain the same, regardless of age (conditional, low).
- When a CIN2 lesion is confirmed, and CIN3 is ruled out, conservative management may be undertaken when childbearing considerations outweigh the risk of invasive disease (strong, moderate).
3.11.2. Pregnancy
- Risk-based threshold for entry to colposcopy are the same, regardless of pregnancy (strong, high).
- Pregnant people should be evaluated by an experienced colposcopist (strong, moderate).
- Pregnant people who are HR-HPV-positive with reflex normal or low-grade referral cytology (ASCUS or LSIL) should have HPV-based screening repeated 3 months post-partum (strong, moderate); pregnant people who are HR-HPV positive with reflex high-grade or glandular cytology (ASC-H, HSIL, AGC) should be seen in colposcopy within 4 weeks (strong, moderate).
- Endometrial biopsy and endocervical curettage are contraindicated in pregnancy. (strong, high)
- Cervical biopsies are indicated when there is a concern for HSIL or cancer; adverse obstetrical outcomes of cervical biopsies are rare (conditional, moderate).
- Excisional procedures for biopsy-proven HSIL or AIS in pregnancy can be delayed until 8–12 weeks post-partum (conditional, low).
- Biopsy-proven carcinoma in pregnancy should be referred urgently to gynecologic oncology (strong, high).
3.11.3. Immunocompromised People
- Colposcopy is recommended for all immunocompromised people who are HPV-positive, regardless of HPV genotype (conditional, low).
3.11.4. Menopausal People
- Menopausal people have higher rates of cervix cancer and unsatisfactory colposcopy. Consider ECC and larger excisions when indicated (conditional, moderate).
- Consider pre-treatment with vaginal estrogen for 6 weeks prior to colposcopy to increase the rates of a satisfactory exam in postmenopausal people (conditional, low).
3.12. Equity in Colposcopy
- Colposcopy providers should be aware of the barriers to access cervical cancer screening and colposcopy, including geographical, socioeconomic, cultural, physical, psychological, provider-related and system-related barriers (strong, low).
- Colposcopy providers are encouraged to seek additional training in cultural safety and trauma-informed care (strong, low).
- Every effort should be made to facilitate access to care for individuals from historically underserved populations, including people with mobility restrictions, obesity, members of the transgender community, immigrants, Indigenous peoples, people from rural communities and those with mental health disorders (strong, low).
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem; World Health Organization: Geneva, Switzerland, 2021; Available online: www.wipo.int/amc/en/mediation/rules/ (accessed on 4 November 2022).
- Brenner, D. Canadian Cancer Statistics Advisory Committee in Collaboration with the Canadian Cancer Society, Statistics Canada and the Public Health Agency of Canada. “Canadian Cancer Statistics 2021”; Canadian Cancer Society: Toronto, ON, Canada, 2021. [Google Scholar]
- Action Plan for the Elimination of cervical cancer in Canada 2020–2030. 2020. Available online: https://www.partnershipagainstcancer.ca/topics/elimination-cervical-cancer-action-plan/ (accessed on 4 November 2022).
- Zigras, T.; Mayrand, M.-H.; Bouchard, C.; Salvador, S.; Eiriksson, L.; Almadin, C.; Kean, S.; Dean, E.; Malhotra, U.; Todd, N.; et al. Canadian Guideline on the Management of a Positive Human Papillomavirus Test and Guidance for Specific Populations. Curr. Oncol. 2023, 30, 5652–5679. [Google Scholar] [CrossRef]
- Perkins, R.B.; Guido, R.S.; Castle, P.E.; Chelmow, D.; Einstein, M.H.; Garcia, F.; Huh, W.K.; Kim, J.J.; Moscicki, A.-B.; Nayar, R.; et al. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J. Low. Genit. Tract Dis. 2020, 24, 102–131. [Google Scholar] [CrossRef] [PubMed]
- Darragh, T.M.; Colgan, T.J.; Cox, J.T.; Heller, D.S.; Henry, M.R.; Luff, R.D.; McCalmont, T.; Nayar, R.; Palefsky, J.M.; Stoler, M.H.; et al. Members of LAST Project Work Groups. The Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions: Background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med. 2012, 136, 1266–1297. [Google Scholar] [CrossRef] [PubMed]
- Carreon, J.D.; Sherman, M.E.; Guillén, D.; Solomon, D.; Herrero, R.; Jerónimo, J.; Wacholder, S.; Rodríguez, A.C.; Morales, J.; Hutchinson, M.; et al. CIN2 is a much less reproducible and less valid diagnosis than CIN3: Results from a histological review of population-based cervical samples. Int. J. Gynecol. Pathol. 2007, 26, 441–446. [Google Scholar] [CrossRef] [PubMed]
- The 1988 Bethesda System for reporting cervical/vaginal cytological diagnoses. National Cancer Institute Workshop. JAMA 1989, 262, 931–934. [CrossRef]
- Richart, R.M. Cervical intraepithelial neoplasia: A review. Pathol. Annu. 1973, 8, 301–328. [Google Scholar]
- Demarco, M.; Egemen, D.; Raine-Bennett, T.R.; Cheung, L.C.; Befano, B.; Poitras, N.E.; Lorey, T.S.; Chen, X.; Gage, J.C.; Castle, P.E.; et al. A Study of Partial Human Papillomavirus Genotyping in Support of the 2019 ASCCP Risk-Based Management Consensus Guidelines. J. Low. Genit. Tract Dis. 2020, 24, 144–147. [Google Scholar] [CrossRef]
- Egemen, D.; Cheung, L.C.; Chen, X.; Demarco, M.; Perkins, R.B.; Kinney, W.; Poitras, N.; Befano, B.; Locke, A.; Guido, R.S.; et al. Risk Estimates Supporting the 2019 ASCCP Risk-Based Management Consensus Guidelines. J. Low. Genit. Tract Dis. 2020, 24, 132–143. [Google Scholar] [CrossRef]
- Perkins, R.B. Summary of Current Guidelines for Cervical Cancer Screening and Management of Abnormal Test Results: 2016–2020. J. Womens Health 2021, 30, 5–13. [Google Scholar] [CrossRef]
- Moscicki, A.-B.; Flowers, L.; Huchko, M.J.; Long, M.E.; MacLaughlin, K.; Murphy, J.; Spiryda, L.B.; Gold, M.A. Guidelines for Cervical Cancer Screening in Immunosuppressed Women without HIV Infection. J. Low. Genit. Tract Dis. 2019, 23, 87–101. [Google Scholar] [CrossRef]
- Wang, J.; Dong, J.; Zhou, Y.; Wang, K.; Pan, M.; Deng, Z.; Wang, P.; Du, Y.; Lu, W. Performance of human papillomavirus (HPV) mRNA testing and HPV 16 and 18/45 genotyping combined with age stratification in the triaging of women with ASC-US cytology. Gynecol. Oncol. 2022, 164, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, J.; Bentley, J.; Bösze, P.; Girardi, F.; Haefner, H.; Menton, M.; Perrotta, M.; Prendiville, W.; Russell, P.; Sideri, M.; et al. 2011 Colposcopic Terminology of the International Federation for Cervical Pathology and Colposcopy. Obstet. Gynecol. 2012, 120, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Bentley, J.; Bertrand, M.; Brydon, L.; Gagné, H.; Hauck, B.; Mayrand, M.-H.; McFaul, S.; Power, P.; Schepansky, A.; Straszak-Suri, M.; et al. RETIRED: Colposcopic Management of Abnormal Cervical Cytology and Histology. J. Obstet. Gynaecol. Can. 2012, 34, 1188–1202. [Google Scholar] [CrossRef] [PubMed]
- Gage, J.C.; Hanson, V.W.; Abbey, K.; Dippery, S.; Gardner, S.; Kubota, J.; Schiffman, M.; Solomon, D.; Jeronimo, J. Number of Cervical Biopsies and Sensitivity of Colposcopy. Obstet. Gynecol. 2006, 108, 264–272. [Google Scholar] [CrossRef]
- Ren, H.; Jia, M.; Zhao, S.; Li, H.; Fan, S. Factors Correlated with the Accuracy of Colposcopy-Directed Biopsy: A Systematic Review and Meta-Analysis. J. Investig. Surg. 2020, 35, 284–292. [Google Scholar] [CrossRef]
- Wentzensen, N.; Schiffman, M.; Silver, M.I.; Khan, M.J.; Perkins, R.B.; Smith, K.M.; Gage, J.C.; Gold, M.A.; Conageski, C.; Einstein, M.H.; et al. ASCCP Colposcopy Standards: Risk-Based Colposcopy Practice. J. Low. Genit. Tract Dis. 2017, 21, 230–234. [Google Scholar] [CrossRef]
- Jing, L.; Dan, W.; Zhunan, L.; Ying, X.; Yi, C. Residual lesions in uterine specimens after loop electrosurgical excision procedure in patients with CIN. Arch. Gynecol. Obstet. 2018, 298, 805–812. [Google Scholar] [CrossRef]
- Cao, D.; Shen, K.; Chen, Y.; Xu, Y.; Wu, D. Value of endocervical curettage in follow-up for patients with cervical intraepithelial neoplasia stage 2+ after loop electrosurgical excision. Gynecol. Oncol. 2020, 158, 584–589. [Google Scholar] [CrossRef]
- Auclair, M.-H.; Yong, P.J.; Salvador, S.; Thurston, J.; Colgan, T.J.; Sebastianelli, A. Guideline No. 390—Classification and Management of Endometrial Hyperplasia. J. Obstet. Gynaecol. Can. 2019, 41, 1789–1800. [Google Scholar] [CrossRef]
- Allen, R.H.; Micks, E.; Edelman, A. Pain Relief for Obstetric and Gynecologic Ambulatory Procedures. Obstet. Gynecol. Clin. N. Am. 2013, 40, 625–645. [Google Scholar] [CrossRef]
- Chan, Y.; Lee, P.W.; Ng, T.; Ngan, H.Y.; Wong, L. The use of music to reduce anxiety for patients undergoing colposcopy: A randomized trial. Gynecol. Oncol. 2003, 91, 213–217. [Google Scholar] [CrossRef]
- Naki, M.; Api, O.; Acioglu, H.C.; Uzun, M.G.; Kars, B.; Unal, O. Analgesic Efficacy of Forced Coughing versus Local Anesthesia during Cervical Punch Biopsy. Gynecol. Obstet. Investig. 2011, 72, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Serati, M.; Cromi, A.; di Naro, E.; Casarin, J.; Pinelli, C.; Rossi, T.; Ghezzi, F. Local anesthetic versus forced coughing at colposcopic-guided biopsy: A prospective study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 181, 15–19. [Google Scholar] [CrossRef]
- Kuhn, T.; Ukazu, A.; Strickland, P.O.; Roche, N.; Taveras, Y.; Kovalenko, O.; Pompeo, L.; Einstein, M. The Effect of Forced Cough to Minimize Pain and Discomfort at the Time of Colposcopy-Guided Cervical Biopsy. J. Low. Genit. Tract Dis. 2020, 24, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Schmid, B.C.; Pils, S.; Heinze, G.; Hefler, L.; Reinthaller, A.; Speiser, P. Forced coughing versus local anesthesia and pain associated with cervical biopsy: A randomized trial. Am. J. Obstet. Gynecol. 2008, 199, 641.e1–641.e3. [Google Scholar] [CrossRef]
- Church, L. Analgesia for colposcopy: Double-masked, randomized comparison of ibuprofen and benzocaine gel. Obstet. Gynecol. 2001, 97, 5–10. [Google Scholar] [CrossRef]
- Darweesh, F.F.; Samy, A.; Mousa, A.M.; Abdelbar, A.T.; Mahmoud, M.; Abdelhakim, A.M.; Metwally, A.A. Role of Oral Tramadol 50 mg in Reducing Pain during Colposcopy-Directed Cervical Biopsy. J. Low. Genit. Tract Dis. 2020, 24, 206–210. [Google Scholar] [CrossRef]
- Ferris, D.G.; Harper, D.M.; Callahan, B.; Robinson, T.; Litaker, M.S.; Messing, M.; Mensah, L. The Efficacy of Topical Benzocaine Gel in Providing Anesthesia Prior to Cervical Biopsy and Endocervical Curettage. J. Low. Genit. Tract Dis. 1997, 1, 221–227. [Google Scholar] [CrossRef]
- Clifton, P.A.; Shaughnessy, A.F.; Andrews, S. Ineffectiveness of topical benzocaine spray during colposcopy. J. Fam. Pract. 1998, 46, 242–247. [Google Scholar] [CrossRef]
- Wong, G.; Li, R.; Wong, T.; Fan, S. The effect of topical lignocaine gel in pain relief for colposcopic assessment and biopsy: Is it useful? BJOG Int. J. Obstet. Gynaecol. 2008, 115, 1057–1060. [Google Scholar] [CrossRef]
- Mattar, O.M.; Samy, A.; Shehata, M.; Ibrahim, A.M.; Abdelaziz, A.; Abdelazeim, N.; Elzemrany, A.A.; Kasem, S.A.; Ros, M.H.; Hamad, L.Y.; et al. The efficacy of local anesthetics in pain relief during colposcopic-guided biopsy: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 237, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Loopik, D.L.; Bekkers, R.L.; Massuger, L.F.; Melchers, W.J.; Siebers, A.G.; Bentley, J.R. Post-Colposcopy Management and Progression Predictors of Biopsy-Proven CIN1 in Women under 25 Years. J. Obstet. Gynaecol. Can. 2019, 41, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Demarco, M.; Cheung, L.C.; Kinney, W.K.; Wentzensen, N.; Lorey, T.S.; Fetterman, B.; Poitras, N.E.; Befano, B.; Castle, P.E.; Schiffman, M. Low Risk of Cervical Cancer/Precancer Among Most Women under Surveillance Postcolposcopy. J. Low. Genit. Tract Dis. 2018, 22, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.M. Stuck in the middle: Diagnostic and clinical management challenges surrounding CIN2. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 737. [Google Scholar] [CrossRef]
- Jordan, J.; Martin-Hirsch, P.; Arbyn, M.; Schenck, U.; Baldauf, J.-J.; Da Silva, D.; Anttila, A.; Nieminen, P.; Prendiville, W. European guidelines for clinical management of abnormal cervical cytology, Part 2. Cytopathology 2009, 20, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Massad, L.S.; Einstein, M.H.; Huh, W.K.; Katki, H.A.; Kinney, W.K.; Schiffman, M.; Solomon, D.; Wentzensen, N.; Lawson, H.W. 2012 Updated Consensus Guidelines for the Management of Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J. Low. Genit. Tract Dis. 2013, 17, S1–S27. [Google Scholar] [CrossRef]
- Jordan, J.; Arbyn, M.; Martin-Hirsch, P.; Schenck, U.; Baldauf, J.-J.; Da Silva, D.; Anttila, A.; Nieminen, P.; Prendiville, W. European guidelines for quality assurance in cervical cancer screening: Recommendations for clinical management of abnormal cervical cytology, part 1. Cytopathology 2008, 19, 342–354. [Google Scholar] [CrossRef]
- Wright, T.C., Jr.; Massad, L.S.; Dunton, C.J.; Spitzer, M.; Wilkinson, E.J.; Solomon, D. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. J. Low. Genit. Tract Dis. 2007, 11, 223–239. [Google Scholar] [CrossRef]
- Cervical Cancer Screening Guidelines|Cancer Council. (n.d.). Retrieved 8 May 2023. Available online: https://www.cancer.org.au/clinical-guidelines/cervical-cancer/cervical-cancer-screening (accessed on 4 November 2022).
- Kyrgiou, M.; Athanasiou, A.; Kalliala, I.E.J.; Paraskevaidi, M.; Mitra, A.; Martin-Hirsch, P.P.; Arbyn, M.; Bennett, P.; Paraskevaidis, E. Obstetric outcomes after conservative treatment for cervical intraepithelial lesions and early invasive disease. Cochrane Database Syst. Rev. 2017, 2017, CD012847. [Google Scholar] [CrossRef]
- Tainio, K.; Athanasiou, A.; Tikkinen, K.; Aaltonen, R.; Cárdenas, J.; Hernándes; Glazer-Livson, S.; Jakobsson, M.; Joronen, K.; Kiviharju, M.; et al. Clinical course of untreated cervical intraepithelial neoplasia grade 2 under active surveillance: Systematic review and meta-analysis. BMJ 2018, 360, k499. [Google Scholar] [CrossRef]
- Bekos, C.; Schwameis, R.; Heinze, G.; Gärner, M.; Grimm, C.; Joura, E.; Horvat, R.; Polterauer, S.; Polterauer, M. Influence of age on histologic outcome of cervical intraepithelial neoplasia during observational management: Results from large cohort, systematic review, meta-analysis. Sci. Rep. 2018, 8, 6383. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, M.A.; Nikolopoulos, M.; Garner, J.E.; Adib, T.R.; Mukhopadhyay, D.; Rains, J.S.; Harper, C.A.; Wuntakal, R. Conservative management of cervical intraepithelial neoplasia grade 2 (CIN2) in women under 30 years of age: A cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 228, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Brun, J.-L.; Letoffet, D.; Marty, M.; Griffier, R.; Ah-Kit, X.; Garrigue, I. Factors predicting the spontaneous regression of cervical high-grade squamous intraepithelial lesions (HSIL/CIN2). Arch. Gynecol. Obstet. 2020, 303, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Silver, M.I.; Gage, J.C.; Schiffman, M.; Fetterman, B.; Poitras, N.E.; Lorey, T.; Cheung, L.C.; Katki, H.A.; Locke, A.; Kinney, W.K.; et al. Clinical Outcomes after Conservative Management of Cervical Intraepithelial Neoplasia Grade 2 (CIN2) in Women Ages 21–39 Years. Cancer Prev. Res. 2018, 11, 165–170. [Google Scholar] [CrossRef]
- Dempster-Rivett, K.; Innes, C.R.; Simcock, B.J.; Harker, D.; Williman, J.A.; Van Der Griend, R.A.; Whitehead, M.; Hibma, M.; Lawton, B.A.; Fitzgerald, P.; et al. Evaluation of guidelines for observational management of cervical intraepithelial neoplasia 2 in young women. Am. J. Obstet. Gynecol. 2020, 223, 408.e1–408.e11. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Finlayson, S.J.; Gukova, K.; Hanley, G.; Miller, D.; Sadownik, L.A. Outcomes of Conservative Management of High Grade Squamous Intraepithelial Lesions in Young Women. J. Low. Genit. Tract Dis. 2018, 22, 212–218. [Google Scholar] [CrossRef]
- Loopik, D.L.; Bekkers, R.L.; Massuger, L.F.; Melchers, W.J.; Siebers, A.G.; Bentley, J. Justifying conservative management of CIN2 in women younger than 25 years—A population-based study. Gynecol. Oncol. 2018, 152, 82–86. [Google Scholar] [CrossRef]
- Li, L.; Chen, C.-X.; Jiang, Y.-M. Meta-analysis of cold-knife conization versus loop electrosurgical excision procedure for cervical intraepithelial neoplasia. OncoTargets Ther. 2016, 9, 3907–3915. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, C.; Li, L. Comparison of Cold-Knife Conization versus Loop Electrosurgical Excision for Cervical Adenocarcinoma In Situ (ACIS): A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0170587. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, H.F.; Tang, Y.; Chen, J.; Lv, J. Pregnancy Outcome after the Treatment of Loop Electrosurgical Excision Procedure or Cold-Knife Conization for Cervical Intraepithelial Neoplasia. Gynecol. Obstet. Investig. 2014, 77, 240–244. [Google Scholar] [CrossRef]
- Prendiville, W. The treatment of CIN: What are the risks? Cytopathology 2009, 20, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.; Taghavi, K.; Hu, S.-Y.; Mogri, S.; Joshi, S. Management of cervical premalignant lesions. Curr. Probl. Cancer 2018, 42, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Santesso, N.; Mustafa, R.A.; Wiercioch, W.; Kehar, R.; Gandhi, S.; Chen, Y.; Cheung, A.; Hopkins, J.; Khatib, R.; Ma, B.; et al. Systematic reviews and meta-analyses of benefits and harms of cryotherapy, LEEP, and cold knife conization to treat cervical intraepithelial neoplasia. Int. J. Gynecol. Obstet. 2015, 132, 266–271. [Google Scholar] [CrossRef]
- Gajjar, K.; Martin-Hirsch, P.P.L.; Bryant, A.; Owens, G. Pain relief for women with cervical intraepithelial neoplasia undergoing colposcopy treatment. Cochrane Database Syst. Rev. 2016, 7, CD006120. [Google Scholar] [CrossRef]
- Cancer Care Ontario Clinical Guidance: Recommended Best Practices for Delivery of Colposcopy Services in Ontario. 2016. Available online: https://www.publications.gov.on.ca/clinical-guidance-recommended-best-practices-for-delivery-of-colposcopy-services-in-ontario (accessed on 4 November 2022).
- NHS Cervical Screening Programme: Detailed Information. 2016. Available online: www.gov.uk/topic/population-screening-programmes (accessed on 4 November 2022).
- Costa-Fagbemi, M.; Yakubu, M.; Meggetto, O.; Moffatt, J.; Walker, M.J.; Koné, A.P.; Murphy, K.J.; Kupets, R. Risk of Cervical Dysplasia after Colposcopy Care and Risk-Informed Return to Population-Based Screening: A Systematic Review. J. Obstet. Gynaecol. Can. 2020, 42, 607–624. [Google Scholar] [CrossRef]
- Clarke, M.A.; Unger, E.R.; Zuna, R.; Nelson, E.; Darragh, T.M.; Cremer, M.; Stockdale, C.K.; Einstein, M.H.; Wentzensen, N. A Systematic Review of Tests for Postcolposcopy and Posttreatment Surveillance. J. Low. Genit. Tract Dis. 2020, 24, 148–156. [Google Scholar] [CrossRef]
- Yang, J.; Yeasman, F.; Kliewer, G.; Nation, J.; Dickinson, J.; Yang, H.; Kopciuk, K. Evidence to Support Change of Clinical Pathway Following Colposcopy Treatment for Cervical Intraepithelial Neoplasia in Canada. J. Obstet. Gynaecol. Can. 2022, 44, 650–657.e1. [Google Scholar] [CrossRef]
- Bjørnerem, M.S.; Sørbye, S.W.; Skjeldestad, F.E. Recurrent disease after treatment for cervical intraepithelial neoplasia—The importance of a flawless definition of residual disease and length of follow-up. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 248, 44–49. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Tao, X.; Guo, L.; Zhang, H.; Sui, L. Risk Factor Analysis of Persistent High-Grade Squamous Intraepithelial Lesion after Loop Electrosurgical Excision Procedure Conization. J. Low. Genit. Tract Dis. 2019, 23, 24–27. [Google Scholar] [CrossRef]
- Leng, F.; Jiang, L.; Nong, L.; Ren, X.; Xie, T.; Dong, Y.; Tao, X. Value of top-hat procedure in management of squamous intraepithelial lesion. J. Obstet. Gynaecol. Res. 2018, 45, 182–188. [Google Scholar] [CrossRef]
- Katki, H.A.; Schiffman, M.; Castle, P.E.; Fetterman, B.; Poitras, N.E.; Lorey, T.; Cheung, L.C.; Raine-Bennett, T.; Gage, J.C.; Kinney, W.K. Five-Year Risk of Recurrence after Treatment of CIN 2, CIN 3, or AIS: Performance of HPV and Pap Cotesting in Posttreatment Management. J. Low. Genit. Tract. Dis. 2013, 17, 78–84. [Google Scholar] [CrossRef]
- Strander, B.; Andersson-Ellström, A.; Milsom, I.; Sparén, P. Long term risk of invasive cancer after treatment for cervical intraepithelial neoplasia grade 3: Population based cohort study. BMJ 2007, 335, 1077. [Google Scholar] [CrossRef]
- Melnikow, J.; McGahan, C.; Sawaya, G.F.; Ehlen, T.; Coldman, A. Cervical Intraepithelial Neoplasia Outcomes after Treatment: Long-Term Follow-up from the British Columbia Cohort Study. Gynecol. Oncol. 2009, 101, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Kalliala, I.; Anttila, A.; Pukkala, E.; Nieminen, P. Risk of cervical and other cancers after treatment of cervical intraepithelial neoplasia: Retrospective cohort study. BMJ 2005, 331, 1183–1185. [Google Scholar] [CrossRef] [PubMed]
- Ullal, A.; Roberts, M.; Bulmer, J.N.; Mathers, M.E.; Wadehra, V. The role of cervical cytology and colposcopy in detecting cervical glandular neoplasia. Cytopathology 2009, 20, 359–366. [Google Scholar] [CrossRef] [PubMed]
- McCluggage, W.G. Endocervical glandular lesions: Controversial aspects and ancillary techniques. J. Clin. Pathol. 2003, 56, 164–173. [Google Scholar] [CrossRef]
- Teshima, S.; Shimosato, Y.; Kishi, K.; Kasamatsu, T.; Ohmi, K.; Uei, Y. Early Stage Adenocarcinoma of the Uterine Cervix Histopathologic Analysis with Consideration of Histogenesis. Cancer 1985, 56, 167–172. [Google Scholar] [CrossRef]
- Willows, K.; Bentley, J.R. Challenges in Detection and Management of Pre-invasive Glandular Lesions of the Cervix. Indian J. Gynecol. Oncol. 2019, 17, 100. [Google Scholar] [CrossRef]
- Geier, C.S.; Wilson, M.; Creasman, W. Clinical evaluation of atypical glandular cells of undetermined significance. Am. J. Obstet. Gynecol. 2001, 184, 64–69. [Google Scholar] [CrossRef]
- Sharpless, K.E.; Schnatz, P.F.; Mandavilli, S.; Greene, J.F.; Sorosky, J.I. Dysplasia Associated with Atypical Glandular Cells on Cervical Cytology. Obstet. Gynecol. 2005, 105, 494–500. [Google Scholar] [CrossRef]
- Castle, P.E.; Fetterman, B.; Poitras, N.; Lorey, T.; Shaber, R.; Kinney, W. Relationship of Atypical Glandular Cell Cytology, Age, and Human Papillomavirus Detection to Cervical and Endometrial Cancer Risks. Obstet. Gynecol. 2010, 115, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.; Li, Z.; Ocque, R.; Patadji, S.; Zhao, C. Clinical significance of atypical glandular cells in Pap tests: An analysis of more than 3000 cases at a large academic women’s center. Cancer Cytopathol. 2016, 124, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-K.; Lee, Y.K.; Hong, S.R.; Lim, K.T. Clinicopathological significance of atypical glandular cells on cervicovaginal Pap smears. Diagn. Cytopathol. 2017, 45, 867–872. [Google Scholar] [CrossRef]
- Daniel, A.; Barreth, D.; Schepansky, A.; Johnson, G.; Capstick, V.; Faught, W. Histologic and clinical significance of atypical glandular cells on pap smears. Int. J. Gynecol. Obstet. 2005, 91, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Nayar, R.; Wilbur, D.C. The Pap test and Bethesda Cancer. Cytopathol 2015, 123, 271–281. [Google Scholar] [CrossRef]
- Schnatz, P.F.; Guile, M.; O’sullivan, D.M.; Sorosky, J.I. Clinical Significance of Atypical Glandular Cells on Cervical Cytology. Obstet. Gynecol. 2006, 107, 701–708. [Google Scholar] [CrossRef]
- Munro, A.; Leung, Y.; Spilsbury, K.; Stewart, C.; Semmens, J.; Codde, J.; Williams, V.; O’Leary, P.; Steel, N.; Cohen, P. Comparison of cold knife cone biopsy and loop electrosurgical excision procedure in the management of cervical adenocarcinoma in situ: What is the gold standard? Gynecol. Oncol. 2015, 137, 258–263. [Google Scholar] [CrossRef]
- Latif, N.A.; Neubauer, N.L.; Helenowski, I.B.; Lurain, J.R. Management of Adenocarcinoma In Situ of the Uterine Cervix: A Comparison of Loop Electrosurgical Excision Procedure and Cold Knife Conization. J. Low. Genit. Tract Dis. 2015, 19, 97–102. [Google Scholar] [CrossRef]
- Ciavattini, A.; Giannella, L.; Carpini, G.D.; Tsiroglou, D.; Sopracordevole, F.; Chiossi, G.; Di Giuseppe, J. Adenocarcinoma in situ of the uterine cervix: Clinical practice guidelines from the Italian society of colposcopy and cervical pathology (SICPCV). Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 240, 273–277. [Google Scholar] [CrossRef]
- Salani, R.; Puri, I.; Bristow, R.E. Adenocarcinoma in situ of the uterine cervix: A metaanalysis of 1278 patients evaluating the predictive value of conization margin status. Am. J. Obstet. Gynecol. 2009, 200, 182.e1–182.e5. [Google Scholar] [CrossRef]
- Costa, S.; Negri, G.; Sideri, M.; Santini, D.; Martinelli, G.; Venturoli, S.; Pelusi, C.; Syrjanen, S.; Syrjanen, K.; Pelusi, G. Human papillomavirus (HPV) test and PAP smear as predictors of outcome in conservatively treated adenocarcinoma in situ (AIS) of the uterine cervix. Gynecol. Oncol. 2007, 106, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Katki, H.A.; Gage, J.C.; Schiffman, M.; Castle, P.E.; Fetterman, B.; Poitras, N.E.; Lorey, T.; Cheung, L.; Raine-Bennett, T.; Kinney, W.K. Follow-up Testing after Colposcopy. J. Low. Genit. Tract Dis. 2013, 17, S69–S77. [Google Scholar] [CrossRef] [PubMed]
- Guido, R.; Schiffman, M.; Solomon, D.; Burke, L. Postcolposcopy management strategies for women referred with low-grade squamous intraepithelial lesions or human papillomavirus DNA-positive atypical squamous cells of undetermined significance: A two-year prospective study. Am. J. Obstet. Gynecol. 2003, 188, 1401–1405. [Google Scholar] [CrossRef] [PubMed]
- Cheung, L.C.; Egemen, D.; Chen, X.; Katki, H.A.; Demarco, M.; Wiser, A.L.; Perkins, R.B.; Guido, R.S.; Wentzensen, N.; Schiffman, M. 2019 ASCCP Risk-Based Management Consensus Guidelines. J. Low. Genit. Tract Dis. 2020, 24, 90–101. [Google Scholar] [CrossRef]
- Moore, R.A.; Ogilvie, G.; Fornika, D.; Moravan, V.; Brisson, M.; Amirabbasi-Beik, M.; Kollar, A.; Burgess, T.; Hsu, R.; Towers, L.; et al. Prevalence and type distribution of human papillomavirus in 5000 British Columbia women—Implications for vaccination. Cancer Causes Control 2009, 20, 1387–1396. [Google Scholar] [CrossRef]
- Moscicki, A.-B.; Shiboski, S.; Hills, N.K.; Powell, K.J.; Jay, N.; Hanson, E.N.; Miller, S.; Canjura-Clayton, L.K.; Farhat, S.; Broering, J.M.; et al. Regression of low-grade squamous intra-epithelial lesions in young women. Lancet 2004, 364, 1678–1683. [Google Scholar] [CrossRef]
- Who Should Be Screened for Cervical Cancer? Canadian Task Force on Preventative Health Care. Available online: https://canadiantaskforce.ca/tools-resources/cervical-cancer-2/clinician-algorithm/ (accessed on 4 November 2022).
- Fader, A.N.; Alward, E.K.; Niederhauser, A.; Chirico, C.; Lesnock, J.L.; Zwiesler, D.J.; Guido, R.S.; Lofgren, D.J.; Gold, M.A.; Moore, K.N. Cervical dysplasia in pregnancy: A multi-institutional evaluation. Am. J. Obstet. Gynecol. 2010, 203, 113.e1–113.e6. [Google Scholar] [CrossRef]
- Ciavattini, A.; Serri, M.; Di Giuseppe, J.; Liverani, C.A.; Fallani, M.G.; Tsiroglou, D.; Papiccio, M.; Carpini, G.D.; Pieralli, A.; Clemente, N.; et al. Reliability of colposcopy during pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 229, 76–81. [Google Scholar] [CrossRef]
- Fleury, A.C.; Birsner, M.L.; Fader, A.N. Management of the abnormal Papanicolaou smear and colposcopy in pregnancy: An evidenced-based review. Minerva Ginecol. 2012, 64, 137–148. [Google Scholar]
- Kärrberg, C.; Brännström, M.; Strander, B.; Ladfors, L.; Rådberg, T. Colposcopically directed cervical biopsy during pregnancy; minor surgical and obstetrical complications and high rates of persistence and regression. Acta Obstet. et Gynecol. Scand. 2013, 92, 692–699. [Google Scholar] [CrossRef]
- Mailath-Pokorny, M.; Schwameis, R.; Grimm, C.; Reinthaller, A.; Polterauer, S. Natural history of cervical intraepithelial neoplasia in pregnancy: Postpartum histo-pathologic outcome and review of the literature. BMC Pregnancy Childbirth 2016, 16, 74. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.; Joura, E.; Kohlberger, P. Natural History of Squamous Intraepithelial Lesions in Pregnancy and Mode of Delivery. Anticancer Res. 2018, 38, 2439–2442. [Google Scholar] [CrossRef] [PubMed]
- Slama, J.; Freitag, P.; Dundr, P.; Duskova, J.; Fischerova, D.; Zikan, M.; Pinkavova, I.; Cibula, D. Outcomes of pregnant patients with Pap smears classified as atypical glandular cells. Cytopathology 2011, 23, 383–388. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Guo, R.X.; Li, B.J.; Wu, Y.; Bai, J.; Li, L.X.; Wang, C.F. Analysis of clinical features of cervical precancerous lesions in postmenopausal women. Zhonghua Fu Chan Ke Za Zhi 2021, 56, 114–120. [Google Scholar] [PubMed]
- Richards, A.; Dalrymple, C. Abnormal cervicovaginal cytology, unsatisfactory colposcopy and the use of vaginal estrogen cream: An observational study of clinical outcomes for women in low estrogen states. J. Obstet. Gynaecol. Res. 2015, 41, 440–444. [Google Scholar] [CrossRef]
- Bruno, M.T.; Coco, A.; Di Pasqua, S.; Bonanno, G. Management of ASC-US/HPV positive post-menopausal woman. Virol. J. 2019, 16, 39. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.W.; You, Z.X.; Qian, X.Y.; Heng, D.; Tang, M.Y. Discussion on the diagnosis and treatment of high-grade squamous intraepithelial lesions in post-menopausal women. Zhonghua Fu Chan Ke Za Zhi 2019, 54, 393–398. [Google Scholar] [PubMed]
- Kiuchi, K.; Hasegawa, K.; Motegi, E.; Kosaka, N.; Udagawa, Y.; Fukasawa, I. Complications of laser conization versus loop electrosurgical excision procedure in pre- and postmenopausal patients. Eur. J. Gynaecol. Oncol. 2016, 37, 803–808. [Google Scholar]
- Lin, J.; Meng, Y.; Chen, Y.; Li, Z.; Xu, Y.; Wu, D. A new approach to prevent cervical stenosis in postmenopausal women after loop electrosurgical excision procedure: A randomized controlled trial. Sci. Rep. 2020, 10, 8512. [Google Scholar] [CrossRef] [PubMed]
- White, M.C.; Shoemaker, M.L.; Benard, V.B. Cervical Cancer Screening and Incidence by Age: Unmet Needs Near and after the Stopping Age for Screening. Am. J. Prev. Med. 2017, 53, 392–395. [Google Scholar] [CrossRef]
- Stillman, M.D.; Bertocci, G.; Smalley, C.; Williams, S.; Frost, K.L. Healthcare utilization and associated barriers experienced by wheelchair users: A pilot study. Disabil. Health J. 2017, 10, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Duff, P.; Ogilvie, G.; Shoveller, J.; Amram, O.; Chettiar, J.; Nguyen, P.; Dobrer, S.; Montaner, J.; Shannon, K. Barriers to Cervical Screening among Sex Workers in Vancouver. Am. J. Public Health 2016, 106, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Wee, C.C.; McCarthy, E.P.; Davis, R.B.; Phillips, R.S. Screening for Cervical and Breast Cancer: Is Obesity an Unrecognized Barrier to Preventive Care? Ann. Intern. Med. 2000, 132, 697–704. [Google Scholar] [CrossRef]
- Mitchell, R.S.; Padwal, R.S.; Chuck, A.W.; Klarenbach, S.W. Cancer Screening among the Overweight and Obese in Canada. Am. J. Prev. Med. 2008, 35, 127–132. [Google Scholar] [CrossRef]
- Aldrich, T.; Hackley, B. The Impact of Obesity on Gynecologic Cancer Screening: An Integrative Literature Review. J. Midwifery Women’s Health 2010, 55, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, A.E.; Moysich, K.B.; Ferrando, C.A.; Starbuck, K.D. Clinical needs for transgender men in the gynecologic oncology setting. Gynecol. Oncol. 2020, 159, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Weyers, S.; Garland, S.; Cruickshank, M.; Kyrgiou, M.; Arbyn, M. Cervical cancer prevention in transgender men: A review. BJOG Int. J. Obstet. Gynaecol. 2020, 128, 822–826. [Google Scholar] [CrossRef]
- Sallans, R.K. Six Tips for Giving Good Health Care to Anyone with a Cervix. AMA J. Ethics 2020, 22, E168–E175. [Google Scholar] [CrossRef]
- Curmi, C.; Peters, K.; Salamonson, Y. Barriers to cervical cancer screening experienced by lesbian women: A qualitative study. J. Clin. Nurs. 2015, 25, 3643–3651. [Google Scholar] [CrossRef]
- Ferdous, M.; Lee, S.; Goopy, S.; Yang, H.; Rumana, N.; Abedin, T.; Turin, T.C. Barriers to cervical cancer screening faced by immigrant women in Canada: A systematic scoping review. BMC Womens Health 2018, 18, 165. [Google Scholar] [CrossRef]
- Hislop, T.G.; Clarke, H.F.; Deschamps, M.; Joseph, R.; Band, P.R.; Smith, J.; Le, N.; Atleo, R. Cervical cytology screening. How can we improve rates among First Nations women in urban British Columbia? Can. Fam. Physician 1996, 42, 1701–1708. [Google Scholar] [PubMed]
- O’Brien, B.A.; Mill, J.; Wilson, T. Cervical Screening in Canadian First Nation Cree Women. J. Transcult. Nurs. 2008, 20, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Tratt, E.; Sarmiento, I.; Gamelin, R.; Nayoumealuk, J.; Andersson, N.; Brassard, P. Fuzzy cognitive mapping with Inuit women: What needs to change to improve cervical cancer screening in Nunavik, northern Quebec? BMC Health Serv. Res. 2020, 20, 529. [Google Scholar] [CrossRef] [PubMed]
- Racey, C.S.; Gesink, D.C. Barriers and Facilitators to Cervical Cancer Screening among Women in Rural Ontario, Canada: The Role of Self-Collected HPV Testing. J. Rural. Health 2015, 32, 136–145. [Google Scholar] [CrossRef]
- Martens, P.J.; Chochinov, H.M.; Prior, H.J.; Fransoo, R.; Burland, E. Are cervical cancer screening rates different for women with schizophrenia? A Manitoba population-based study. Schizophr. Res. 2009, 113, 101–106. [Google Scholar] [CrossRef]
| HPV-Related Abbreviations | |
|---|---|
| HPV | Human papillomavirus |
| HR-HPV | High-risk HPV as identified on HPV genotyping |
| HPV 16/18 | HPV 16 and/or 18 |
| Positive HPV test | HPV test showing high-risk HPV types on genotyping |
| HSIL+ | HSIL or cervical cancer |
| VaIN | Vaginal intraepithelial neoplasia |
| 2014 Bethesda System for Reporting Cervical Cytology [8] | |
| Normal | Negative for intraepithelial lesion and malignancy |
| LSIL | Low-grade squamous intraepithelial lesion |
| ASCUS | Abnormal squamous cells of undetermined significance |
| ASC-H | Abnormal squamous cells cannot rule out high-grade dysplasia |
| HSIL | High-grade squamous intraepithelial lesion |
| AIS | Adenocarcinoma in situ |
| AGC | Abnormal glandular cells |
| AGC-NOS | Abnormal glandular cells, not otherwise specified |
| AGC-N | Abnormal glandular cells, favoring neoplasia |
| Cervical Intraepithelial Neoplasia Naming System for Cervical Pathology [9] | |
| CIN 1 | Cervical intraepithelial neoplasia 1 |
| CIN 2 | Cervical intraepithelial neoplasia 2 |
| CIN 3 | Cervical intraepithelial neoplasia 3 |
| Colposcopy Terminology | |
| CKC | Cold knife conization |
| ECC | Endocervical curettage |
| LEEP | Loop electrosurgical excisional procedure |
| LLETZ | Large loop excision of the transformation zone |
| HPV | ||||
|---|---|---|---|---|
| Cytology | Pos HR-HPV (Any) | Pos HPV 16 | Pos HPV 18 | Pos HPV Other |
| Normal | 3.4% [10] | 5.3% [10] | 3% [5] | 2% |
| ASCUS | 4.4% [11] | 9% [10]–12.9% [14] | 5% [14] | 2.7% [14]–4.4% [11] |
| LSIL | 4.3% [11] | 11% [10] | 3% [5] | 4.3% [5,11] |
| ASC-H | 26% [5,11] | 28% [5,10] | 15% [10] | 26% [5,11] |
| HSIL | 49% [5,11] | 60% [5,10] | 30% [5,10] | 49% [5,11] |
| General Assessment: | Squamocolumnar Junction Visibility: Completely Visible, Partially Visible, Not Visible Transformation Zone Types 1,2,3 (Figure 1) |
|---|---|
| Normal Findings: | Original squamous epithelium: mature or atrophic columnar epithelium, ectopy, metaplastic squamous epithelium, nabothian cysts, crypt (gland) openings, deciduosis in pregnancy |
| Grade 1/Minor Findings: | Thin aceto-white epithelium; irregular, geographic border Fine mosaic, fine punctation |
| Grade 2/Major Findings | Dense aceto-white epithelium, rapid appearance of acetowhitening, cuffed crypt (gland) openings, coarse mosaic, coarse punctuation, sharp border, inner border sign, ridge sign |
| Findings Suspicious for Invasion | Atypical vessels, fragile vessels, irregular surface, exophytic lesion, necrosis, ulceration (necrosis), tumor/gross neoplasm |
| The transformation zone must be fully visible (type 1); |
| The lesion should not extend to the endocervix or vagina; |
| The lesion should not occupy more than 75% of the ectocervix; |
| The transformation zone can be covered by the largest ablative probe; |
| There is no cytological/histological disparity; |
| The person has not had previous treatment; |
| There is no suspicion of cancer or glandular lesion. |
| Atypical Endocervical cells, not otherwise specified (-NOS) Atypical Endometrial cells, not otherwise specified (-NOS) Atypical Glandular cells, not otherwise specified (-NOS) |
| Atypical Endocervical cells, favoring neoplastic (-N) Atypical Glandular cells, favoring neoplastic (-N) |
| Endocervical adenocarcinoma in situ (AIS) |
| Adenocarcinoma—endocervical Adenocarcinoma—endometrial Adenocarcinoma—extrauterine Adenocarcinoma—not otherwise specified (NOS) |
| HPV Status at Referral | Referral Cytology | Pre-Colposcopy 3-Year Risk of HSIL+, Percent | Colposcopy Findings | Post-Colposcopy (Normal or LSIL) 3-Year Risk of HSIL+, Percent |
|---|---|---|---|---|
| HPV-positive | HSIL+ | 45.4% (43.6, 47.3) | Normal or LSIL | 9.3 (0.27, 18.3) |
| HPV-positive | ASC-H | 23.9 (22.4, 25.4) | Normal or LSIL | 6.5 (2.2, 10.8) |
| HPV-positive | AGC | 26.0 (23.3, 28.9) | Normal or LSIL | 8.0 (1.5, 14.5) |
| HPV-positive | LSIL | 4.6 (4.3, 5.0) | Normal or LSIL | 1.8 (1.1, 2.6) |
| HPV-positive | ASCUS | 5.2 (4.9, 5.4) | Normal or LSIL | 2.2 (1.6, 2.8) |
| HPV-positive | NILM | 4.5 (4.1, 4.9) | Normal or LSIL | 2.1 (1.2, 3.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willows, K.; Selk, A.; Auclair, M.-H.; Jim, B.; Jumah, N.; Nation, J.; Proctor, L.; Iazzi, M.; Bentley, J. 2023 Canadian Colposcopy Guideline: A Risk-Based Approach to Management and Surveillance of Cervical Dysplasia. Curr. Oncol. 2023, 30, 5738-5768. https://doi.org/10.3390/curroncol30060431
Willows K, Selk A, Auclair M-H, Jim B, Jumah N, Nation J, Proctor L, Iazzi M, Bentley J. 2023 Canadian Colposcopy Guideline: A Risk-Based Approach to Management and Surveillance of Cervical Dysplasia. Current Oncology. 2023; 30(6):5738-5768. https://doi.org/10.3390/curroncol30060431
Chicago/Turabian StyleWillows, Karla, Amanda Selk, Marie-Hélène Auclair, Brent Jim, Naana Jumah, Jill Nation, Lily Proctor, Melissa Iazzi, and James Bentley. 2023. "2023 Canadian Colposcopy Guideline: A Risk-Based Approach to Management and Surveillance of Cervical Dysplasia" Current Oncology 30, no. 6: 5738-5768. https://doi.org/10.3390/curroncol30060431
APA StyleWillows, K., Selk, A., Auclair, M.-H., Jim, B., Jumah, N., Nation, J., Proctor, L., Iazzi, M., & Bentley, J. (2023). 2023 Canadian Colposcopy Guideline: A Risk-Based Approach to Management and Surveillance of Cervical Dysplasia. Current Oncology, 30(6), 5738-5768. https://doi.org/10.3390/curroncol30060431





