Olaparib versus Placebo in Maintenance Treatment of Germline BRCA-Mutated Metastatic Pancreatic Cancer: A Cost–Utility Analysis from the Canadian Public Payer’s Perspective
Abstract
1. Introduction
2. Materials and Methods
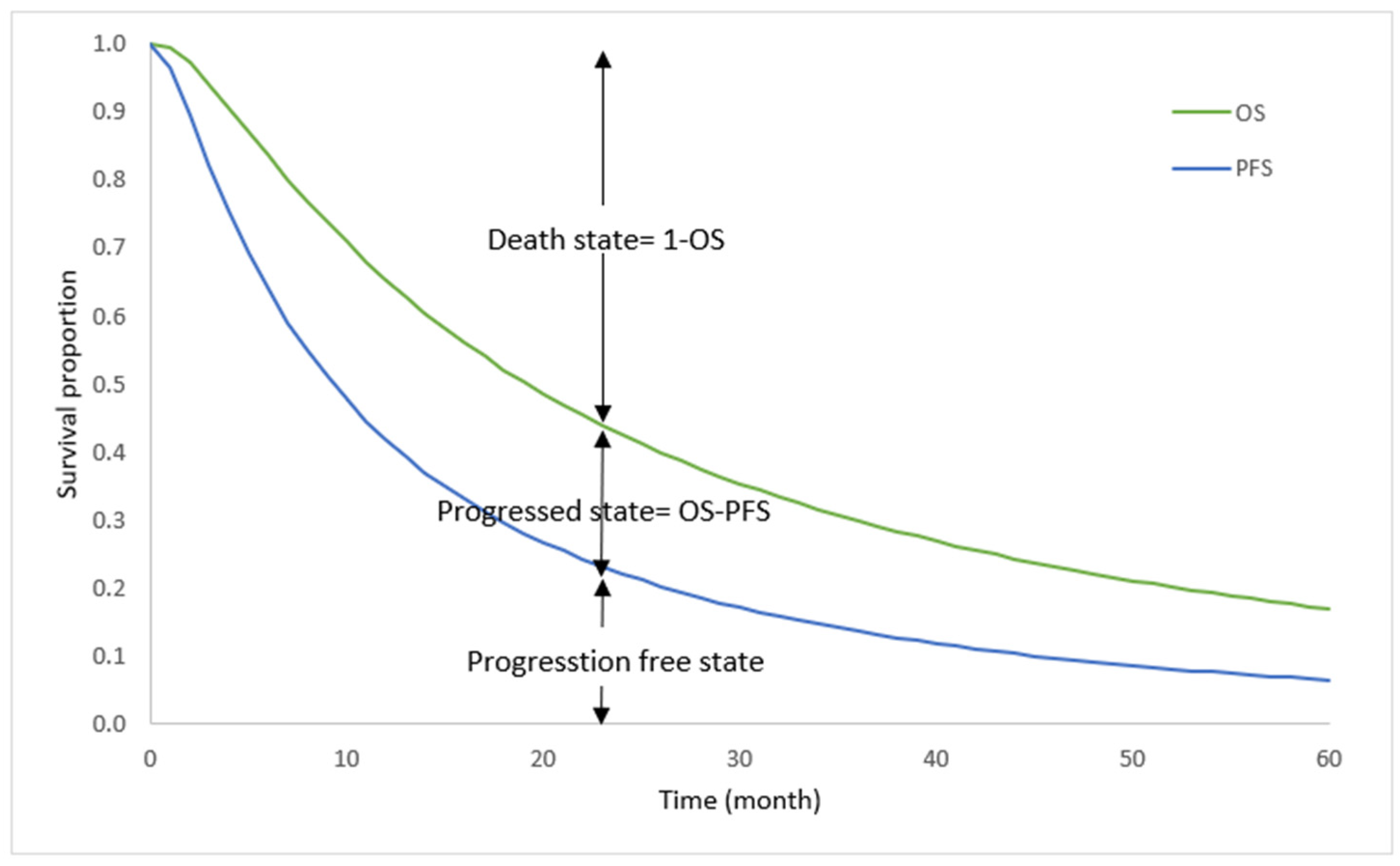
2.1. Model Overview
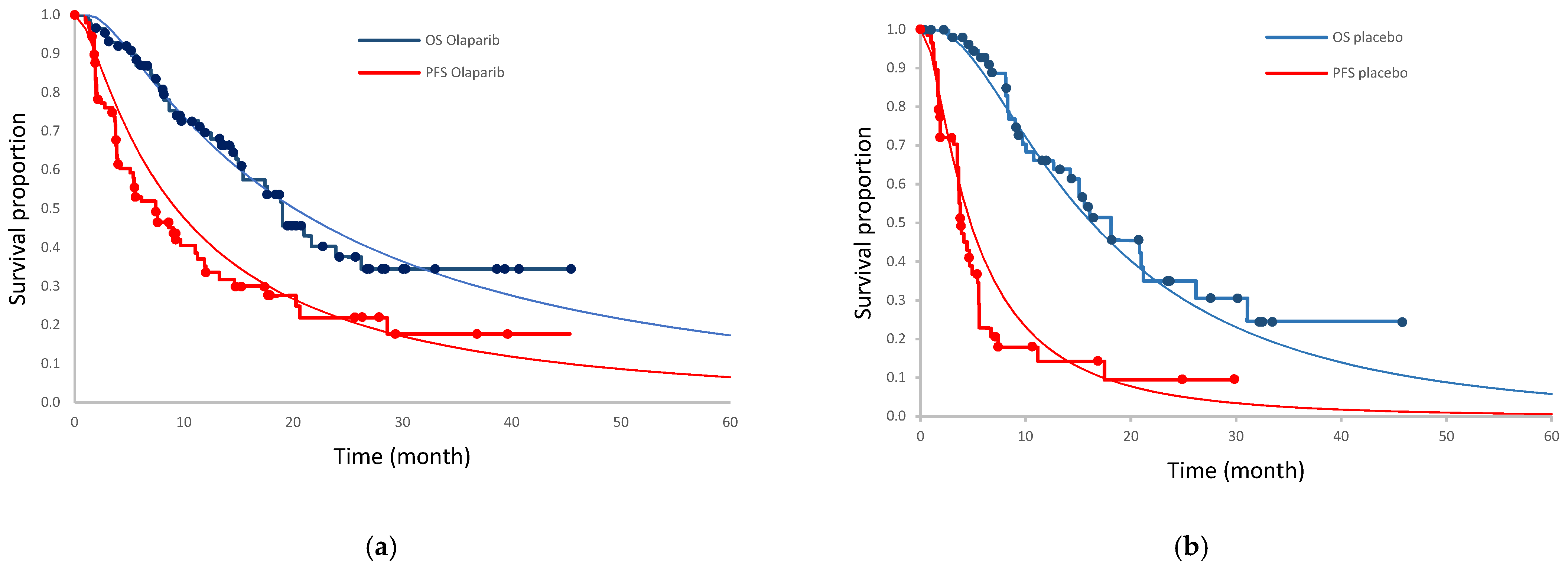
2.2. Clinical Input
2.3. Health State Utility
2.4. Resource Use and Costs
2.5. Discounting
2.6. Analysis
2.7. Sensitivity Analysis
3. Results
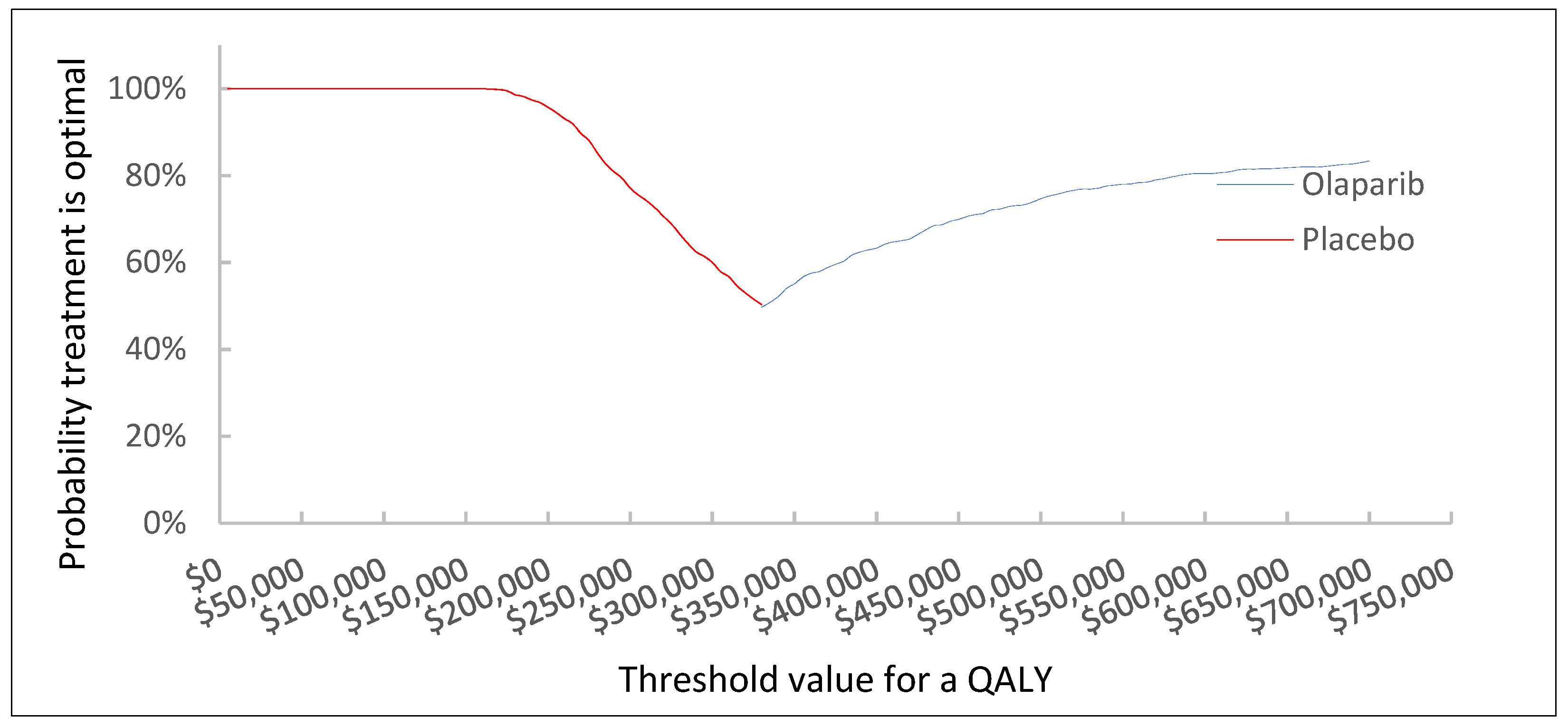
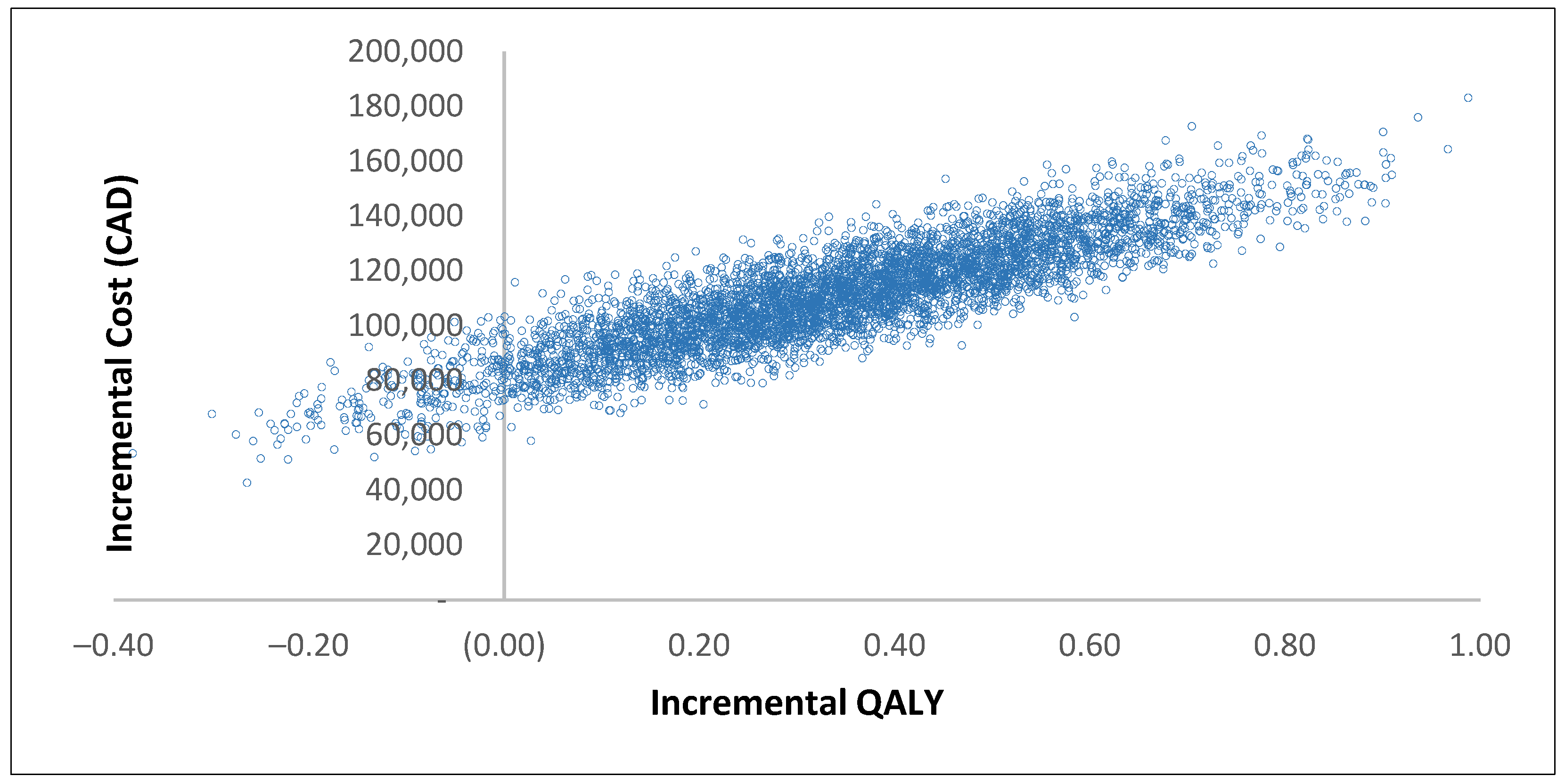
3.1. Base Case Results
3.2. Results of Scenario Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brenner, D.R.; Poirier, A.; Woods, R.R.; Ellison, L.F.; Billette, J.M.; Demers, A.A.; Zhang, S.X.; Yao, C.; Finley, C.; Fitzgerald, N.; et al. Projected estimates of cancer in Canada in 2022. Cmaj 2022, 194, E601–E607. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, C.; Weir, S.; Rangrej, J.; Krahn, M.D.; Mittmann, N.; Hoch, J.S.; Chan, K.K.W.; Peacock, S. The economic burden of cancer care in Canada: A population-based cost study. Can. Med. Assoc. Open Access J. 2018, 6, E1–E10. [Google Scholar] [CrossRef]
- Ellison, L. Age-Specific Patterns in the Diagnosis of, and Survival from, Pancreatic Cancer in Canada. Heal. a Glance. Stat. Canada Cat. 2017. Available online: https://www150.statcan.gc.ca/n1/pub/82-624-x/2017001/article/14799-eng.htm (accessed on 27 April 2023).
- Flook, R.; Van Zanten, S.V. Pancreatic cancer in Canada: Incidence and mortality trends from 1992 to 2005. Can. J. Gastroenterol. 2009, 23, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Anthes, L.E.; Hajizadeh, M. Socioeconomic inequalities in pancreatic cancer incidence in Canada: Evidence from Cancer Registry data. J. Public Health 2022, 30, 801–810. [Google Scholar] [CrossRef]
- Soefje, S.A. Managing the economic impact of advanced pancreatic cancer. Am. J. Manag. Care 2019, 25, S11–S16. [Google Scholar]
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic Cancer. Lancet 2011, 378, 607–620. [Google Scholar] [CrossRef]
- Maitra, A.; Hruban, R.H. Pancreatic cancer. Annu. Rev. Pathol. Mech. Dis. 2008, 3, 157–188. [Google Scholar] [CrossRef]
- Hidalgo, M. Pancreatic Cancer: Pancreatic Cancer: Overview. Lancet 2011, 378, 1605–1617. Available online: https://www.sciencedirect.com/science/article/pii/S0140673610623070?via%3Dihub (accessed on 27 April 2023).
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 18, 1691–1703. Available online: https://www.nejm.org/doi/10.1056/NEJMoa1304369?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200www.ncbi.nlm.nih.gov (accessed on 27 April 2023). [CrossRef]
- Smith, A.L.; Wong, C.; Cuggia, A.; Borgida, A.; Holter, S.; Hall, A.; Connor, A.A.; Bascuñana, C.; Asselah, J.; Bouganim, N.; et al. Reflex Testing for Germline BRCA1, BRCA2, PALB2, and ATM Mutations in Pancreatic Cancer: Mutation Prevalence and Clinical Outcomes From Two Canadian Research Registries. JCO Precis. Oncol. 2018, 2, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Bryan, S.; De, P.; Rahal, R.; Shaw, A.; Turner, D. Canadian Cancer Statistics Advisory Committee. Can. Cancer Soc. 2022; epub ahead of printing. Available online: https://www.cancer.gov/about-cancer/causes-prevention/genetics/brca-genes-hp-pdq (accessed on 27 April 2023).
- Anonymous. Cancer Risks in BRCA2 Mutation Carriers. The breast cancer linkage consortium. J. Natl. Cancer Inst. 1999, 91, 1310–1316. [Google Scholar] [CrossRef]
- Goggins, M.; Sehutte, M.; Lu, J.; Moskaluk, C.A.; Weinstein, C.L.; Petersen, G.M.; Yeo, C.J.; Jackson, C.E.; Lynch, H.T.; Hruban, R.H.; et al. Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Cancer Res. 1996, 56, 5360–5364. [Google Scholar] [PubMed]
- LYNPARZA FDA Approved Package Insert. 2022. Available online: https://den8dhaj6zs0e.cloudfront.net/50fd68b9-106b-4550-b5d0-12b045f8b184/00997c3f-5912-486f-a7db-930b4639cd51/00997c3f-5912-486f-a7db-930b4639cd51_viewable_rendition__v.pdf (accessed on 27 April 2023).
- FDA. FDA Approves Olaparib for gBRCAm Metastatic Pancreatic Adenocarcinoma. 2019. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olaparib-gbrcam-metastatic-pancreatic-adenocarcinoma (accessed on 27 April 2023).
- de Oliveira, C.; Pataky, R.; Bremner, K.E.; Rangrej, J.; Chan, K.K.W.; Cheung, W.Y.; Hoch, J.S.; Peacock, S.; Krahn, M.D. Phase-specific and lifetime costs of cancer care in Ontario, Canada. BMC Cancer. BMC Cancer 2016, 16, 1–12. [Google Scholar] [CrossRef]
- Wu, B.; Shi, L. Cost-Effectiveness of Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. J. Natl. Compr. Cancer Netw. 2020, 18, 1528–1536. Available online: https://jnccn.org/view/journals/jnccn/18/11/article-p1528.xml (accessed on 27 April 2023). [CrossRef]
- Zhan, M.; Zheng, H.; Yang, Y.; He, Z.; Xu, T.; Li, Q. Cost-effectiveness analysis of maintenance olaparib in patients with metastatic pancreatic cancer and a germline BRCA1/2 mutation based on the polo trial. Cancer Manag. Res. 2020, 12, 12919–12926. [Google Scholar] [CrossRef]
- Li, N.; Zheng, H.; Huang, Y.; Zheng, B.; Cai, H.; Liu, M. Cost-Effectiveness Analysis of Olaparib Maintenance Treatment for Germline BRCA-Mutated Metastatic Pancreatic Cancer. Front. Pharmacol. 2021, 12, 632818. [Google Scholar] [CrossRef]
- Sohal, D.P.S.; Kennedy, E.B.; Cinar, P.; Conroy, T.; Copur, M.S.; Crane, C.H.; Garrido-Laguna, I.; Lau, M.W.; Johnson, T.; Krishnamurthi, S.; et al. Metastatic pancreatic cancer: ASCO guideline update. J. Clin. Oncol. 2020, 38, 3217–3230. [Google Scholar] [CrossRef]
- Golan, T.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.O.; Hochhauser, D.; Arnold, D.; Oh, D.Y.R.-S.A. Do not use main. Physiol. Behav. 2016, 176, 139–148. [Google Scholar]
- Yao, J.; Wang, Y.; Mao, T.; Liang, Y.; Zhang, X.; Yang, H.; Hu, J.; Jiao, F.; Cui, J.; Wang, L. A narrative review of Safety management of 1 L platinum-based chemotherapy and maintenance olaparib in BRCA mutated advanced pancreatic cancer. Transl. Cancer Res. 2021, 10, 2488–2495. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, A. WebPlotDigitizer. Version 4.3. Available online: https://automeris.io/WebPlotDigitizer/citation.html (accessed on 26 March 2023).
- Hoyle, M.W.; Henley, W. Improved curve fits to summary survival data: Application to economic evaluation of health technologies. BMC Med. Res. Methodol. 2011, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hammel, P.; Kindler, H.L.; Reni, M.; Van Cutsem, E.; MacArulla, T.; Hall, M.J.; Park, J.O.; Hochhauser, D.; Arnold, D.; Oh, D.Y.; et al. Health-related quality of life in patients with a germline BRCA mutation and metastatic pancreatic cancer receiving maintenance olaparib. Ann. Oncol. 2019, 30, 1959–1968. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.H.; Das, S. Health-related quality of life outcomes from the POLO study: A commentary. Dig. Med. Res. 2020, 3, 23. [Google Scholar] [CrossRef]
- Romanus, D.; Kindler, H.; Archer, L.; Basch, E.; Niedzwiecki, D.; Weeks, J.; Schrag, D. Does Health-Related Quality of Life Improve for Advanced Pancreas Cancer Patients Who Respond to Gemcitabine? J. Pain. Symptom. Manag. 2012, 43, 205–217. [Google Scholar] [CrossRef] [PubMed]
- pCODR Expert Review Committee for Olaparib. 2020, pp. 1–17. Available online: https://www.cadth.ca/sites/default/files/pcodr/Reviews2021/10223OlaparibmCRPC_fnRec_REDACT_EC21Apr2021_final.pdf (accessed on 27 April 2023).
- OHIP Schedule of Benefits and Fees, Physician Services Under the Health Insurance Act. 2022. Available online: https://www.health.gov.on.ca/en/pro/programs/ohip/sob/physserv/sob_master.pdf (accessed on 27 April 2023).
- Ministry of Health. Ontario Schedule of Benefits for Laboratory Services. 2020. Available online: https://www.health.gov.on.ca/en/pro/programs/ohip/sob/lab/lab_mn2020.pdf (accessed on 27 April 2023).
- Chan, D.L.; Cheung, M.; Earle, C.C.; Coburn, N.; Mittmann, N.; Rahman, F.; Liu, N.; Singh, S. Are We Choosing Surveillance Imaging in Gastric and Pancreatic Cancers Wisely? A Population-Based Study. J. Gastrointest. Cancer 2020, 51, 189–195. [Google Scholar] [CrossRef]
- Han, D. Pan-Canadian Oncology Drug Review. Pan-Canadian Oncology Drug Review Final Economic Guidance Report Nab-Paclitaxel (Abraxane) for Pancreatic Cancer; Policy Commons: Amsterdam, The Netherlands, 2014; 13p, Available online: https://www.cadth.ca/sites/default/files/pcodr/pcodr-xtandi-mcrpc-fn-egr.pdf (accessed on 27 April 2023).
- Canadian Agency for Drugs and Technologies in Health. Pan-Canadian Oncology Drug Review Final Economic Guidance Report Pertuzumab-Trastuzumab for Early Breast Cancer; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2018. Available online: https://www.cadth.ca/sites/default/files/pcodr/pcodr_pertuzumab-trastuzumab_perjeta-herceptin-combo_ebc_fn_egr.pdf (accessed on 27 April 2023).
- Yu, M.; Guerriere, D.N.; Coyte, P.C. Health Social Care Comm-2014-Yu-Societal costs of home and hospital end-of-life care for palliative care patients in Ontario, Canada. Health Soc. Care Community 2015, 23, 605–618. [Google Scholar] [CrossRef]
- Gharaibeh, M.; McBride, A.; Alberts, D.S.; Erstad, B.; Slack, M.; Alsaid, N.; Bootman, J.L.; Abraham, I. Economic Evaluation for the UK of Systemic Chemotherapies as First-Line Treatment of Metastatic Pancreatic Cancer. Pharmacoeconomics 2018, 36, 1333–1343. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Louvet, C.; Labianca, R.; Hammel, P.; Lledo, G.; Zampino, M.G.; André, T.; Zaniboni, A.; Ducreux, M.; Aitini, E.; Taïeb, J.; et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: Results of a GERCOR and GISCAD phase III trial. J. Clin. Oncol. 2005, 23, 3509–3516. [Google Scholar] [CrossRef]
- De Dosso, S.; Siebenhüner, A.R.; Winder, T.; Meisel, A.; Fritsch, R.; Astaras, C.; Szturz, P.; Borner, M. Treatment landscape of metastatic pancreatic cancer. Cancer Treat. Rev. 2021, 96, 102180. [Google Scholar] [CrossRef]
- Perri, G.; Prakash, L.; Qiao, W.; Varadhachary, G.R.; Wolff, R.; Fogelman, D.; Overman, M.; Pant, S.; Javle, M.; Koay, E.J.; et al. Response and Survival Associated with First-line FOLFIRINOX vs Gemcitabine and nab-Paclitaxel Chemotherapy for Localized Pancreatic Ductal Adenocarcinoma. JAMA Surg. 2020, 155, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.K.W.; Guo, H.; Cheng, S.; Beca, J.M.; Redmond-Misner, R.; Isaranuwatchai, W.; Qiao, L.; Earle, C.; Berry, S.R.; Biagi, J.J.; et al. Real-world outcomes of FOLFIRINOX vs gemcitabine and nab-paclitaxel in advanced pancreatic cancer: A population-based propensity score-weighted analysis. Cancer Med. 2020, 9, 160–169. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, J.; chan Kim, J.H.; Kim, J.; Hwang, J.H. Pharmacoethnicity of FOLFIRINOX versus gemcitabine plus nab-paclitaxel in metastatic pancreatic cancer: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 20152. [Google Scholar] [CrossRef] [PubMed]
- Seow, H.; O’Leary, E.; Perez, R.; Tanuseputro, P. Access to palliative care by disease trajectory: A population-based cohort of Ontario decedents. BMJ Open 2018, 8, e021147. [Google Scholar] [CrossRef] [PubMed]
- Binder, L.; Ghadban, M.; Sit, C.; Barnard, K. Health Technology Assessment Process for Oncology Drugs: Impact of CADTH Changes on Public Payer Reimbursement Recommendations. Curr. Oncol. 2022, 29, 1514–1526. [Google Scholar] [CrossRef]
- Health Canada Product Monograph for Lynparza. 2022. Available online: https://pdf.hres.ca/dpd_pm/00066912.PDF (accessed on 27 April 2023).
- The pCODR Expert Review Committee (pERC) Final Recommendation: Olaparib as Monotherapy Maintenance Treatment of Adult Patients with Platinum-Sensitive Relapsed BRCA-Mutated Epithelial Ovarian, Fallopian Tube or Primary Peritoneal Cancer Who Are in Response to Platinum-Based Chemotherapy; Canadian Agency for Drugs & Technology in Health: Ottawa, ON, Canada, 2017; pp. 1–17. Available online: https://www.cadth.ca/sites/default/files/pcodr/pcodr_olaparib_lynparza_resub_fn_rec.pdf (accessed on 27 April 2023).
- Pharmacoeconomic Report. Olaparib: As Monotherapy Maintenance Treatment of Adult Patients with Platinum-Sensitive Relapsed BRCA-Mutated Epithelial Ovarian, Fallopian Tube or Primary Peritoneal Cancer Who Are in Response to Platinum-Based Chemotherapy; Canadian Agency for Drugs & Technology in Health: Ottawa, ON, Canada, 2016; Available online: https://www.cadth.ca/sites/default/files/pcodr/pcodr_olaparib_lynparza_oc_fn_rec.pdf (accessed on 27 April 2023).
- The pCODR Expert Review Committee (pERC) Final Recommendation: Olaparib as Monotherapy for the Treatment of Adult Patients with Metastatic Castration-Resistant Prostate Cancer (mCRPC) and Homologous Recombination Repair (HRR) Gene Mutations (Germline and/or Somatic) Who Have Progressed Following Prior Treatment with a New Hormonal Agent (NHA); Canadian Agency for Drugs & Technology in Health: Ottawa, ON, Canada, 2021; Available online: https://www.cadth.ca/sites/default/files/pcodr/Reviews2021/10223OlaparibmCRPC_fnEGR_NOREDACT-ABBREV_EC21Apr2021_final.pdf (accessed on 27 April 2023).
- Exceptional Access Program Reimbursement Criteria for Frequently Requested Drugs; Ministry of Health and Health-Long Term Care: Toronto, ON, Canada, 2014; pp. 1–166. Available online: https://www.health.gov.on.ca/en/pro/programs/drugs/docs/frequently_requested_drugs.pdf (accessed on 27 April 2023).
| Parameters | Base Estimate | Probability Distribution | Source |
|---|---|---|---|
| Monthly costs of progression-free health state in olaparib group | |||
| Olaparib, 150 mg, 2 tablets, b.i.d 1 | CAD 7906.8 | Fixed | [30] |
| Consultation by medical oncologist 2 | CAD 87.5 | Gamma (16, 4.91) | [31] |
| Biochemistry test | CAD 121.0 | Gamma (16, 7.56) | [32] |
| Complete blood count | CAD 3.9 | Gamma (16, 0.25) | [32] |
| CT scan | CAD 429.9 | Gamma (16, 26.86) | [33] |
| Monthly costs of progression-free health state in placebo group | |||
| Consultation by medical oncologist | CAD 87.5 | Gamma (16, 4.91) | [31] |
| CT scan | CAD 429.9 | Gamma (16, 26.86) | [33] |
| Monthly costs of progressed health state in olaparib group | |||
| Third-line therapy | CAD 2377 | Gamma (16, 148.56) | [34,35] |
| Biochemistry test | CAD 121.0 | Gamma (16, 7.56) | [32] |
| Complete blood count | CAD 3.9 | Gamma (16, 0.25) | [32] |
| Consultation by medical oncologist | CAD 87.5 | Gamma (16, 4.91) | [31] |
| CT scan | CAD 429.9 | Gamma (16, 26.86) | [33] |
| ER visit | CAD 43.3 | Gamma (16, 2.71) | [36] |
| Palliative care | CAD 1890.0 | Gamma (16, 118.13) | [36] |
| Monthly costs of progressed health state in placebo group | |||
| Third-line therapy | CAD 2377 | Gamma (16, 148.56) | [34,35] |
| Biochemistry test | CAD 121.0 | Gamma (16, 7.56) | [32] |
| Complete blood count | CAD 3.9 | Gamma (16, 0.25) | [32] |
| Consultation by medical oncologist | CAD 87.5 | Gamma (16, 4.91) | [31] |
| CT scan | CAD 429.9 | Gamma (16, 26.86) | [33] |
| ER visit | CAD 43.3 | Gamma (16, 2.71) | [36] |
| Palliative care | CAD 1890.0 | Gamma (16, 118.13) | [36] |
| Utility values | |||
| Progression-free state | 0.81 | Beta (4.73, 1.11) | [29] |
| Progressed state | 0.73 | Beta (3.71, 1.37) | [29] |
| Outcome | Olaparib | Placebo | Incremental |
|---|---|---|---|
| Cost of progression-free state | CAD 131,586 | CAD 3727 | CAD 127,859 |
| Cost of progressed state | CAD 47,891 | CAD 64,842 | -CAD 16,951 |
| Total cost | CAD 179,477 | CAD 68,569 | CAD 110,909 |
| Progression-free life years | 1.28 | 0.61 | 0.67 |
| Progressed life years | 0.81 | 1.09 | −0.28 |
| Total life years | 2.09 | 1.70 | 0.39 |
| QALY of progression-free state | 1.11 | 0.56 | 0.53 |
| QALY of progressed state | 0.59 | 0.80 | −0.21 |
| Total QALY | 1.70 | 1.36 | 0.34 |
| ICUR | CAD 329,517 | ||
| Scenario | Outcome | Olaparib | Placebo | Incremental | ICUR 1 |
|---|---|---|---|---|---|
| Base case | Cost | CAD 179,477 | CAD 68,569 | CAD 110,909 | CAD 329,517 |
| QALY | 1.70 | 1.36 | 0.34 | ||
| Discount rate of 0% | Cost | CAD 182,912 | CAD 69,973 | CAD 112,940 | CAD 322,143 |
| QALY | 1.66 | 1.31 | 0.35 | ||
| Discount rate of 3% | Cost | CAD 176,482 | CAD 67,582 | CAD 108,900 | CAD 339,229 |
| QALY | 1.67 | 1.34 | 0.32 | ||
| 4-year time horizon | Cost | CAD 165,353 | CAD 64,954 | CAD 100,339 | CAD 405,348 |
| QALY | 1.56 | 1.31 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirzayeh Fashami, F.; Levine, M.; Xie, F.; Blackhouse, G.; Tarride, J.-E. Olaparib versus Placebo in Maintenance Treatment of Germline BRCA-Mutated Metastatic Pancreatic Cancer: A Cost–Utility Analysis from the Canadian Public Payer’s Perspective. Curr. Oncol. 2023, 30, 4688-4699. https://doi.org/10.3390/curroncol30050354
Mirzayeh Fashami F, Levine M, Xie F, Blackhouse G, Tarride J-E. Olaparib versus Placebo in Maintenance Treatment of Germline BRCA-Mutated Metastatic Pancreatic Cancer: A Cost–Utility Analysis from the Canadian Public Payer’s Perspective. Current Oncology. 2023; 30(5):4688-4699. https://doi.org/10.3390/curroncol30050354
Chicago/Turabian StyleMirzayeh Fashami, Fatemeh, Mitchell Levine, Feng Xie, Gordon Blackhouse, and Jean-Eric Tarride. 2023. "Olaparib versus Placebo in Maintenance Treatment of Germline BRCA-Mutated Metastatic Pancreatic Cancer: A Cost–Utility Analysis from the Canadian Public Payer’s Perspective" Current Oncology 30, no. 5: 4688-4699. https://doi.org/10.3390/curroncol30050354
APA StyleMirzayeh Fashami, F., Levine, M., Xie, F., Blackhouse, G., & Tarride, J.-E. (2023). Olaparib versus Placebo in Maintenance Treatment of Germline BRCA-Mutated Metastatic Pancreatic Cancer: A Cost–Utility Analysis from the Canadian Public Payer’s Perspective. Current Oncology, 30(5), 4688-4699. https://doi.org/10.3390/curroncol30050354





