Adverse Effects of Immune-Checkpoint Inhibitors: A Comprehensive Imaging-Oriented Review
Abstract
1. Introduction
| ICIs | Mechanism of Action | Indication |
|---|---|---|
| Ipilimumab [13] | Anti-CTLA-4 antibody |
|
| Tremelimumab [14] | Human IgG2 monoclonal antibody that blocks CTLA-4 |
|
| Pembrolizumab [15] | Anti-PD-1 antibody |
|
| Nivolumab [13] | Anti-PD-1 antibody |
|
| Atezolizumab [7] | Anti-PD-L1 antibody |
|
| Avelumab [16] | Anti-PD-L1 antibody |
|
| Durvalumab [4] | Anti-PD-L1 antibody |
|
| Relatlimab [17] | Anti-LAG3 antibody |
|
| Organs | Symptoms |
|---|---|
| Systemic Adverse Effects |
|
| Skin [19,20] |
|
| Intestinal [21,22] |
|
| Hepatic [23] |
|
| Pulmonary [24] |
|
| Endocrine system [25] |
|
| Cardiac [26] |
|
| Renal [27] |
|
| Rheumatologic [18] |
|
| Nervous system [28] |
|
| Ocular [18] |
|
| Hematologic [18] |
|
2. Gastrointestinal
2.1. Incidence
2.2. Sign and Symptoms
2.3. Diagnosis and Imaging
2.4. Differential Diagnosis
2.5. Management
3. Liver
3.1. Incidence
3.2. Sign and Symptoms
3.3. Diagnosis and Imaging [16,41,42,43,44]
3.4. Differential Diagnosis
3.5. Management
4. Pancreas
4.1. Incidence
4.2. Sign and Symptoms
4.3. Diagnosis and Imaging
| Imaging Modality | ICIPI Imaging Findings (IEP > NP) |
|---|---|
| US | Limited role (hindered by patient’s habitus and bowel gas)
|
| CT | IEP
|
| MRI | IEP With edematous pancreatic regions:
with necrotic areas involving pancreatic gland and/or peripancreatic spaces:
|
| PET-CT (18F-FDG) |
|
4.4. Differential Diagnosis
4.5. Management
5. Lung
5.1. Incidence
5.2. Sign and Symptoms
5.3. Diagnosis and Imaging and Differential Diagnosis
5.4. Management
6. Endocrine System
7. Thyroid
7.1. Incidence
7.2. Sign and Symptoms
7.3. Diagnosis and Imaging
7.4. Differential Diagnosis
7.5. Management
8. Pituitary Gland
8.1. Incidence
8.2. Sign and Symptoms
8.3. Diagnosis and Imaging
8.4. Differential Diagnosis
8.5. Management
9. Adrenal Gland
9.1. Incidence
9.2. Sign and Symptoms
9.3. Diagnosis and Imaging
9.4. Differential Diagnosis
9.5. Management
10. Endocrine Pancreas
10.1. Incidence
10.2. Sign and Symptoms
10.3. Diagnosis and Imaging
10.4. Differential Diagnosis
10.5. Management
11. Renal Toxicity
11.1. Incidence
11.2. Sign and Symptoms
11.3. Diagnosis and Imaging
11.4. Differential Diagnosis
11.5. Management
12. Cardiac Toxicity
12.1. Incidence
12.2. Sign and Symptoms
12.3. Diagnosis and Imaging
12.4. Differential Diagnosis
12.5. Management
13. Neurological Toxicity
13.1. Incidence
13.2. Sign and Symptoms
13.3. Diagnosis and Imaging
13.4. Differential Diagnosis
13.5. Management
14. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Quattrocchi, C.C.; Giona, A.; Di Martino, A.; Gaudino, F.; Mallio, C.A.; Errante, Y.; Occhicone, F.; Vitali, M.A.; Zobel, B.B.; Denaro, V. Lumbar subcutaneous edema and degenerative spinal disease in patients with low back pain: A retrospective MRI study. Musculoskelet. Surg. 2015, 99, 159–163. [Google Scholar] [CrossRef]
- Greco, F.; Mallio, C.A. Relationship between visceral adipose tissue and genetic mutations (VHL and KDM5C) in clear cell renal cell carcinoma. Radiol. Med. 2021, 126, 645–651. [Google Scholar] [CrossRef]
- Mallio, C.A.; Napolitano, A.; Castiello, G.; Giordano, F.M.; D’Alessio, P.; Iozzino, M.; Sun, Y.; Angeletti, S.; Russano, M.; Santini, D.; et al. Deep Learning Algorithm Trained with COVID-19 Pneumonia Also Identifies Immune Checkpoint Inhibitor Therapy-Related Pneumonitis. Cancers 2021, 13, 652. [Google Scholar] [CrossRef]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Freites-Martinez, A.; Santana, N.; Arias-Santiago, S.; Viera, A. Using the Common Terminology Criteria for Adverse Events (CTCAE-Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermosifiliogr. 2021, 112, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Dromain, C.; Beigelman, C.; Pozzessere, C.; Duran, R.; Digklia, A. Imaging of tumour response to immunotherapy. Eur. Radiol. Exp. 2020, 4, 2. [Google Scholar] [CrossRef]
- Wang, P.-F.; Chen, Y.; Song, S.-Y.; Wang, T.-J.; Ji, W.-J.; Li, S.-W.; Liu, N.; Yan, C.-X. Immune-Related Adverse Events Associated with Anti-PD-1/PD-L1 Treatment for Malignancies: A Meta-Analysis. Front. Pharmacol. 2017, 8, 730. [Google Scholar] [CrossRef] [PubMed]
- Tirumani, S.H.; Ramaiya, N.H.; Keraliya, A.; Bailey, N.D.; Ott, P.A.; Hodi, F.S.; Nishino, M. Radiographic Profiling of Immune-Related Adverse Events in Advanced Melanoma Patients Treated with Ipilimumab. Cancer Immunol. Res. 2015, 3, 1185–1192. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Zang, X.-Y.; Wang, J.-C.; Huang, S.-S.; Xu, J.; Zhang, P. Diagnosis and Management of Immune Related Adverse Events (irAEs) in Cancer Immunotherapy. Biomed. Pharmacother. 2019, 120, 109437. [Google Scholar] [CrossRef]
- Marthey, L.; Mateus, C.; Mussini, C.; Nachury, M.; Nancey, S.; Grange, F.; Zallot, C.; Peyrin-Biroulet, L.; Rahier, J.F.; Bourdier de Beauregard, M.; et al. Cancer Immunotherapy with Anti-CTLA-4 Monoclonal Antibodies Induces an Inflammatory Bowel Disease. J. Crohns Colitis 2016, 10, 395–401. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, Y.-A.; Sim, C.K.; Heo, S.-H.; Song, I.H.; Park, H.S.; Park, S.Y.; Bang, W.S.; Park, I.A.; Lee, M.; et al. Expansion of tumor-infiltrating lymphocytes and their potential for application as adoptive cell transfer therapy in human breast cancer. Oncotarget 2017, 8, 113345–113359. [Google Scholar] [CrossRef]
- Kraehenbuehl, L.; Weng, C.-H.; Eghbali, S.; Wolchok, J.D.; Merghoub, T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat. Rev. Clin. Oncol. 2022, 19, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef]
- Keam, S.J. Tremelimumab: First Approval. Drugs 2023, 83, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Kwok, G.; Yau, T.C.C.; Chiu, J.W.; Tse, E.; Kwong, Y.-L. Pembrolizumab (Keytruda). Hum. Vaccines Immunother. 2016, 12, 2777–2789. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Sun, J.-M.; Lee, S.-H.; Ahn, J.S.; Park, K.; Ahn, M.-J. Late-Onset Cholecystitis with Cholangitis after Avelumab Treatment in Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, e34–e36. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Palmieri, D.J.; Carlino, M.S. Immune Checkpoint Inhibitor Toxicity. Curr. Oncol. Rep. 2018, 20, 72. [Google Scholar] [CrossRef]
- Sibaud, V. Dermatologic Reactions to Immune Checkpoint Inhibitors: Skin Toxicities and Immunotherapy. Am. J. Clin. Dermatol. 2018, 19, 345–361. [Google Scholar] [CrossRef]
- Nikolaou, V.; Tsimpidakis, A.; Stratigos, A. Cutaneous Adverse Reactions of Immunotherapy in Patients with Advanced Melanoma. Cancers 2023, 15, 2084. [Google Scholar] [CrossRef]
- Wang, D.Y.; Ye, F.; Zhao, S.; Johnson, D.B. Incidence of immune checkpoint inhibitor-related colitis in solid tumor patients: A systematic review and meta-analysis. Oncoimmunology 2017, 6, e1344805. [Google Scholar] [CrossRef]
- Cappello, G.; Molea, F.; Campanella, D.; Galioto, F.; Russo, F.; Regge, D. Gastrointestinal adverse events of immunotherapy. BJR Open 2021, 3, 20210027. [Google Scholar] [CrossRef]
- Zen, Y.; Chen, Y.-Y.; Jeng, Y.-M.; Tsai, H.-W.; Yeh, M.M. Immune-related adverse reactions in the hepatobiliary system: Second-generation check-point inhibitors highlight diverse histological changes. Histopathology 2020, 76, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, J.; Wang, X.; Woo, K.M.; Iyriboz, T.; Halpenny, D.; Cunningham, J.; Chaft, J.E.; Segal, N.H.; Callahan, M.K.; Lesokhin, A.M.; et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J. Clin. Oncol. 2017, 35, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.J. Endocrinopathies Associated with Immune Checkpoint Inhibitors. Acta Med. Port. 2022, 35, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; Yousaf, N.; Battisti, N.M.L.; Moslehi, J.; Larkin, J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018, 19, e447–e458. [Google Scholar] [CrossRef]
- Hu, R.; Chen, M.; Xu, Y.; Wang, M.; Zheng, K.; Li, X. Renal immune-related adverse events of immune checkpoint inhibitor. Asia Pac. J. Clin. Oncol. 2020, 16, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Cuzzubbo, S.; Javeri, F.; Tissier, M.; Roumi, A.; Barlog, C.; Doridam, J.; Lebbe, C.; Belin, C.; Ursu, R.; Carpentier, A.F. Neurological adverse events associated with immune checkpoint inhibitors: Review of the literature. Eur. J. Cancer 2017, 73, 1–8. [Google Scholar] [CrossRef]
- Gupta, A.; De Felice, K.M.; Loftus, E.V.; Khanna, S. Systematic review: Colitis associated with anti-CTLA-4 therapy. Aliment. Pharmacol. Ther. 2015, 42, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Boike, J.; Dejulio, T. Severe Esophagitis and Gastritis from Nivolumab Therapy. ACG Case Rep. J. 2017, 4, e57. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Richards, C.J.; Boyle, K.; Faust, G. Severe inflammatory ileitis resulting in ileal perforation in association with combination immune checkpoint blockade for metastatic malignant melanoma. BMJ Case Rep. 2018, 2018, bcr-2018–224913. [Google Scholar] [CrossRef] [PubMed]
- Geukes Foppen, M.H.; Rozeman, E.A.; van Wilpe, S.; Postma, C.; Snaebjornsson, P.; van Thienen, J.V.; van Leerdam, M.E.; van den Heuvel, M.; Blank, C.U.; van Dieren, J.; et al. Immune checkpoint inhibition-related colitis: Symptoms, endoscopic features, histology and response to management. ESMO Open 2018, 3, e000278. [Google Scholar] [CrossRef] [PubMed]
- Rajha, E.; Chaftari, P.; Kamal, M.; Maamari, J.; Chaftari, C.; Yeung, S.-C.J. Gastrointestinal adverse events associated with immune checkpoint inhibitor therapy. Gastroenterol. Rep. 2020, 8, 25–30. [Google Scholar] [CrossRef]
- Radulescu, L.; Crisan, D.; Grapa, C.; Radulescu, D. Digestive Toxicities Secondary to Immune Checkpoint Inhibition Therapy-Reports of Rare Events. A Systematic Review. J. Gastrointestin Liver Dis. 2021, 30, 506–516. [Google Scholar] [CrossRef]
- Wang, Y.; Abu-Sbeih, H.; Mao, E.; Ali, N.; Qiao, W.; Trinh, V.A.; Zobniw, C.; Johnson, D.H.; Samdani, R.; Lum, P.; et al. Endoscopic and Histologic Features of Immune Checkpoint Inhibitor-Related Colitis. Inflamm. Bowel Dis. 2018, 24, 1695–1705. [Google Scholar] [CrossRef]
- Grover, S.; Rahma, O.E.; Hashemi, N.; Lim, R.M. Gastrointestinal and Hepatic Toxicities of Checkpoint Inhibitors: Algorithms for Management. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 13–19. [Google Scholar] [CrossRef]
- Barina, A.R.; Bashir, M.R.; Howard, B.A.; Hanks, B.A.; Salama, A.K.; Jaffe, T.A. Isolated recto-sigmoid colitis: A new imaging pattern of ipilimumab-associated colitis. Abdom. Radiol. 2016, 41, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Bellaguarda, E.; Hanauer, S. Checkpoint Inhibitor-Induced Colitis. Am. J. Gastroenterol. 2020, 115, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Peeraphatdit, T.B.; Wang, J.; Odenwald, M.A.; Hu, S.; Hart, J.; Charlton, M.R. Hepatotoxicity From Immune Checkpoint Inhibitors: A Systematic Review and Management Recommendation. Hepatology 2020, 72, 315–329. [Google Scholar] [CrossRef]
- Anderson, M.A.; Kurra, V.; Bradley, W.; Kilcoyne, A.; Mojtahed, A.; Lee, S.I. Abdominal immune-related adverse events: Detection on ultrasonography, CT, MRI and 18F-Fluorodeoxyglucose positron emission tomography. Br. J. Radiol. 2021, 94, 20200663. [Google Scholar] [CrossRef]
- Widmann, G.; Nguyen, V.A.; Plaickner, J.; Jaschke, W. Imaging Features of Toxicities by Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Radiol. Rep. 2016, 5, 59. [Google Scholar] [CrossRef]
- Pourvaziri, A.; Parakh, A.; Biondetti, P.; Sahani, D.; Kambadakone, A. Abdominal CT manifestations of adverse events to immunotherapy: A primer for radiologists. Abdom Radiol. 2020, 45, 2624–2636. [Google Scholar] [CrossRef] [PubMed]
- Mekki, A.; Dercle, L.; Lichtenstein, P.; Marabelle, A.; Michot, J.-M.; Lambotte, O.; Le Pavec, J.; De Martin, E.; Balleyguier, C.; Champiat, S.; et al. Detection of immune-related adverse events by medical imaging in patients treated with anti-programmed cell death 1. Eur. J. Cancer 2018, 96, 91–104. [Google Scholar] [CrossRef]
- Gelsomino, F.; Vitale, G.; Ardizzoni, A. A case of nivolumab-related cholangitis and literature review: How to look for the right tools for a correct diagnosis of this rare immune-related adverse event. Investig. New Drugs 2018, 36, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Vani, V.; Regge, D.; Cappello, G.; Gabelloni, M.; Neri, E. Imaging of Adverse Events Related to Checkpoint Inhibitor Therapy. Diagnostics 2020, 10, 216. [Google Scholar] [CrossRef]
- Porcu, M.; Solinas, C.; Migali, C.; Battaglia, A.; Schena, M.; Mannelli, L.; Addeo, A.; Willard-Gallo, K.; Saba, L. Immune Checkpoint Inhibitor-Induced Pancreatic Injury: Imaging Findings and Literature Review. Target. Oncol. 2020, 15, 25–35. [Google Scholar] [CrossRef]
- Incidence of Pancreatitis with the Use of Immune Checkpoint Inhibitors (ICI) in Advanced Cancers: A Systematic Review and Meta-Analysis–PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/31076344/ (accessed on 10 March 2023).
- Abu-Sbeih, H.; Tang, T.; Lu, Y.; Thirumurthi, S.; Altan, M.; Jazaeri, A.A.; Dadu, R.; Coronel, E.; Wang, Y. Clinical characteristics and outcomes of immune checkpoint inhibitor-induced pancreatic injury. J. Immunother. Cancer 2019, 7, 31. [Google Scholar] [CrossRef]
- Das, S.; Johnson, D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 306. [Google Scholar] [CrossRef] [PubMed]
- Das, J.P.; Postow, M.A.; Friedman, C.F.; Do, R.K.; Halpenny, D.F. Imaging findings of immune checkpoint inhibitor associated pancreatitis. Eur. J. Radiol. 2020, 131, 109250. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shi, Y.; Liu, X.; Zhang, D.; Zhang, H.; Chen, M.; Xu, Y.; Zhao, J.; Zhong, W.; Wang, M. Clinical characteristics and outcomes of immune checkpoint inhibitor-induced diabetes mellitus. Transl. Oncol. 2022, 24, 101473. [Google Scholar] [CrossRef]
- Manikkavasakar, S.; AlObaidy, M.; Busireddy, K.K.; Ramalho, M.; Nilmini, V.; Alagiyawanna, M.; Semelka, R.C. Magnetic resonance imaging of pancreatitis: An update. World J. Gastroenterol. 2014, 20, 14760–14777. [Google Scholar] [CrossRef]
- Kalisz, K.R.; Ramaiya, N.H.; Laukamp, K.R.; Gupta, A. Immune Checkpoint Inhibitor Therapy-related Pneumonitis: Patterns and Management. Radiographics 2019, 39, 1923–1937. [Google Scholar] [CrossRef]
- Russo, L.; Avesani, G.; Gui, B.; Trombadori, C.M.L.; Salutari, V.; Perri, M.T.; Di Paola, V.; Rodolfino, E.; Scambia, G.; Manfredi, R. Immunotherapy-Related Imaging Findings in Patients with Gynecological Malignancies: What Radiologists Need to Know. Korean J. Radiol. 2021, 22, 1310–1322. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.; Voong, K.R.; Shankar, B.; Forde, P.M.; Ettinger, D.S.; Marrone, K.A.; Kelly, R.J.; Hann, C.L.; Levy, B.; Feliciano, J.L.; et al. Pneumonitis in Non-Small Cell Lung Cancer Patients Receiving Immune Checkpoint Immunotherapy: Incidence and Risk Factors. J. Thorac. Oncol. 2018, 13, 1930–1939. [Google Scholar] [CrossRef]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Ramaiya, N.H.; Awad, M.M.; Sholl, L.M.; Maattala, J.A.; Taibi, M.; Hatabu, H.; Ott, P.A.; Armand, P.F.; Hodi, F.S. PD-1 Inhibitor-Related Pneumonitis in Advanced Cancer Patients: Radiographic Patterns and Clinical Course. Clin. Cancer Res. 2016, 22, 6051–6060. [Google Scholar] [CrossRef]
- Kucukarda, A.; Sayın, S.; Gokyer, A.; Aykan, M.B.; Karadurmuş, N.; Cicin, İ. Secondary pneumothorax during immunotherapy in two patients with metastatic solid tumors; a new entity. Immunotherapy 2021, 13, 565–570. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, L.; Zhou, Y.; He, W.; Li, W. Immune Checkpoint Inhibitor-Associated Pneumonitis in Non-Small Cell Lung Cancer: Current Understanding in Characteristics, Diagnosis, and Management. Front. Immunol. 2021, 12, 663986. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Sholl, L.M.; Awad, M.M.; Hatabu, H.; Armand, P.; Hodi, F.S. Sarcoid-Like Granulomatosis of the Lung Related to Immune-Checkpoint Inhibitors: Distinct Clinical and Imaging Features of a Unique Immune-Related Adverse Event. Cancer Immunol. Res. 2018, 6, 630–635. [Google Scholar] [CrossRef]
- Wright, J.J.; Powers, A.C.; Johnson, D.B. Endocrine toxicities of immune checkpoint inhibitors. Nat. Rev. Endocrinol 2021, 17, 389–399. [Google Scholar] [CrossRef]
- Alessandrino, F.; Shah, H.J.; Ramaiya, N.H. Multimodality imaging of endocrine immune related adverse events: A primer for radiologists. Clin. Imaging 2018, 50, 96–103. [Google Scholar] [CrossRef]
- Abdel-Rahman, O.; ElHalawani, H.; Fouad, M. Risk of endocrine complications in cancer patients treated with immune check point inhibitors: A meta-analysis. Future Oncol. 2016, 12, 413–425. [Google Scholar] [CrossRef] [PubMed]
- de Filette, J.; Andreescu, C.E.; Cools, F.; Bravenboer, B.; Velkeniers, B. A Systematic Review and Meta-Analysis of Endocrine-Related Adverse Events Associated with Immune Checkpoint Inhibitors. Horm. Metab. Res. 2019, 51, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Friedman, D.L.; Berry, E.; Decker, I.; Ye, F.; Zhao, S.; Morgans, A.K.; Puzanov, I.; Sosman, J.A.; Lovly, C.M. Survivorship in Immune Therapy: Assessing Chronic Immune Toxicities, Health Outcomes, and Functional Status among Long-term Ipilimumab Survivors at a Single Referral Center. Cancer Immunol. Res. 2015, 3, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Sakakida, T.; Ishikawa, T.; Uchino, J.; Chihara, Y.; Komori, S.; Asai, J.; Narukawa, T.; Arai, A.; Kobayashi, T.; Tsunezuka, H.; et al. Clinical features of immune-related thyroid dysfunction and its association with outcomes in patients with advanced malignancies treated by PD-1 blockade. Oncol. Lett. 2019, 18, 2140–2147. [Google Scholar] [CrossRef]
- Osorio, J.C.; Ni, A.; Chaft, J.E.; Pollina, R.; Kasler, M.K.; Stephens, D.; Rodriguez, C.; Cambridge, L.; Rizvi, H.; Wolchok, J.D.; et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann. Oncol. 2017, 28, 583–589. [Google Scholar] [CrossRef]
- Muir, C.A.; Clifton-Bligh, R.J.; Long, G.V.; Scolyer, R.A.; Lo, S.N.; Carlino, M.S.; Tsang, V.H.M.; Menzies, A.M. Thyroid Immune-related Adverse Events Following Immune Checkpoint Inhibitor Treatment. J. Clin. Endocrinol. Metab. 2021, 106, e3704–e3713. [Google Scholar] [CrossRef]
- Bai, X.; Chen, X.; Wu, X.; Huang, Y.; Zhuang, Y.; Chen, Y.; Feng, C.; Lin, X. Immune checkpoint inhibitor-associated pituitary adverse events: An observational, retrospective, disproportionality study. J. Endocrinol. Investig. 2020, 43, 1473–1483. [Google Scholar] [CrossRef]
- Faje, A. Immunotherapy and hypophysitis: Clinical presentation, treatment, and biologic insights. Pituitary 2016, 19, 82–92. [Google Scholar] [CrossRef]
- Sasaki, K.; Kobayashi, S.; Kudo, M.; Sugimoto, M.; Takahashi, S.; Nakamura, Y.; Kawazoe, A.; Shitara, K.; Kinoshita, T.; Gotohda, N. Hypothyroidism and hypopituitarism as immune-related adverse events due to lenvatinib plus pembrolizumab therapy in the immediate postoperative period after laparoscopic hepatectomy for liver metastases from gastric cancer: A case report. Surg. Case Rep. 2021, 7, 267. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Yang, K.; Ma, Y.; Fu, S.; Tang, X.; Wang, Y.; Zhou, L. Endocrine Adverse Events Caused by Different Types and Different Doses of Immune Checkpoint Inhibitors in the Treatment of Solid Tumors: A Meta-Analysis and Systematic Review. J. Clin. Pharmacol. 2021, 61, 282–297. [Google Scholar] [CrossRef] [PubMed]
- Tsoli, M.; Kaltsas, G.; Angelousi, A.; Alexandraki, K.; Randeva, H.; Kassi, E. Managing Ipilimumab-Induced Hypophysitis: Challenges and Current Therapeutic Strategies. Cancer Manag. Res. 2020, 12, 9551–9561. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Go, M. Nivolumab Induced Adrenal Insufficiency: Rare Side-effect of a New Anti-cancer Therapy-Immune-checkpoint Inhibitors. Cureus 2020, 12, e7625. [Google Scholar] [CrossRef]
- González-Rodríguez, E.; Rodríguez-Abreu, D. Spanish Group for Cancer Immuno-Biotherapy (GETICA) Immune Checkpoint Inhibitors: Review and Management of Endocrine Adverse Events. Oncologist 2016, 21, 804–816. [Google Scholar] [CrossRef]
- Bacanovic, S.; Burger, I.A.; Stolzmann, P.; Hafner, J.; Huellner, M.W. Ipilimumab-Induced Adrenalitis: A Possible Pitfall in 18F-FDG-PET/CT. Clin. Nucl. Med. 2015, 40, e518–e519. [Google Scholar] [CrossRef]
- Trainer, H.; Hulse, P.; Higham, C.E.; Trainer, P.; Lorigan, P. Hyponatraemia secondary to nivolumab-induced primary adrenal failure. Endocrinol. Diabetes Metab. Case Rep. 2016, 2016, 16–0108. [Google Scholar] [CrossRef]
- Mellati, M.; Eaton, K.D.; Brooks-Worrell, B.M.; Hagopian, W.A.; Martins, R.; Palmer, J.P.; Hirsch, I.B. Anti-PD-1 and Anti-PDL-1 Monoclonal Antibodies Causing Type 1 Diabetes. Diabetes Care 2015, 38, e137–e138. [Google Scholar] [CrossRef] [PubMed]
- Stamatouli, A.M.; Quandt, Z.; Perdigoto, A.L.; Clark, P.L.; Kluger, H.; Weiss, S.A.; Gettinger, S.; Sznol, M.; Young, A.; Rushakoff, R.; et al. Collateral Damage: Insulin-Dependent Diabetes Induced With Checkpoint Inhibitors. Diabetes 2018, 67, 1471–1480. [Google Scholar] [CrossRef]
- Anderson, M.A.; Khauli, M.A.; Furtado, F.; Pourvaziri, A.; Catalano, O. Immunotherapy-related renal toxicity causes reversible renal enlargement. Abdom. Radiol. 2022, 47, 3301–3307. [Google Scholar] [CrossRef]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Mikami, T.; Liaw, B.; Asada, M.; Niimura, T.; Zamami, Y.; Green-LaRoche, D.; Pai, L.; Levy, M.; Jeyapalan, S. Neuroimmunological adverse events associated with immune checkpoint inhibitor: A retrospective, pharmacovigilance study using FAERS database. J. Neurooncol. 2021, 152, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Shroff, S.; Kamiya-Matsuoka, C.; Tummala, S. Atypical neurological complications of ipilimumab therapy in patients with metastatic melanoma. Neuro Oncol. 2014, 16, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Coward, J.; McCaffrey, E.; Coucher, J.; Kalokerinos, P.; O’Byrne, K. Pembrolizumab-Induced Encephalopathy: A Review of Neurological Toxicities with Immune Checkpoint Inhibitors. J. Thorac. Oncol. 2017, 12, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
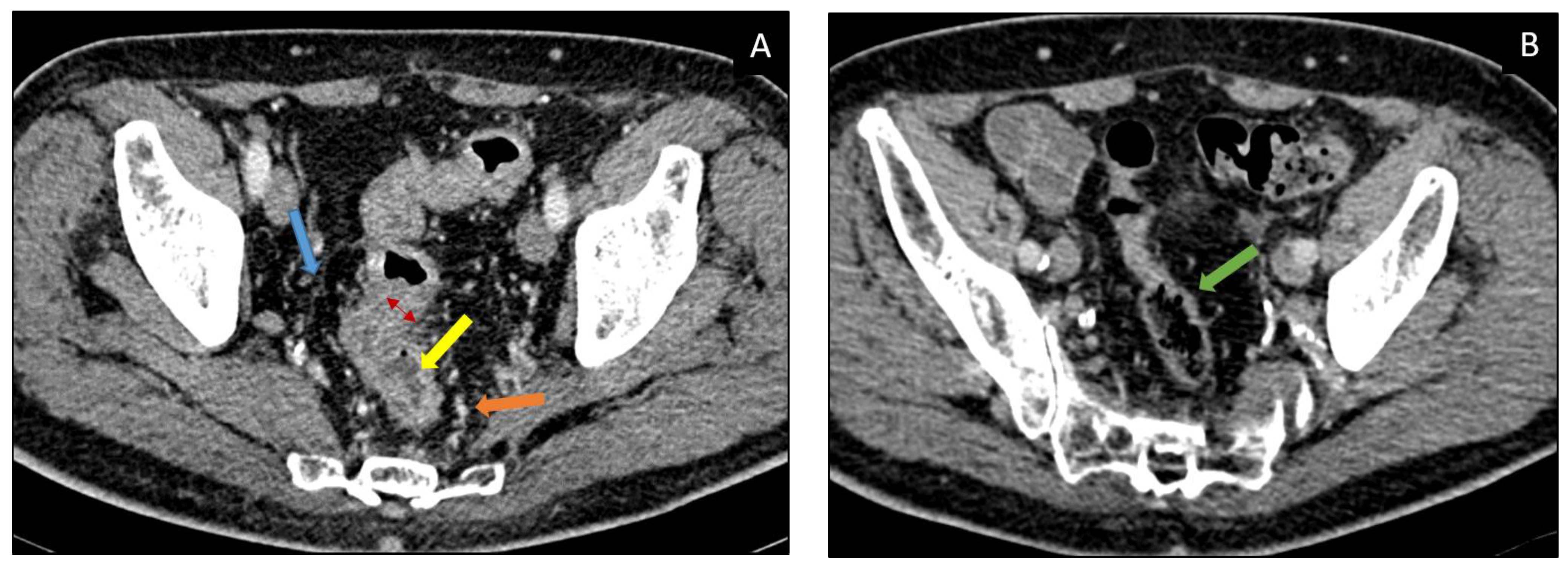
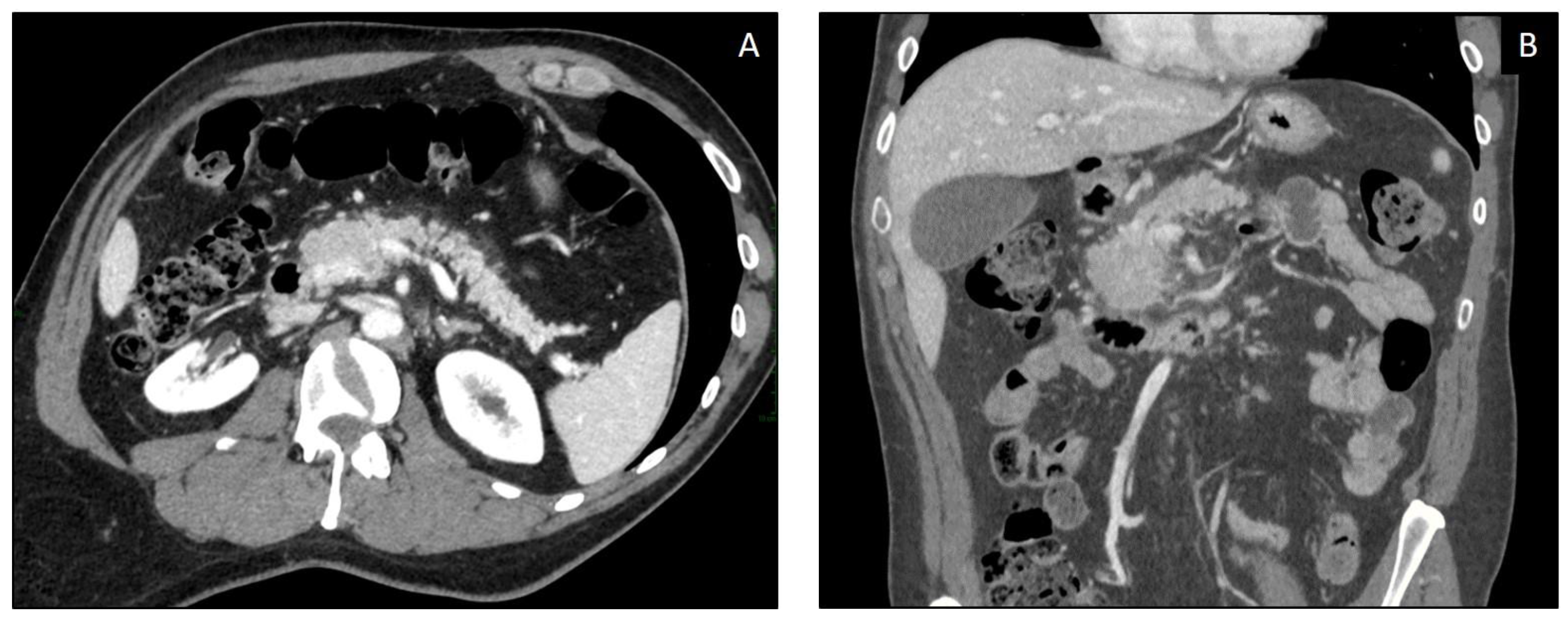
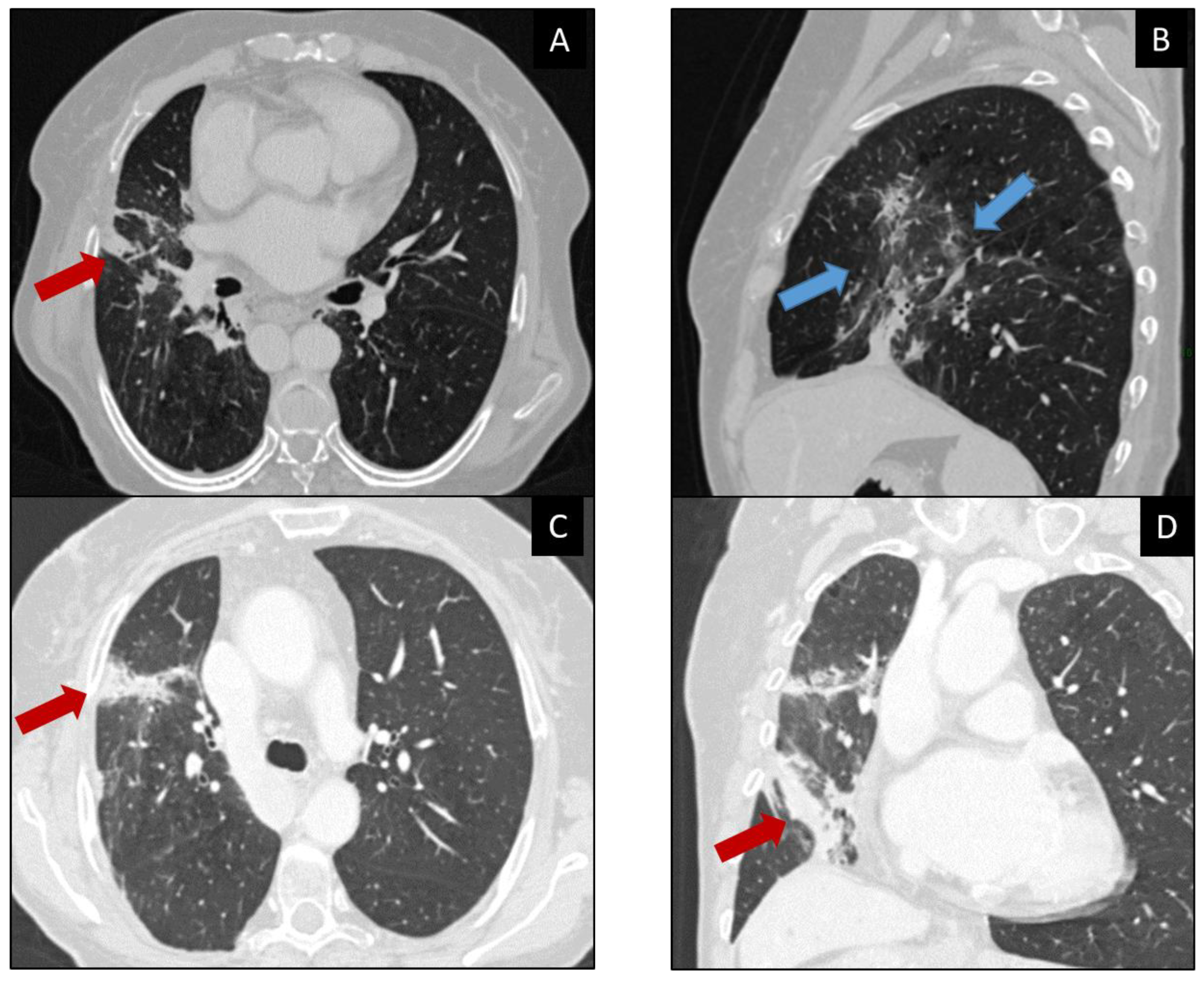
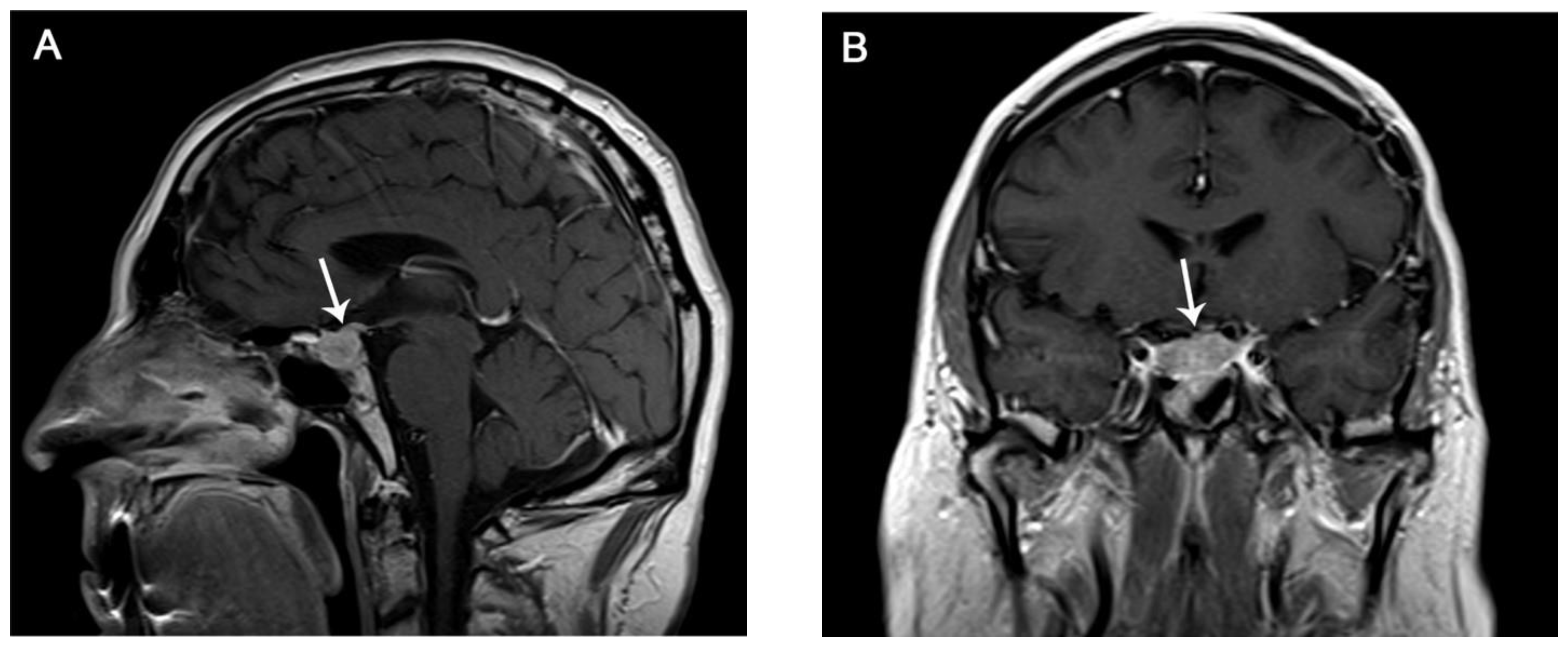

| CT Findings | Description |
|---|---|
Bowel wall thickening
|
|
| Mucosal hyperemia |
|
| Mesenteric vessel engorgement |
|
| Distended colon, fluid filled and air-fluid levels |
|
| Pericolic fat stranding |
|
| PET-CT (18F-FDG) | Description |
| FDG-uptake | Diffuse or segmental (possible false positive in patients in treatment with metformin; consider suspension 48 h before of the PET-CT) |
| Imaging Modality | Findings |
|---|---|
| US, CT, MRI |
|
| Cholangiopancreatography (or endoscopic US) | Extra-hepatic predominant cholangitis
|
| Biopsy with histologic analysis |
|
| CT Patterns of Pneumonitis (in Order of Incidence) | CT Findings | Location | Differential Diagnosis |
|---|---|---|---|
| COP |
|
|
|
| NSIP |
|
| |
| HP |
|
| |
| AIP/ARDSuncommon and severe |
|
| |
| Sarcoid-like reaction |
|
|
|
| Imaging Modality | Findings |
|---|---|
| US Similar to Hashimoto’s thyroiditis |
|
| TC |
|
| PET-CT (18F-FDG) |
|
| Imaging Modality | Findings |
|---|---|
| CT |
|
| MR Necessary if suspected |
|
| PET-CT (18F-FDG) |
|
| Differential Diagnosis | Findings |
|---|---|
| Pituitary adenoma |
|
| Pituitary metastases (melanoma, breast, and lung cancer) |
|
| Lymphocytic hypophysitis |
|
| Imaging Modality | Findings |
|---|---|
| CT MRI |
|
| PET-CT (18F-FDG) |
|
| Imaging Modality | Findings |
|---|---|
| CT |
|
| PET-CT (18F-FDG) |
|
| Imaging Modality | Findings |
|---|---|
| Echocardiography |
|
| Cardiac MR |
|
| 18F-FDG PET-CT |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallio, C.A.; Bernetti, C.; Cea, L.; Buoso, A.; Stiffi, M.; Vertulli, D.; Greco, F.; Zobel, B.B. Adverse Effects of Immune-Checkpoint Inhibitors: A Comprehensive Imaging-Oriented Review. Curr. Oncol. 2023, 30, 4700-4723. https://doi.org/10.3390/curroncol30050355
Mallio CA, Bernetti C, Cea L, Buoso A, Stiffi M, Vertulli D, Greco F, Zobel BB. Adverse Effects of Immune-Checkpoint Inhibitors: A Comprehensive Imaging-Oriented Review. Current Oncology. 2023; 30(5):4700-4723. https://doi.org/10.3390/curroncol30050355
Chicago/Turabian StyleMallio, Carlo Augusto, Caterina Bernetti, Laura Cea, Andrea Buoso, Massimo Stiffi, Daniele Vertulli, Federico Greco, and Bruno Beomonte Zobel. 2023. "Adverse Effects of Immune-Checkpoint Inhibitors: A Comprehensive Imaging-Oriented Review" Current Oncology 30, no. 5: 4700-4723. https://doi.org/10.3390/curroncol30050355
APA StyleMallio, C. A., Bernetti, C., Cea, L., Buoso, A., Stiffi, M., Vertulli, D., Greco, F., & Zobel, B. B. (2023). Adverse Effects of Immune-Checkpoint Inhibitors: A Comprehensive Imaging-Oriented Review. Current Oncology, 30(5), 4700-4723. https://doi.org/10.3390/curroncol30050355








