Abstract
Prostate cancer (PC) is the most common type of tumor in men. In the early stage of the disease, it is sensitive to androgen deprivation therapy. In patients with metastatic castration-sensitive prostate cancer (mHSPC), chemotherapy and second-generation androgen receptor therapy have led to increased survival. However, despite advances in the management of mHSPC, castration resistance is unavoidable and many patients develop metastatic castration-resistant disease (mCRPC). In the past few decades, immunotherapy has dramatically changed the oncology landscape and has increased the survival rate of many types of cancer. However, immunotherapy in prostate cancer has not yet given the revolutionary results it has in other types of tumors. Research into new treatments is very important for patients with mCRPC because of its poor prognosis. In this review, we focus on the reasons for the apparent intrinsic resistance of prostate cancer to immunotherapy, the possibilities for overcoming this resistance, and the clinical evidence and new therapeutic perspectives regarding immunotherapy in prostate cancer with a look toward the future.
1. Introduction
Prostate cancer (PCa) is one of the malignancies that affects middle-aged men between the ages of 45 and 60 and contributes significantly to the increase in mortality rates in men globally [1]. PCa-related risk factors include family risk, ethnicity, obesity, and age. PCa tumorigenesis is dependent on epidemiology and genetics [1]. Chromosomal rearrangements have an important role in the development of cancer and, in this context, the role of genetic alterations on androgen synthesis and metabolism has already been investigated as PCA tumorigenesis is closely related to the androgen receptor signaling pathway. Many men with PCa are diagnosed through prostate-specific antigen (PSA) testing, digital rectal examination, prostate biopsy and analysis, and magnetic resonance imaging [2,3]. PCa can be classified as androgen-sensitive (HSPC) or castration-resistant (CRPC), based on the sensitivity to androgen deprivation therapy [4]. There are several treatment options for patients with PCa that can be used alone or in combination with each other; these are chosen based on stage, potential side effects, and patient choice, and include surgery, radiation therapy, cryotherapy, active surveillance, hormone therapy, and chemotherapy [5,6,7,8,9,10,11]. However, each treatment should be chosen taking into account the side effects, which can also be serious, such as incontinence, erectile dysfunction, hematological toxicity, asthenia, and peripheral neuropathy. Metastasis and the eventual development of resistance can occur during the initial treatment [6,7,8,9,10,11,12]. For all of these reasons, the introduction of new well-tolerated and effective treatment strategies is necessary. Immunotherapy has changed the natural history of several cancers and revolutionized modern oncology. It generally consists of the stimulation of the immune system against tumor cells [13]. It has also been investigated in Pca, both with drugs against the PD1-PDL1 axis, CTLA, and with the use of vaccines and adoptive cell immunotherapy. PCa is traditionally considered an immunologically “cold” tumor with low expression of PD-L1 and an immunosuppressive tumor microenvironment (TME). In this review, we aim to provide a holistic overview of immunotherapy in PCa, including a description of the molecular mechanisms, the studies in progress, and future treatment options.
2. PCa as a Cold Tumor: Mechanisms of Immune Tolerance
PCa is traditionally regarded as a cold tumor. This is due to the characteristics of the tumor microenvironment and its genetic expression. The main mechanisms responsible for the intrinsic resistance of PCa to immunotherapy are listed below and are shown in Figure 1.
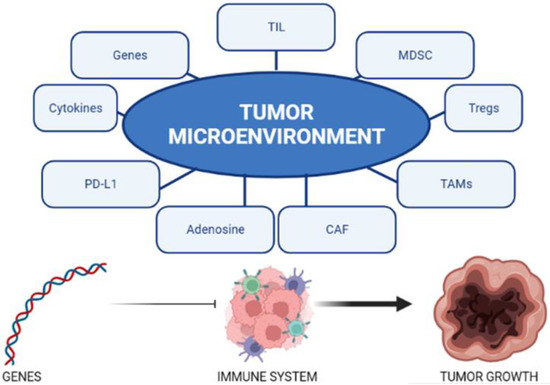
Figure 1.
The tumor microenvironment is influenced by multiple factors. The genetics of PCA influence the interaction between the immune system and the tumor.
2.1. Tumor Microenvironment (TME)
The TME consists of tumor cells, the surrounding blood vessels, an extracellular matrix, immune cells, and signaling molecules that regulate growth and communication between the tumor cells and their surroundings [14]. The composition of the inflammatory microenvironment is crucial in the antitumor immune response, and vascularization has a role both in causing hypoxia (which causes an immunosuppressive environment) and in changing the metabolic state of the TME [15,16]. The TME in PCa tends to be non-inflammatory and with a low expression of neoantigens, which are crucial in the stimulation of the immune system.
Tumor immune evasion is highly correlated with the upregulation of B7 inhibitory molecules in the TME. CD276 is a member of the B7 family that is involved in tumorigenesis. It has stimulating roles, as it is involved in the activation of T cells and the production of interferon-gamma (IFN-γ), but it is also suppressive in immune responses because it promotes metastasis [17].
CD276 is correlated with the reduced function of TMJ and BRCA, as well as low tumor-infiltrating lymphocytes (TIL), and it is highly expressed in advanced Pca [18,19,20].
The elements involved in the composition of the anti-inflammatory TME in PCa are listed below.
2.1.1. Tumor-Infiltrating Lymphocytes (TIL)
The presence of TIL is related to a better prognosis [16]. However, PCa’s TIL populations are composed primarily of M2-type tumor-associated macrophage cells (TAM) and of CD4+ FOXP3+ CD25+ Treg cells, which contribute to the production of immunosuppressive cytokines [18,19,20]. Effector T cells are divided into two types: Th1 and Th2. TILs increase during androgen-deprivation therapy in AR-dependent tumors and, in addition, a reduction in Treg cells occurs [21]. The upregulation of immune checkpoints and TILs has been observed in tissues from patients receiving ADT therapy. TILs can be used as a therapy that involves the use of specific lymphocytes around the tumor. T cells against malignant tumor cells are extracted and they are stimulated to proliferate around the tumor. This therapy is highly effective in patients with metastatic melanoma, with response rates of approximately 40% and complete response rates of 10% to 20% [22]. Furthermore, it has also demonstrated efficacy in preclinical studies in patients with lung cancer [23]. TIL therapy is divided into several phases, starting with surgically resected tumor tissue, from which lymphocytes are extracted and expanded in an interleukin (IL)-2 medium for 4 weeks. Subsequently, the TILs are expanded for 14 days with a procedure that allows their rapid expansion with the anti-CD3 antibody. The TILs are then prepared for infusion.
It is difficult to effectively integrate TIL-based immunotherapy into prostate cancer treatments for several reasons, including the immunosuppressive PCa microenvironment and high tumor heterogeneity. Prostate cancer has a relatively low mutation rate (~0.9 mutations/Mb); however, in advanced stages, alterations in the DNA damage repair mechanisms increase the mutational burden in patients. In several studies, adoptive immunotherapy with direct T cells has demonstrated efficacy, even in tumors with a lower mutational burden [24]. Experiments on TILs in prostate cancer have recently been performed and they indicate a feasibility of tumor-responsive TIL extraction within prostate cancer. In an in vitro study, cultures of prostate-TIL were extracted and proliferated with an expansion rate of approximately 50%. An expression of chemokine receptors was present after expansion. However, proliferating CAR and TIL T cells in immunosuppressive environments for long periods of time remains a major challenge and, in this regard, the goal may be to increase the CAR T cell survival rates. This could occur through the incorporation of TIL 4-1BB and CD137 receptors [25,26].
2.1.2. Myeloid-Derived Suppressor Cells (MDSC)
MDSCs are a diverse group of immune cells of the myeloid lineage [27]. MDSCs are hematopoietic cells that suppress the antitumor activity of the immune system, and when present in TME, are associated with a poor prognosis in several tumor subtypes [28,29,30,31]. The role of MDSC in Pca’s immune evasion is very important [32]. Their immunosuppressive properties include the induction of NK cell anergy, the inhibition of T cell activation, the promotion of the de novo expansion of Tregs, and the inhibition of the maturation of dendritic cells [33]. Some data have shown that the cytokine IL23 secreted by MDSCs can promote cell proliferation by activating AR signaling in PCa cells, and the IL23 blockade restores sensitivity to androgen therapy [34,35,36,37]. MDSCs have been found in the peripheral blood of patients with PCa compared to age-matched healthy controls and the MDSC level was associated with disease burden and PSA level [38]. MDSCs represent a major barrier to many cancer immunotherapies, and targeting them may be an advantageous strategy to improve the efficiency of immunotherapy [38].
2.1.3. Treg Cells
T-cell regulators/T-cell suppressors (Tregs) have the main role of stopping the T-cell-mediated immune response, and thereby promoting immune tolerance [39]. The CD80 and CD86 present on dendritic cells (DC) bind to the CTLA-4 expressed on Tregs, and in this way, the activity of T cells is inhibited [40,41]. Through galectin-1, they can prevent the proliferation of T cells by increasing apoptosis and also produce immunosuppressive cytokines (TGF-β and IL-10) [40,41,42]. In the PCa’s TME, Tregs support MDSCs and M2 macrophages downregulating cytotoxic lymphocytes and NK cells (CTLs) [43]. A histone deacetylase inhibitor, called Entinostat, activates STAT3 acetylation and reduces FoxP3 expression, which inhibits the proliferation and growth of Treg populations, suggesting a novel approach to modulating the TME in PCa [44].
2.1.4. Tumor-Associated Macrophages (TAMs)
TAMs are a key component of the inflammatory TME [45]. In general, they can be classified into an M1 anticancer phenotype or an M2 protumor phenotype. However, mixed phenotypes have been observed. TAMs can stimulate the genetic instability of cancer cells and, consequently, their proliferation and migration [46,47]. In PCa, higher TAM density has been associated with shorter cancer-specific survival and a higher Gleason score [48,49,50]. In addition, they increase the activation of osteoclast-related pathways and have been associated with the activity of TAM, and this is very interesting given the particular tropism of the PCa for bone [48,49,50,51].
2.1.5. Stromal Cells and Cancer-Associated Fibroblasts (CAF)
Various cell types take part in the TME, each with a different task, such as: mesenchymal stromal cells (MSCs), pericytes, fibroblasts, endothelial cells, pericytes, and other innate and adaptive immune cells [52]. CAFs and endothelial cells promote extracellular matrix remodeling (leading to tumor cell invasion), growth factor expression, and neo-angiogenesis [52,53,54]. Among the most important growth factors are bFGF, PDGF, and TNF-α. In addition, MMP and VEGF promote a more aggressive drug-resistant PCa phenotype [53,55]. CAFs perform their antitumor activity on cytotoxic lymphocytes with the development of hypoxia and local inflammation and, therefore, the infiltration of immunosuppressive MDSCs and Tregs in the TME [52,53,56], the overexpression of CD73 and CD39—which generates immunosuppressive adenine, the release of lactate, the expression of PD-L1 mediated by IL-1, and the activation of TGF-β signaling, which involves resistance to therapy with anti PD1 [56,57,58].
2.1.6. Adenosine in PCa
Adenosine is a small anti-inflammatory mediator that suppresses the cytotoxic function of TILs and can increase Tregs, MDSC, fibroblasts, and the function of dendritic cells [59,60]. Adenosine is highly expressed in PCa and causes immune evasion. It is produced by dephosphorylation from ATP that is catalyzed by ectonucleotidases, such as prostatic acid phosphatase (PAP), CD39, CD73, and alkaline phosphatase, and it is associated with immunosuppressive activity, while the hazard-associated molecular pattern (DAMP) promotes immune responses [61,62,63,64,65].
However, studies of vaccination against PAP have been quite disappointing. Early clinical trials are now investigating the efficacy of A2UN and/or A2B receptor antagonists and inhibitors of CD73 ectonucleotidase in PCa [64,65,66,67,68].
2.1.7. Expression of Programmed Death Ligand-1 (PD-L1)
PD-L1 is a protein expressed at low levels in different cell types, such as T lymphocytes, epithelial cells, macrophage endothelial cells, and dendritic cells; it is part of the B7 protein family and binds the PD-1 receptor. The expression of PD-L1 has been detected in only 12% of patients with MSI-H/dMMR disease.
In approximately 10% of tumors with MSI-H/dMMR deletion of PTEN, there is an increased PD-L1 expression. PD-L1 is less expressed in PCa than in other tumors and it also varies according to the histological subtype (it was detected in 46% of neuroendocrine PCa, 29% of acinar Pca, and 7% of ductal PCa) [69].
In addition, the expression level of PD-L1 may be related to the Gleason score, metastasis, infiltration, and biochemical recurrence; however, the data in this regard are not unique [70,71,72,73,74].
2.1.8. Androgen Receptor Signaling
Androgen receptor (AR) signaling is involved in the growth of the survival and tumorigenesis of prostate cells. Deprivative androgen therapy (ADT) that reduces testosterone and AR signaling may be a therapeutic opportunity [75].
The effect of ADT on antitumor immune response and tolerance is controversial [76,77]. In castration-sensitive tumors, ADT leads to a reduction in Tregs and an increase in TIL, while in patients with castration-resistance disease, there is a simultaneous increase in immune tolerance [78].
2.1.9. Tumoral Cytokines
Cytokines are involved in the immune response [79] and participate in the progression of PCa [80]. Pro-inflammatory cytokines lead to increased immune cell infiltration of both cytotoxic T lymphocytes and immunosuppressive MDSCs and Tregs (mainly due to IL-1β and IL-2 production) [81,82]. Many anti-inflammatory cytokines participate in PCA tumorigenesis, such as IL-4, IL-6, and IL-10, which have been found to be elevated in the serum of CRPC patients (82). Tumor necrosis factor α (TNF-α) and IL-17 increase the expression of PD-L1, which has an immunosuppressive role [82]. Furthermore, TGF-β and IL-10 are associated with a weak autoimmune response [83,84].
2.2. Genes Involved in the Regulation of the Immune System in PCa
2.2.1. PTEN
PTEN is a tumor suppressor gene that plays an important role in carcinogenesis and metastasis as it regulates migration and infiltration. About 10% of PCa patients have a homozygous loss of PTEN and 50% have a heterozygous loss [85,86]. In addition to its function on PCa biology, PTEN affects the composition of the inflammatory microenvironment of the TME extension [87,88].
PTEN deficiency leads to increased MDSC infiltration and expression of immunosuppressive molecules IDO1 and CD276 and several chemokines (CXCL12 and CXCL8). It is also related to a high Gleason’s score on ADT sensitivity [87,88,89]. Developing novel PI3K-AKT-mTOR inhibitors and reactivating PTEN function could be a novel therapeutic strategy in CRPC patients with PTEN loss. In a preclinical study, mice genetically modified for the absence of PTEN/p53 were subjected to ADT (degarelix), an anti-PD-1 antibody, or a PI3K inhibitor (copanlisib) in monotherapy or in combination therapy. TAMs with PD-1 expression counteract the antitumor action of ADT/PI3Ki. The combination of ADT/PI3Ki with anti-PD-1 induced an increase in TAM-dependent anti-tumor responses. Furthermore, the tumor cells undergoing PI3Ki had a decrease in lactate production and, consequently, histone lactylation within the TAM. This resulted in the phagocytic activation of TAMs against tumor cells, further stimulated by anti-PD1 and ADT therapy [90].
2.2.2. Forkhead-Box A1 (FOXA1)
FOXA1 has a regulatory function on chromatin and can also influence the TME. FOXA1 causes immunosuppression and promotes the infiltration of M2-type macrophages [91].
Moreover, it was discovered that, in PCa patients with low expression of FOXA1, there is an upregulation of the genes that cause the inflammatory response [91].
FOXA1 overexpression in patients with PCa is inversely correlated with NFI signaling activity and with the expression of the genes that regulate the presentation of antigens, contributing to the resistance to immune checkpoint inhibitors [92,93].
2.2.3. EZH2
EZH2 is a key mediator related to an immunosuppressive TME, the inhibition of T cell differentiation and infiltration, and resistance to immunotherapy. It also regulates the expression of the major histocompatibility complex (MHC) and is involved in Treg and CD4+T differentiation. Furthermore, EZH2 negatively rules INF-induced genes [94,95].
2.2.4. Dickkopf-1 (DKK-1)
DKK-1 is a member of the Dickkopf bone factor family and promotes angiogenesis through VEGFR2. Elevated levels of DKK-1 are associated with a poor prognosis in various tumors. This could be due to its immunosuppressive effect [96,97].
The increase in DKK-1 expression in PCa may have direct effects on the cell cycle, thus increasing tumor proliferation. Furthermore, high serum DKK-1 levels dosed at the time of diagnosis have been correlated with a worse prognosis. Analysis of PCa immune cells revealed that the levels of DKK1 led to an increase in M2 macrophages and lower levels of activated NK and CD8+ T cells [98,99].
In human models of PCa (PC3), DKK1 inhibition blocked tumor growth through the activation of NK cells [98,99,100,101].
A parallel arm Phase 1b/IIa study of DKN-01, a DKK1 inhibitor, as a monotherapy or in combination with docetaxel in patients with advanced PCa and elevated DKK-1 levels is underway (NCT03837353).
2.2.5. Wolf-Hirschhorn Syndrome Candidate Protein 1 (WHSC1, Also Known as MMSET and NSD2)
WHSC1 is correlated with an immunosuppressive TME and has a role in tumor progression in PCa as it mimics lymphocyte infiltration and reduces antigen presentation [102]. The pharmacological inhibition of WHSC1 in a mouse model of PCa (TRAMP C-2) upregulated MHC-II expression on CD45+CD11c+ Dcs and reduced CD4+CD25+ Treg infiltration. It also alters DNA methylation and chromatin accessibility in order to interact with the ubiquitinase and proteasome genes, and it is involved in the expression of CD276 and PD-L1 [103,104,105].
2.2.6. NKG2D
NKG2D is an activating receptor that has a role in killing cancer and virus-infected cells and is expressed on the surface of NK, CD56+, and CD8+ T cells. In patients with Pca, there is a dysfunction of NK cells expressing activating receptors and inhibitors on their surfaces, which leads to poor pharmacological results [106,107]. The blood levels of the NK activating receptor (NKp46) were decreased in patients with PCa and they relate in an inversely proportional way with PSA levels [108,109]. PCa cells, through exosomes containing the surface ligand MICA/B and ULBP-2, negatively regulate the cytotoxic function of NK cells, reducing the expression of NKG2D [110]. One promising approach could be restoring NK cell activation.
2.2.7. CD38
CD38 is part of the ADP-ribosylcyclase family and has an important role in the immune evasion of tumors. In fact, it alters the TIL function by depleting NAD+; on the contrary, the inhibition of CD38 causes the metabolic reorganization of T cells [111,112,113]. Targeted therapies against this CD38/NAD+ axis may make anticancer T-cell adoptive therapy more effective. The combination of anti-PD-1 (cemiplimab) and anti-CD38 (isatuximab) monoclonal antibodies resulted in the activation of peripheral T cells in patients with mCRPC and a median reduction in CD38+ tumor-infiltrating immune cells, from 40% to 3% (NCT03367819) [114].
2.2.8. Polycomb Repressive Complex 1 (PRC1)
The repressive complex Polycomb 1 (PRC1) plays a role in transcriptional regulation. PRC1 promotes PCa immunosuppression through the recruitment of MDSC, TAM, and Tregs in the TME. In contrast, PRC1 inhibition had an antiangiogenic effect and reduced bone metastasis in a DNPC model (Ptenpc−/−Smad4pc−/−) [115].
2.2.9. PIKfyve
PIKFYVE belongs to a family of lipid kinases that are related to immune checkpoint inhibition. The inhibition of PIKfyve blocks Toll-like receptors (TLRs) and the IL12/IL13 signaling pathways inhibit the immune response [116]. Using the drug ESK981, a multi-tyrosine kinase inhibitor that primarily targets VEGFR-1 and 2, the activity of PIKfyve has been inhibited.
Combined treatments of PIKfyve knockdown with anti-PD-1 therapy have been investigated and, in preclinical studies, this led to a significant increase in tumor response [117]. Phase II clinical trials are investigating the efficacy of ESK981 in combination with nivolumab (NCT04159896) or as a monotherapy (NCT03456804) [117].
3. Immunotherapy in PCa: Studies Evaluating Single Agent Immune Checkpoint Inhibitors (ICI) in mCRPC and mHSPC
Immune checkpoints function as controllers of immune activation, and are crucial for preserving immune balance and averting autoimmune reactions. In cancer, tumor cells frequently trigger these checkpoint processes to inhibit the immune system’s anti-cancer responses. The development of checkpoint inhibitory antibodies has led to a significant transformation in the treatment of various hematologic and solid malignancies, with unprecedented response rates reported [118,119].
However, not all solid tumors have reported equally encouraging results. The emphasis on immune checkpoint inhibitors (ICI) has also led to many trials in PCa, particularly in metastatic disease, but unfortunately, the results have been disappointing thus far. The role of the tumor microenvironment is key to understanding the functioning of ICI; it needs to be investigated qualitatively rather than quantitatively, given the genomic determinants of PCa cells. PD-L1, TMB, or dMMR/MSI-high can be considered biomarkers of immunotherapy, and new biomarkers are being added, such as specific pathway aberrations (e.g., AR-V7, HRD, CDK12 inactivated tumors) [120].
Phase I and II studies have explored antibodies targeting the programmed death receptor 1 (PD-1) and T-cell antigen-4 (CTLA-4) inhibitors in metastatic castration-resistant prostate cancer (mCRPC); however, the results obtained were not brilliant [121]. A 2007 study was conducted in patients with hormone refractory prostate cancer; the objective was to evaluate the efficacy of a single 3 mg/kg dose of the humanized anti-CTLA-4 IgG monoclonal antibody, ipilimumab. The endpoints were serologic measures of autoimmunity and the evaluation of T-cell activation, and the treatment with ipilimumab resulted in polyclonal T-cell activation. Unfortunately, there was no objective response, but benefits in the biochemical response were observed. Immune adverse events were limited to one patient with grade 3 rash and pruritus [122]. A randomized phase III study conducted in 2017 (CA184-095) used high-dose (10 mg/kg) ipilimumab monotherapy and had OS as its primary endpoint OS, and PFS and safety as the secondary endpoints. This study did not detect an advantage in the median OS over the placebo (28.7 months vs. 29.7 months; HR = 1.11, 95% CI 26.1–34.2 months, p = 0.3667) in chemotherapy-naïve patients with minimally symptomatic mCRPC [123]. Conversely, ipilimumab dose escalation lengthened the median PFS (5.6 months vs. 3.8 months; HR = 0.67, 95.87% CI 0.55–0.81) and decreased the PSA levels (23% vs. 8%); however, unfortunately, the incidence of grade 3–4 adverse reactions and treatment-related deaths also increased.
Similar outcomes were seen in a phase III study (CA184-043) in which patients with CRPC bone metastases progressing after docetaxel therapy were randomized to receive bone-targeted radiotherapy, followed by ipilimumab 10 mg/kg or a placebo [124].
Countless studies have also investigated anti-PDL-1 monoclonal antibodies in mCRPC. Among the best known, the phase Ib study KEYNOTE-028 considered a cohort of 23 pretreated mCRPC patients with measurable disease and PD-L1 expression ≥1% in tumor or stromal cells. In this study, the primary endpoint was ORR, and the secondary endpoints were OS, PFS, and DOR. The anti-PDL-1 pembrolizumab generated an objective response rate (ORR) of 17.4%, with four patients exhibiting a partial response and three of the four patients exhibiting a biochemical response. As in similar studies, the monoclonal antibody was very well tolerated, prompting further investigation [125].
The KEYNOTE-199 study evaluated the efficacy of pembrolizumab monotherapy in three patient cohorts: PD-L1 positive tumor and measurable disease; PD-L1 negative tumors and measurable disease; and non-measurable metastatic bone disease regardless of PD-L1 status. The primary endpoint of the study was ORR and the secondary endpoints were safety, DCR, DOR, PSA response rate, PFS, OS, and duration of PSA response. The median overall survival and objective response rates were modest, but pembrolizumab demonstrated activity in both RECIST measurable diseases and bone-predominant diseases, regardless of PD-L1 expression.
The DNA damage repair (DDR) gene mutation status, as determined by whole-exome sequencing and analysis, has not revealed a clear relationship between pembrolizumab response and DDR gene mutations [126]. DDR gene alterations, found in 23% of mCRPC patients, mainly involve the BRCA2, ATM, CHEK2, and BRCA1 genes. These alterations generate genomic instability and reactivity of the antitumor immune response by increasing the mutational load of the DNA [127,128,129]. Atezolizumab (anti-PDL-1) has been used (schedule 1200 mg IV every three weeks) in patients with mCRPC previously treated with enzalutamide and/or sipuleucel-T, demonstrating a good safety profile, an overall survival rate of twelve months survival of 55.6%, and a six-month progression-free survival rate of 26.7%. The median overall survival is still under definition [130]. The JAVELIN solid tumor study tested Avelumab, another human IgG1 monoclonal antibody that binds to PD-L1, at different doses administered every 2 weeks [131]. Among the various dosages, the 10 mg/kg dose was chosen as the most effective; this was evaluated in an expansion cohort of 18 mCRPC patients who had progressed on previous treatments. Avelumab had a manageable safety profile. The results were not impressive: seven patients evaluated by RECISTv1.1 achieved stable disease after 24 weeks of treatment and six patients experienced disease progression at 6 weeks. The prolonged PSA doubling time (PSADT) was stable or declining [132].
However, while encouraging in the preclinical stages, the activity of the immune checkpoint blockade in prostate cancer is not convincing, especially with ICI monotherapies in real-world experiences, compared to that observed in other cancers. There are several biological causes for this failure. From an immunological point of view, PCa has a much lower tumor mutation burden (TMB) than other solid tumors that are considered “immunogenic”. In addition, we must consider the lower PD-L1 expression levels compared to other cancers.
The tumor microenvironment is made hostile to the survival of T cells and TIL through various mechanisms due to hypoxic zones within the PCa. It is further made immunosuppressive through the prevalence of myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages, at the expense of immature myeloid cells. CD4+ FOXP3+ CD25+ and CD8+ FOXP3+ CD25+ T cells release inhibitory cytokines, and the IFN-1 pathway associated with PTEN gene loss directs the microenvironment to suppress the immune response. These conditions result in a poor response to the immune checkpoint blockade [133]. All of the studies evaluating single agent immune checkpoint inhibitors (ICI) in mCRPC and mHSPC are summarized in Table 1.

Table 1.
Studies evaluating single agent immune checkpoint inhibitors (ICI) in CRPC.
4. Vaccine-Based Immunotherapy
Sipuleucel-T is a vaccine that contains autologous peripheral blood mononuclear cells (PBMCs) that are reinfused in patients after in vitro culture. They are prepared in vitro with a recombinant fusion protein (PA2024) containing prostatic acid phosphatase, prostate antigen, and granulocyte-macrophage colony stimulating factor (GM-CSF) by activating APCs to expand the antigen [134,135]. A double-blind phase III, randomized, multicenter study (IMPACT: NCT00065442) of 512 patients in a 2:1 ratio received sipuleucel-T or a placebo with overall survival as the primary endpoint. A relative reduction in the risk of death of 22% was observed in the experimental group compared to the placebo group, with a 4.1 month increase in median survival (25.8 months vs. 21.7 months, respectively, in the experimental group vs. placebo). The 36-month survival probability was 31.7% vs. 23.0% in the sipuleucel-T vs. placebo group, respectively. Regarding safety, the most frequent adverse events in the sipuleucel-T group were headache and fever [134]. Studies on the combination of this vaccine and target agents AR [136], atezolizumab [137], or radio-223 [138] in patients with mCRPC are ongoing. It is the only vaccine against PCa approved by the FDA.
GVAX is another vaccine and is based on genetically modified PCa cells that produce the granulocyte-macrophage colony-stimulating factor [139]. GVAX is a safe cytokine and causes an immune response in a dose-dependent manner. The patients showed only fever and flu-like symptoms during treatment. However, considering several failed phase III studies of this vaccination, further trials have been abandoned [140,141].
PROSTVAC is a vaccine that uses a recombinant vaccine strain coupled with transgenes and costimulatory molecules to elicit an immune response [142]. Patients who were treated with PROSTVAC showed increased levels of specific PSA T cells [142]. In a phase II study, 125 patients with mCRPC and a Gleason score ≤7 were randomized to receive PROSTVAC or a placebo [143], with a median OS of 24.4 months in the investigational arm and 16.3 months in the placebo arm [143]. A recent phase III study of PROSTVAC was conducted, but it is considered negative [144,145].
DNA vaccines have been tested in animals but their use in humans remains controversial because of the high risk–benefit ratio [135]. They offer a novel approach [146] compared to other cancer vaccinations in terms of the absence of infectious agents. Several phase I clinical trials are currently ongoing [147,148].
5. Adoptive Immune Cell Immunotherapy
Adoptive cell therapy is based on the use of autologous immune effector cells activated to attack the cancer cells and it has obtained promising results in a variety of hematologic malignancies [149]. However, T cell activity is reduced due to the immunosuppressive environment of the TME caused by the expression of inhibitory molecules (such as PD-1) by cancer cells. The epithelial cell adhesion molecule (EpCAM) is effective in a broad range of cancer immunotherapies involving this stem cell antigen [150]. To more specifically target PCa, CAR-T cells contain the prostate-specific membrane antigen (PSMA). In an initial phase I study of PSMA targeting TGFβ-insensitive armored CAR T cells (NCT0308-9203) in patients with CRPC, 5 of 13 patients developed grade 2 or higher cytokine release (CRS) and 4 had ≥30% reductions in PSA. One patient achieved a >98% reduction in PSA. However, this patient developed enterococcal sepsis 30 days after infusion, leading to multiorgan dysfunction and death [151].
Recent experiments on TILs in PCa have indicated the potential to expand TILs that are reactive against PCa. In one study, 28 cultures of TILs resulting from PCa were successfully extracted and expanded in vitro. The analysis revealed the expression of chemokine receptors after expansion [25,152]. The results of these studies are promising, but CAR-T cell therapy and TILs still face many challenges because the TME in PCa is immunosuppressive and it limits the efficacy of adoptive immunotherapy.
Bispecific T-cell engagement (BiTE) antibodies target PSMA and use T-cells via the CD3 receptor. AMG 212 (pasotuxizumab) has been studied in a phase I clinical trial in 68 patients after disease progression from abiraterone or enzalutamide and at least one taxane-based chemotherapy. There have been dose-dependent reductions in PSA and objective answers in one-third of patients [153]. The limitation of this study included the development of drug-neutralizing antibodies with subcutaneous injection and the short serum half-life of the molecule. In this regard, there is an ongoing development of BiTE molecules with an extended half-life [154]. Other targets for BiT therapy that are expressed in different PCa subtypes are being studied, including delta-like protein 3 (DLL3), human carcinoembryonic antigen (CEA)-related cell adhesion molecule 5, and six-transmembrane prostate epithelial antigen (STEAP) [155,156].
6. Immunotherapy Combination Strategies
Considering the poor results of immunotherapy as single agents, immune checkpoint inhibitors have been studied in combination with each other or with other drugs. These studies aim to overcome the inherent resistance of PCa to immunotherapy by stimulating the immune system in a combined way and improving cancer immunotherapy. Combination therapies with multiple strategies or drug combinations targeting specific mechanisms in the TME aim to overcome the resistance mechanisms in PCa.
6.1. Immunocheckpoint Inhibitors in Combination with ARSI
Considering the role of the AR blockade on the TME, in a phase I study in mHSPC patients with recurrent non-metastatic PCa, tremelimumab (a humanized anti-CTLA-4 antibody) was administered in combination with bicalutamide. The primary endpoint of the study was safety and the secondary was measures of PSA kinetics and the identification of a maximum tolerated dose. Three of the eleven patients experienced a stretch of double-time PSA [157].
AR is a negative regulator of CD8+ T cells in patients with mCRPC; thus, it decreases the response to anti-PD1/PD-L1 treatment [76,77].
A phase II single-arm study in 28 patients with mCRPC progressing after enzalutamide investigated the combination of pembrolizumab with enzalutamide. The primary outcome was a prostate-specific antigen (PSA) decline of ≥50% and the secondary endpoints included ORR, PSA-PFS, OS, and time to subsequent treatment. The PSA response rate was 18% (5/28) and the OS was 21.9 months in all patients. In the responder subgroup (three patients), the OS was 41.7 months [158], one was MSI-high, and none had detectable PD-L1 expression in biopsy tissues [158].
In the randomized phase III IMbassador 250 (#NCT03016312) study in 759 patients progressing after abiraterone or docetaxel, enzalutamide was studied in combination with atezolizumab or as a monotherapy. The primary endpoint (OS) was not met [159]. However, the results are interesting as they suggest that the combination of atezolizumab plus enzalutamide may be more effective in patients with high levels of CD8+ T cells and high PDL1 expression [159].
KEYNOTE-641, a multicenter, randomized, double-blind, phase III study is currently underway in approximately 1200 patients with mCRPC to evaluate the efficacy and safety of enzalutamide plus pembrolizumab+ versus enzalutamide plus placebo (NCT03834493). The primary outcomes of this study are OS and rPFS, and the results have not been published [160].
Further ongoing studies are summarized in Table 2.

Table 2.
Combination therapy trial ongoing.
6.2. Immunocheckpoint Inhibitors in Combination with Cryotherapy or Radiotherapy
ICIs have not been successful in the early stages of the hormone-sensitive disease. Ross and colleagues conducted a single-institution, single-arm pilot study in patients with oligometastatic HSPC to evaluate pembrolizumab in combination with prostate cryotherapy and ADT. Exploiting the increased response to ICI caused by local ablative treatment, the study allowed good local control of the disease with a significant reduction in PSA in 42% of the 12 enrolled patients [161].
The other ongoing phase Ib to phase III studies in mHSPC have evaluated the combination of ICIs with other drugs. Several data have also confirmed the role of radiotherapy in the production of neoantigens. These could stimulate the immune response. Based on this rationale, a Phase I/II study in 50 patients with mCRPC evaluated the treatment with ipilimumab alone or in combination with radiation therapy. One of them had a complete answer, eight had a PSA drop of ≥50%, and six had stable disease. These responding patients had increased intratumoral T-cell infiltration [162].
A phase II study in 31 men with mCRPC previously treated with anti-androgenic agents of avelumab with stereotactic radiotherapy showed a median OS of 14.1 months [163].
In a double-blind, phase III study, patients with mCRPC were randomized to receive radiotherapy followed by ipilimumab or a placebo. There was no statistically significant difference in the OS, but only an increase in progression-free survival between the ipilimumab group and the placebo group. However, in the long-term follow-up study, the OS in the ipilimumab arm was 7.9% vs. 2.7% in the 5-year placebo arm [164].
Further ongoing studies are summarized in Table 2.
6.3. Immunocheckpoint Inhibitors in Combination with Chemotherapy
The association of chemotherapy with immunotherapy has also been investigated to overcome the resistance of PCa to immunotherapy. The advantage of this association consists of the fact that the cytotoxic effect of chemotherapy changes the molecular arrangement of tumor cells, thus facilitating the action of immunotherapy.
KEYNOTE-365 (NCT-02861573), a phase 1b/2 study in patients with mCRPC not pretreated with abiraterone or enzalutamide and not with chemotherapy, investigated the combination of docetaxel and prednisone plus pembrolizumab and demonstrated an OS of 20.2 months, an ORR of 23%, a median radiographic PFS (rPFS) of 8.5 months, and a PSA response rate of 34% [165].
KEYNOTE-921, a multicenter, randomized, double-blind phase III study in this patient population is ongoing to evaluate docetaxel and prednisone or prednisolone plus pembrolizumab versus docetaxel plus a placebo with the primary endpoints of OS and rPFS [166]. Further ongoing studies are summarized in Table 2.
6.4. PARP- Inhibitors and Immunocheckpoint Inhibitors
Most CRPC patients with DDR gene alterations have unsatisfactory responses to immunotherapy and it is not clear whether there is a correlation between the PD-L1 and BRCA status [167].
PARP inhibitors can induce replication stress that causes the entrance into the cytosol of genetic material and the formation of highly permeable micronuclei, even in HR-proficient cells [168,169,170]. This causes a potent immune response because of the transcription of a Type I Interferon. This is one of the possible mechanisms of the synergic efficacy of PARP inhibitors and immunotherapy. PARP inhibitors also upregulate immune checkpoints such as the programmed death-ligand 1 (PD-L1) [171]. The combination of PARP inhibitors and immune checkpoint inhibitors can increase PD-L1 expression in cells with BRCA2 mutation and allows a higher release of neoantigens and consequent increases in T-cell responses against tumors [172,173]. PARP/PI3K inhibition causes the release of DNA-DSB-containing microvesicles that activate the macrophages with an anti-tumor effect in mouse models; this suggests that PARP/PI3K inhibition can augment the response of ICI [174,175].
Clinical studies have investigated the combination of PARP inhibitors and PD1 and PD-L1 blockers in patients with mCRPC. Rucaparib plus nivolumab has been investigated in patients with mCRPC who progressed to at least one line of NHT in a phase I study (NCT03572478) [176]. PSA response to treatment was observed in only one patient (who has a pathogenic BRCA2 mutation). The KEYNOTE-365 trial (NCT0286-1573) was a phase 1–2 trial conducted in post-docetaxel patients with no detectable HR mutation who progressed after at least two lines of NHT. The study had ten cohorts and evaluated pembrolizumab in combination with other drugs. The primary endpoint was the percentage of participants with a decrease of ≥50% in PSA, Aes, and ORR. The cohort A patients received pembrolizumab + olaparib, and this combination of agents showed activity in docetaxel-pretreated patients [177,178]. A phase I/II study showed the efficacy of the combination of olaparib with durvalumab in mCRPC patients. The median radiographic PFS was greater in patients with DDR defects than in patients without DDR defects (16.1 months vs. 4.8 months) [179] and were found to be correlated to a higher amount of Ki-67+/PD-1+/CD8+ T-cells, a decrease in circulating tumor cells after treatment, and an increase in dendritic cells [179]. The combination of olaparib and durvalumab is being studied in a phase II clinical trial in patients with biallelic CDK12 mutations (Cohort A), mismatch repair deficiency (Cohort B), or HRR gene mutations (Cohort C), and the primary outcome is undetectable PSA. Patients in cohorts A and B will receive three cycles of durvalumab followed by three cycles of olaparib in combination with durvalumab; cohort C will receive six cycles of durvalumab plus olaparib [180]. A three-arm non-randomized phase II study in patients with mCRPC, Checkmate 9KD (NCT03338790), examined the combination of nivolumab with rucaparib. The study shows greater objective responses for HRD+ vs. HRD– tumors, and in mutated BRCA2, the ORR was 37.5% [181]. Different drugs kill cells in different ways and the combination can, on one hand, kill cells with targeted drugs (PARP inhibitors) and cytotoxic drugs (chemotherapy), and on the other hand, augment the neoantigen load and help the immune system to attack the tumor. In this regard, combination therapies are effective in a lot of tumor types [182]. A phase II clinical trial in patients with metastatic PCa is evaluating the therapy with cabazitaxel, carboplatin, and cetrelimab in the first phase, followed by niraparib with or without cetrelimab [183]. The primary endpoint is PFS and the study will be completed in 2025 [183].
6.5. Immunocheckpoint Inhibitors in Combination Therapies
Another plausible strategy could be to overcome resistance to mono-immunotherapy by associating an anti-PD1 with an anti-CTLA4.
In CheckMate 650, a Phase II clinical study of the combination of nivolumab and ipilimumab in patients with mCRPC, the ORR was 25% in the experimental treatment [184].
In another phase II study, patients with ARV7 were subjected to a combination of nivolumab and ipilimumab and treated with or without enzalutamide. Overall, 20% (3/15) of patients without enzalutamide and 26.7% (4/15) of patients with enzalutamide achieved a progression-free survival of greater than two years. In the non-enzalutamide arm, the PSA response was 13% and the ORR was 2%, and in the enzalutamide arm, the PSA response rate and ORR were demonstrated to be 0% and 0% [185]. Further ongoing studies are summarized in Table 2.
6.6. Immunochechpoint Inhibitors with Vaccine and Other Drugs
Immunotherapy has also been studied in combination with vaccines containing costimulatory molecules.
In a phase I study, Ipilimumab was combined with a vaccine containing transgenes for costimulatory molecules and for PSA in patients with mCRPC (PROSTVAC), and demonstrated a reduction in PSA in 14 of 24 (58%) patients not pretreated with chemotherapy [186].
Pembrolizumab was studied in phase I and II studies in mHSPC newly diagnosed oligometastatic or mCPRC patients in combination with cryotherapy, with ADXS31-142 (a cancer vaccine containing a live attenuated strain of Listeria monocytogenes encoding a PSA fusion protein and a fragment of listeriolysin O) and MVI-816 (a DNA vaccine encoding prostatic acid phosphatase) [161,187,188,189,190].
In a phase I study of 24 mCRPC patients with a fixed dose of GM-CSF with an escalating dose of ipilimumab, the treatment induced the expansion of specific T cells against the tumor and 50% of patients treated with the higher dose had a PSA drop >50% [191].
A combination of ipilimumab with evophosphamide, a pro-drug that relieves hypoxia, resulted in stable disease in 18 patients and in 3 patients (16.7%) with partial responses; all responders had an augmentation of intratumoral T-cell infiltration [192].
Other phase I and II trials in mCRPC patients have investigated the combination therapy of anti-PD-1 with IDO1 inhibitors, anti-IL6, anti-TGF-β, B7-H3 inhibitors enoblituzumab, LAG-3, OX40, and 4-1BBL [193,194,195,196,197] (NCT03821246, NCT-02628535, NCT02923180, NCT01391143).
Further ongoing studies are summarized in Table 2.
7. Side Effects of Treatments
All of the therapies mentioned above are not free from side effects. Each treatment has side effects related to the properties of the drug. Immunotherapy is associated with autoimmune side effects such as arthritis, colitis, skin toxicity, hypophysitis, thyroiditis, hepatitis, and renal and cardiological toxicity. However, the use of CAR-T therapies is associated with the risk of even more serious adverse events. In particular, cytokine release syndrome (CRS), which is characterized by the possible occurrence of fever, a drop in blood pressure, an increase in heart rate, chills, and low blood oxygen; these are caused by the intense inflammatory response that develops following the activation of CAR-T cells in the body. In the majority of cases, it occurs within 7–14 days after the administration of the therapy. Patients with a severe CRS require interruption of treatment, and in rare cases, it proves fatal. In cases of severe CRS, specific drugs are administered to reduce the levels of inflammatory substances in the circulation (for example tocilizumab or cortisone). Another adverse event is the reduction in B lymphocytes and antibodies (hypogammaglobulinemia) because CAR-T therapies involve, together with the tumor cells, the destruction of B lymphocytes, with a reduction in the level of antibodies. In some cases, to achieve a normal concentration of antibodies and reduce the infectious risk, it is necessary to subject the patient to periodic infusions of human immunoglobulins. Severe adverse events are related to neurological adverse reactions, and the most commonly observed are CAR-T-cell-related encephalopathy syndrome (CRES), tremor, aphasia, and delirium. Most cases occur within 8 weeks following the administration of the CAR-T therapy. The average duration of these alterations is 14 days, with the complete disappearance of symptoms in 98% of patients [198]. Even classic treatments are not free from side effects [199]: chemotherapy is associated with asthenia, diarrhea, vomiting, pain, fatigue, among the most common side effects, which can often affect quality of life; ARSI is associated with reduced sexual desire, erection difficulties, low number of red blood cells, liver and neurological toxicity; radiotherapy can lead to various side effects, with skin toxicity, lymphedema, and hematological toxicity among the most common. The side effects of the various treatments are added together in combination therapies and, although they are promising from the point of view of survival outcomes, the choice of treatment and lines of research cannot ignore the adverse events in this older patient setting.
8. Discussion
Immunotherapy can be an excellent opportunity for patients with prostate cancer for several reasons. For example, it is associated with manageable side effects compared to chemotherapy in this population of often elderly patients, as well as the possibility of having an extra weapon for patients who are not responsive to other treatments. The role of the tumor microenvironment in the poor response of prostate cancer to immunotherapy is well established. The most important questions regarding the resistance mechanisms concern the interaction between the tumor microenvironment and tumor cells, which are governed by various genetic mechanisms. There is a common thread between the composition of the tumor microenvironment and genetic alterations.
Tumor immune evasion is highly correlated with the upregulation of B7 inhibitory molecules in the TME. CD276 is a B7 family member related to impaired TMJ and BRCA function and low tumor infiltrating lymphocytes (TILs), and it is closely related to PTEN deficiency [87,88,89]. In this interpretation, it is clear how modifying the genetics of PCa would also lead to a modification of the tumor microenvironment. Combination therapies are hopeful as, by combining the different treatments, it would seem possible to overcome the resistance. An important aspect is also related to costs; for example, immunotherapy with sipuleucel-T added to standard treatment led to an increase in quality-adjusted life years (QALYs) of 0.37, with an additional cost of around USD 104,536. In this regard, the incremental cost–utility ratio, which is important for estimating costs, was USD 283,000 per QALY saved. These data demonstrate the high costs of immunotherapy in prostate cancer [200]. We believe in immunotherapy as a great opportunity [201]; however, the costs of this treatment should be balanced with its effectiveness. In this regard, the most promising lines of research on immunotherapy must, above all, take into account patient selection: the real key to balancing the costs with the benefits and side effects would be to identify a defined subgroup of patients who respond well to each treatment. This is, from our perspective, the research key to be associated with trials on combination therapies.
9. Conclusions
Immunotherapies have transformed oncology and improved the outcome of a lot of cancer types. This approach allowed us to achieve durable immune control, but it seemed to be less effective in PCa patients. PCa has been considered an immunologically “cold” tumor because of the immunosuppressive characteristics of the TME. The high hopes about immunotherapy in PCa did not initially receive confirmation. This failure has stimulated scientific research to investigate the composition of the TME of PCa and possible solutions to modify it and make it sensitive to immunotherapy in the future. Various combination therapies are underway and this strategy is promising in this setting of patients. These approaches aim to transform the PCa TME from “cold” to “hot” in order to improve strategy and rewrite the treatment of PCa.
Author Contributions
Conceptualization, F.M.M. and P.F.; methodology, F.G.; software, C.L.; validation, M.P., P.D.S. and A.N.S.; formal analysis, C.G.; investigation, M.L.I.; resources, A.M.; data curation, F.G.; writing—original draft preparation, F.M.M. and A.M.; writing—review and editing, P.F.; visualization, F.G.; supervision, P.F.; project administration, F.M.M. and P.F.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, J.; Zhang, D.; Yan, W.; Yang, D.; Shen, B. Translational Bioinformatics for Diagnostic and Prognostic Prediction of Prostate Cancer in the Next-Generation Sequencing Era. BioMed Res. Int. 2013, 2013, 901578. [Google Scholar] [CrossRef] [PubMed]
- Cittadini, A.; Isidori, A.M.; Salzano, A. Testosterone therapy and cardiovascular diseases. Cardiovasc. Res. 2021, 118, 2039–2057. [Google Scholar] [CrossRef] [PubMed]
- Bluemn, E.G.; Nelson, P.S. The androgen/androgen receptor axis in prostate cancer. Curr. Opin. Oncol. 2012, 24, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.-I. Splicing Factors Have an Essential Role in Prostate Cancer Progression and Androgen Receptor Signaling. Biomolecules 2019, 9, 131. [Google Scholar] [CrossRef]
- Bill-Axelson, A.; Holmberg, L.; Garmo, H.; Rider, J.R.; Taari, K.; Busch, C.; Nordling, S.; Häggman, M.; Andersson, S.-O.; Spångberg, A.; et al. Radical Prostatectomy or Watchful Waiting in Early Prostate Cancer. N. Engl. J. Med. 2014, 370, 932–942. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Azad, A.; Alcaraz, A.; Alekseev, B.; Iguchi, T.; Shore, N.D.; et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy with Enzalutamide or Placebo in Men with Metastatic Hormone-Sensitive Prostate Cancer. J. Clin. Oncol. 2019, 37, 2974–2986. [Google Scholar] [CrossRef]
- Chi, K.N.; Agarwal, N.; Bjartell, A.; Chung, B.H.; Gomes, A.J.P.D.S.; Given, R.; Soto, A.J.; Merseburger, A.S.; Özgüroglu, M.; Uemura, H.; et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2019, 381, 13–24. [Google Scholar] [CrossRef]
- Tan, P.S.; Aguiar, P., Jr.; Haaland, B.; Lopes, G. Addition of abiraterone, docetaxel, bisphosphonate, celecoxib or combinations to androgen-deprivation therapy (ADT) for metastatic hormone-sensitive prostate cancer (mHSPC): A network meta-analysis. Prostate Cancer Prostatic Dis. 2018, 21, 516–523. [Google Scholar] [CrossRef]
- Smith, M.R.; Hussain, M.; Saad, F.; Fizazi, K.; Sternberg, C.N.; Crawford, D.; Manarite, J.; Muslin, D.; Farrington, T.; Tombal, B. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer: A patient and caregiver perspective and plain language summary of the ARASENS trial. Futur. Oncol. 2022, 18, 2585–2597. [Google Scholar] [CrossRef]
- Fizazi, K.; Foulon, S.; Carles, J.; Roubaud, G.; McDermott, R.; Fléchon, A.; Tombal, B.; Supiot, S.; Berthold, D.; Ronchin, P.; et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): A multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet 2022, 399, 1695–1707. [Google Scholar] [CrossRef]
- Kluth, L.A.; Shariat, S.F.; Kratzik, C.; Tagawa, S.; Sonpavde, G.; Rieken, M.; Scherr, D.S.; Pummer, K. The hypothalamic–pituitary–gonadal axis and prostate cancer: Implications for androgen deprivation therapy. World J. Urol. 2013, 32, 669–676. [Google Scholar] [CrossRef]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef]
- Haanen, J.B.A.G.; Baars, A.; Gomez, R.; Weder, P.; Smits, M.; de Gruijl, T.D.; von Blomberg, B.M.E.; Bloemena, E.; Scheper, R.J.; van Ham, S.M.; et al. Melanoma-specific tumor-infiltrating lymphocytes but not circulating melanoma-specific T cells may predict survival in resected advanced-stage melanoma patients. Cancer Immunol. Immunother. 2005, 55, 451–458. [Google Scholar] [CrossRef]
- Strasner, A.; Karin, M. Immune Infiltration and Prostate Cancer. Front. Oncol. 2015, 5, 128. [Google Scholar] [CrossRef]
- Tourinho-Barbosa, R.R.; De La Rosette, J.; Sanchez-Salas, R. Prostate cancer multifocality, the index lesion, and the microenvironment. Curr. Opin. Urol. 2018, 28, 499–505. [Google Scholar] [CrossRef]
- Adekoya, T.O.; Richardson, R.M. Cytokines and Chemokines as Mediators of Prostate Cancer Metastasis. Int. J. Mol. Sci. 2020, 21, 4449. [Google Scholar] [CrossRef]
- Page, S.T.; Plymate, S.R.; Bremner, W.J.; Matsumoto, A.M.; Hess, D.L.; Lin, D.W.; Amory, J.K.; Nelson, P.S.; Wu, J.D. Effect of medical castration on CD4+CD25+ T cells, CD8+ T cell IFN-γ expression, and NK cells: A physiological role for testosterone and/or its metabolites. Am. J. Physiol. Metab. 2006, 290, E856–E863. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.; Donia, M.; Ellebaek, E.; Borch, T.H.; Kongsted, P.; Iversen, T.Z.; Hölmich, L.R.; Hendel, H.W.; Met, O.; Andersen, M.H.; et al. Long-Lasting Complete Responses in Patients with Metastatic Melanoma after Adoptive Cell Therapy with Tumor-Infiltrating Lymphocytes and an Attenuated IL2 Regimen. Clin. Cancer Res. 2016, 22, 3734–3745. [Google Scholar] [CrossRef] [PubMed]
- Ben-Avi, R.; Farhi, R.; Ben-Nun, A.; Gorodner, M.; Greenberg, E.; Markel, G.; Schachter, J.; Itzhaki, O.; Besser, M.J. Establishment of adoptive cell therapy with tumor infiltrating lymphocytes for non-small cell lung cancer patients. Cancer Immunol. Immunother. 2018, 67, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Johnson, B.A., III; Lutz, E.R.; Laheru, D.A.; Jaffee, E.M. Targeting neoantigens to augment antitumour immunity. Nat. Rev. Cancer 2017, 17, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Yunger, S.; Bar El, A.; Zeltzer, L.-A.; Fridman, E.; Raviv, G.; Laufer, M.; Schachter, J.; Markel, G.; Itzhaki, O.; Besser, M.J. Tumor-infiltrating lymphocytes from human prostate tumors reveal anti-tumor reactivity and potential for adoptive cell therapy. Oncoimmunology 2019, 8, e1672494. [Google Scholar] [CrossRef]
- Milone, M.C.; Fish, J.D.; Carpenito, C.; Carroll, R.G.; Binder, G.K.; Teachey, D.; Samanta, M.; Lakhal, M.; Gloss, B.; Danet-Desnoyers, G.; et al. Chimeric Receptors Containing CD137 Signal Transduction Domains Mediate Enhanced Survival of T Cells and Increased Antileukemic Efficacy In Vivo. Mol. Ther. 2009, 17, 1453–1464. [Google Scholar] [CrossRef]
- Talmadge, J.E.; Gabrilovich, D.I. History of myeloid-derived suppressor cells. Nat. Rev. Cancer 2013, 13, 739–752. [Google Scholar] [CrossRef]
- Bronte, V.; Brandau, S.; Chen, S.-H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef]
- Jiang, J.; Guo, W.; Liang, X. Phenotypes, accumulation, and functions of myeloid-derived suppressor cells and associated treatment strategies in cancer patients. Hum. Immunol. 2014, 75, 1128–1137. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012, 12, 253–268. [Google Scholar] [CrossRef]
- Greten, T.F.; Manns, M.P.; Korangy, F. Myeloid derived suppressor cells in human diseases. Int. Immunopharmacol. 2011, 11, 802–807. [Google Scholar] [CrossRef]
- Wang, J.; McGuire, T.R.; Britton, H.C.; Schwarz, J.K.; Loberiza, F.R.; Meza, J.L.; Talmadge, J.E. Lenalidomide and cyclophosphamide immunoregulation in patients with metastatic, castration-resistant prostate cancer. Clin. Exp. Metastasis 2015, 32, 111–124. [Google Scholar] [CrossRef]
- Idorn, M.; Køllgaard, T.; Kongsted, P.; Sengeløv, L.; Straten, P.T. Correlation between frequencies of blood monocytic myeloid-derived suppressor cells, regulatory T cells and negative prognostic markers in patients with castration-resistant metastatic prostate cancer. Cancer Immunol. Immunother. 2014, 63, 1177–1187. [Google Scholar] [CrossRef]
- Garcia, A.J.; Ruscetti, M.; Arenzana, T.L.; Tran, L.M.; Bianci-Frias, D.; Sybert, E.; Priceman, S.J.; Wu, L.; Nelson, P.S.; Smale, S.T.; et al. Pten Null Prostate Epithelium Promotes Localized Myeloid-Derived Suppressor Cell Expansion and Immune Suppression during Tumor Initiation and Progression. Mol. Cell. Biol. 2014, 34, 2017–2028. [Google Scholar] [CrossRef]
- Calcinotto, A.; Spataro, C.; Zagato, E.; Di Mitri, D.; Gil, V.; Crespo, M.; De Bernardis, G.; Losa, M.; Mirenda, M.; Pasquini, E.; et al. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature 2018, 559, 363–369. [Google Scholar] [CrossRef]
- Hossain, D.M.S.; Pal, S.K.; Moreira, D.; Duttagupta, P.; Zhang, Q.; Won, H.; Jones, J.; D’Apuzzo, M.; Forman, S.; Kortylewski, M. TLR9-Targeted STAT3 Silencing Abrogates Immunosuppressive Activity of Myeloid-Derived Suppressor Cells from Prostate Cancer Patients. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 3771–3782. [Google Scholar] [CrossRef]
- Feng, S.; Cheng, X.; Zhang, L.; Lu, X.; Chaudhary, S.; Teng, R.; Frederickson, C.; Champion, M.M.; Zhao, R.; Cheng, L.; et al. Myeloid-derived suppressor cells inhibit T cell activation through nitrating LCK in mouse cancers. Proc. Natl. Acad. Sci. USA 2018, 115, 10094–10099. [Google Scholar] [CrossRef]
- Koinis, F.; Xagara, A.; Chantzara, E.; Leontopoulou, V.; Aidarinis, C.; Kotsakis, A. Myeloid-Derived Suppressor Cells in Prostate Cancer: Present Knowledge and Future Perspectives. Cells 2021, 11, 20. [Google Scholar] [CrossRef]
- Askenasy, N.; Kaminitz, A.; Yarkoni, S. Mechanisms of T regulatory cell function. Autoimmun. Rev. 2008, 7, 370–375. [Google Scholar] [CrossRef]
- Garín, M.I.; Chu, C.-C.; Golshayan, D.; Cernuda-Morollón, E.; Wait, R.; Lechler, R.I. Galectin-1: A key effector of regulation mediated by CD4+CD25+ T cells. Blood 2007, 109, 2058–2065. [Google Scholar] [CrossRef]
- Peach, R.; Bajorath, J.; Brady, W.; Leytze, G.; Greene, J.; Naemura, J.; Linsley, P.S. Complementarity determining region 1 (CDR1)- and CDR3-analogous regions in CTLA-4 and CD28 determine the binding to B7-1. J. Exp. Med. 1994, 180, 2049–2058. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Mathur, R.; Farooque, A.; Kaul, V.; Gupta, S.; Dwarakanath, B.S. T-Regulatory Cells in Tumor Progression and Therapy. Cancer Manag. Res. 2019, 11, 10731–10747. [Google Scholar] [CrossRef] [PubMed]
- Karpisheh, V.; Mousavi, S.M.; Sheykholeslami, P.N.; Fathi, M.; Saray, M.M.; Aghebati-Maleki, L.; Jafari, R.; Zolbanin, N.M.; Jadidi-Niaragh, F. The role of regulatory T cells in the pathogenesis and treatment of prostate cancer. Life Sci. 2021, 284, 119132. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Ciesielski, M.; Ramakrishnan, S.; Miles, K.M.; Ellis, L.; Sotomayor, P.; Shrikant, P.; Fenstermaker, R.; Pili, R. Class I Histone Deacetylase Inhibitor Entinostat Suppresses Regulatory T Cells and Enhances Immunotherapies in Renal and Prostate Cancer Models. PLoS ONE 2012, 7, e30815. [Google Scholar] [CrossRef]
- Larionova, I.; Tuguzbaeva, G.; Ponomaryova, A.; Stakheyeva, M.; Cherdyntseva, N.; Pavlov, V.; Choinzonov, E.; Kzhyshkowska, J. Tumor-Associated Macrophages in Human Breast, Colorectal, Lung, Ovarian and Prostate Cancers. Front. Oncol. 2020, 10, 566511. [Google Scholar] [CrossRef]
- Erlandsson, A.; Carlsson, J.; Lundholm, M.; Fält, A.; Andersson, S.-O.; Andrén, O.; Davidsson, S. M2 macrophages and regulatory T cells in lethal prostate cancer. Prostate 2019, 79, 363–369. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Lissbrant, I.F.; Stattin, P.; Wikstrom, P.; Damber, J.E.; Egevad, L.; Bergh, A. Tumor associated macrophages in human prostate cancer: Relation to clinicopathological variables and survival. Int. J. Oncol. 2000, 17, 445–496. [Google Scholar] [CrossRef]
- Shimura, S.; Yang, G.; Ebara, S.; Wheeler, T.M.; Frolov, A.; Thompson, T.C. Reduced infiltration of tumor-associated macrophages in human prostate cancer: Association with cancer progression. Cancer Res. 2000, 60, 5857–5861. [Google Scholar]
- Seif, F.; Sharifi, L.; Khoshmirsafa, M.; Mojibi, Y.; Mohsenzadegan, M. A Review of Preclinical Experiments Toward Targeting M2 Macrophages in Prostate Cancer. Curr. Drug Targets 2019, 20, 789–798. [Google Scholar] [CrossRef]
- Joyce, J.A.; Pollard, J.W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 2009, 9, 239–252. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Kiosses, W.B. Intratumoral Cancer Cell Intravasation Can Occur Independent of Invasion into the Adjacent Stroma. Cell Rep. 2017, 19, 601–616. [Google Scholar] [CrossRef]
- Bonollo, F.; Thalmann, G.N.; Kruithof-de Julio, M.; Karkampouna, S. The Role of Cancer-Associated Fibroblasts in Prostate Cancer Tumorigenesis. Cancers 2020, 12, 1887. [Google Scholar] [CrossRef]
- Monteran, L.; Erez, N. The Dark Side of Fibroblasts: Cancer-Associated Fibroblasts as Mediators of Immunosuppression in the Tumor Microenvironment. Front. Immunol. 2019, 10, 1835. [Google Scholar] [CrossRef]
- Giannoni, E.; Bianchini, F.; Masieri, L.; Serni, S.; Torre, E.; Calorini, L.; Chiarugi, P. Reciprocal Activation of Prostate Cancer Cells and Cancer-Associated Fibroblasts Stimulates Epithelial-Mesenchymal Transition and Cancer Stemness. Cancer Res. 2010, 70, 6945–6956. [Google Scholar] [CrossRef]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340. [Google Scholar] [CrossRef]
- Ziani, L.; Ben Safta-Saadoun, T.; Gourbeix, J.; Cavalcanti, A.; Robert, C.; Favre, G.; Chouaib, S.; Thiery, J. Melanoma-associated fibroblasts decrease tumor cell susceptibility to NK cell-mediated killing through matrix-metalloproteinases secretion. Oncotarget 2017, 8, 19780–19794. [Google Scholar] [CrossRef]
- Li, T.; Yang, Y.; Hua, X.; Wang, G.; Liu, W.; Jia, C.; Tai, Y.; Zhang, Q.; Chen, G. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett. 2012, 318, 154–161. [Google Scholar] [CrossRef]
- Ohta, A.; Sitkovsky, M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 2001, 414, 916–920. [Google Scholar] [CrossRef]
- Beavis, P.A.; Stagg, J.; Darcy, P.K.; Smyth, M.J. CD73: A potent suppressor of antitumor immune responses. Trends Immunol. 2012, 33, 231–237. [Google Scholar] [CrossRef]
- Stagg, J.; Divisekera, U.; Duret, H.; Sparwasser, T.; Teng, M.W.; Darcy, P.K.; Smyth, M.J. CD73-Deficient Mice Have Increased Antitumor Immunity and Are Resistant to Experimental Metastasis. Cancer Res. 2011, 71, 2892–2900. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Guo, G.; Huang, L.; Deng, L.; Chang, C.-S.; Achyut, B.R.; Canning, M.; Xu, N.; Arbab, A.S.; Bollag, R.J.; et al. CD73 on cancer-associated fibroblasts enhanced by the A2B-mediated feedforward circuit enforces an immune checkpoint. Nat. Commun. 2020, 11, 515. [Google Scholar] [CrossRef] [PubMed]
- Graddis, T.J.; McMahan, C.J.; Tamman, J.; Page, K.J.; Trager, J.B. Prostatic acid phosphatase expression in human tissues. Int. J. Clin. Exp. Pathol. 2011, 4, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Vigano, S.; Alatzoglou, D.; Irving, M.; Ménétrier-Caux, C.; Caux, C.; Romero, P.; Coukos, G. Targeting Adenosine in Cancer Immunotherapy to Enhance T-Cell Function. Front. Immunol. 2019, 10, 925. [Google Scholar] [CrossRef]
- Leone, R.D.; Emens, L.A. Targeting adenosine for cancer immunotherapy. J. Immunother. Cancer 2018, 6, 57. [Google Scholar] [CrossRef]
- Allard, B.; Longhi, M.S.; Robson, S.C.; Stagg, J. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol. Rev. 2017, 276, 121–144. [Google Scholar] [CrossRef]
- Bendell, J.; Bauer, T.; Patel, M.; Falchook, G.; Karlix, J.L.; Lim, E.; Mugundu, G.; Mitchell, P.D.; Pouliot, G.P.; Moorthy, G.; et al. Abstract CT026: Evidence of immune activation in the first-in-human Phase Ia dose escalation study of the adenosine 2a receptor antagonist, AZD4635, in patients with advanced solid tumors. Cancer Res. 2019, 79, CT026. [Google Scholar] [CrossRef]
- Wise, D.R.; Gardner, O.; Gilbert, H.N.; Rieger, A.; Paoloni, M.C.; Krishnan, K. A phase Ib/II, open-label, platform study evaluating the efficacy and safety of AB928-based treatment combinations in participants with metastatic castrate-resistant prostate cancer. J. Clin. Oncol. 2020, 38, TPS272. [Google Scholar] [CrossRef]
- Haffner, M.C.; Guner, G.; Taheri, D.; Netto, G.J.; Palsgrove, D.N.; Zheng, Q.; Guedes, L.B.; Kim, K.; Tsai, H.; Esopi, D.M.; et al. Comprehensive Evaluation of Programmed Death-Ligand 1 Expression in Primary and Metastatic Prostate Cancer. Am. J. Pathol. 2018, 188, 1478–1485. [Google Scholar] [CrossRef]
- Gevensleben, H.; Dietrich, D.; Golletz, C.; Steiner, S.; Jung, M.; Thiesler, T.; Majores, M.; Stein, J.; Uhl, B.; Müller, S.; et al. The Immune Checkpoint Regulator PD-L1 Is Highly Expressed in Aggressive Primary Prostate Cancer. Clin. Cancer Res. 2016, 22, 1969–1977. [Google Scholar] [CrossRef]
- Baas, W.; Gershburg, S.; Dynda, D.; Delfino, K.; Robinson, K.; Nie, D.; Yearley, J.H.; Alanee, S. Immune Characterization of the Programmed Death Receptor Pathway in High Risk Prostate Cancer. Clin. Genitourin. Cancer 2017, 15, 577–581. [Google Scholar] [CrossRef]
- Calagua, C.; Russo, J.; Sun, Y.; Schaefer, R.; Lis, R.; Zhang, Z.; Mahoney, K.; Bubley, G.J.; Loda, M.; Taplin, M.-E.; et al. Expression of PD-L1 in Hormone-naïve and Treated Prostate Cancer Patients Receiving Neoadjuvant Abiraterone Acetate plus Prednisone and Leuprolide. Clin. Cancer Res. 2017, 23, 6812–6822. [Google Scholar] [CrossRef]
- Ness, N.; Andersen, S.; Khanehkenari, M.R.; Nordbakken, C.V.; Valkov, A.; Paulsen, E.-E.; Nordby, Y.; Bremnes, R.M.; Donnem, T.; Busund, L.-T.; et al. The prognostic role of immune checkpoint markers programmed cell death protein 1 (PD-1) and programmed death ligand 1 (PD-L1) in a large, multicenter prostate cancer cohort. Oncotarget 2017, 8, 26789–26801. [Google Scholar] [CrossRef]
- Fankhauser, C.D.; Schüffler, P.J.; Gillessen, S.; Omlin, A.; Rupp, N.J.; Rueschoff, J.H.; Hermanns, T.; Poyet, C.; Sulser, T.; Moch, H.; et al. Comprehensive immunohistochemical analysis of PD-L1 shows scarce expression in castration-resistant prostate cancer. Oncotarget 2018, 9, 10284–10293. [Google Scholar] [CrossRef]
- Formaggio, N.; Rubin, M.A.; Theurillat, J.-P. Loss and revival of androgen receptor signaling in advanced prostate cancer. Oncogene 2021, 40, 1205–1216. [Google Scholar] [CrossRef]
- Ben-Batalla, I.; Vargas-Delgado, M.E.; Von Amsberg, G.; Janning, M.; Loges, S. Influence of Androgens on Immunity to Self and Foreign: Effects on Immunity and Cancer. Front. Immunol. 2020, 11, 1184. [Google Scholar] [CrossRef]
- Gamat, M.; McNeel, D.G. Androgen deprivation and immunotherapy for the treatment of prostate cancer. Endocr.-Relat. Cancer 2017, 24, T297–T310. [Google Scholar] [CrossRef]
- Long, X.; Hou, H.; Wang, X.; Liu, S.; Diao, T.; Lai, S.; Hu, M.; Zhang, S.; Liu, M.; Zhang, H. Immune signature driven by ADT-induced immune microenvironment remodeling in prostate cancer is correlated with recurrence-free survival and immune infiltration. Cell Death Dis. 2020, 11, 779. [Google Scholar] [CrossRef]
- Chulpanova, D.S.; Kitaeva, K.V.; Green, A.R.; Rizvanov, A.A.; Solovyeva, V.V. Molecular Aspects and Future Perspectives of Cytokine-Based Anti-cancer Immunotherapy. Front. Cell Dev. Biol. 2020, 8, 402. [Google Scholar] [CrossRef]
- Mao, C.; Ding, Y.; Xu, N. A Double-Edged Sword Role of Cytokines in Prostate Cancer Immunotherapy. Front. Oncol. 2021, 11, 688489. [Google Scholar] [CrossRef]
- Wang, X.; Yang, L.; Huang, F.; Zhang, Q.; Liu, S.; Ma, L.; You, Z. Inflammatory cytokines IL-17 and TNF-α up-regulate PD-L1 expression in human prostate and colon cancer cells. Immunol. Lett. 2017, 176, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Wise, G.J.; Marella, V.K.; Talluri, G.; Shirazian, D. Cytokine variations in patients with hormone treated prostate cancer. J. Urol. 2000, 164, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E., III; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Kloss, C.C.; Lee, J.; Zhang, A.; Chen, F.; Melenhorst, J.J.; Lacey, S.F.; Maus, M.V.; Fraietta, J.A.; Zhao, Y.; June, C.H. Dominant-Negative TGF-β Receptor Enhances PSMA-Targeted Human CAR T Cell Proliferation and Augments Prostate Cancer Eradication. Mol. Ther. 2018, 26, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Freije, D.; Nusskern, D.R.; Okami, K.; Cairns, P.; Sidransky, D.; Isaacs, W.B.; Bova, G.S. Interfocal heterogeneity of PTEN/MMAC1 gene alterations in multiple metastatic prostate cancer tissues. Cancer Res. 1998, 58, 204–209. [Google Scholar]
- Naderali, E.; Khaki, A.A.; Rad, J.S.; Ali-Hemmati, A.; Rahmati, M.; Charoudeh, H.N. Regulation and modulation of PTEN activity. Mol. Biol. Rep. 2018, 45, 2869–2881. [Google Scholar] [CrossRef]
- Vidotto, T.; Saggioro, F.P.; Jamaspishvili, T.; Chesca, D.L.; De Albuquerque, C.G.P.; Reis, R.B.; Graham, C.H.; Berman, D.M.; Siemens, D.R.; Squire, J.; et al. PTEN-deficient prostate cancer is associated with an immunosuppressive tumor microenvironment mediated by increased expression of IDO1 and infiltrating FoxP3+ T regulatory cells. Prostate 2019, 79, 969–979. [Google Scholar] [CrossRef]
- Shen, H.; Lentsch, A.B. Progressive dysregulation of transcription factors NF-κB and STAT1 in prostate cancer cells causes proangiogenic production of CXC chemokines. Am. J. Physiol.-Cell Physiol. 2004, 286, C840–C847. [Google Scholar] [CrossRef]
- Vidotto, T.; Melo, C.M.; Castelli, E.; Koti, M.; dos Reis, R.B.; Squire, J.A. Emerging role of PTEN loss in evasion of the immune response to tumours. Br. J. Cancer 2020, 122, 1732–1743. [Google Scholar] [CrossRef]
- Chaudagar, K.; Hieromnimon, H.M.; Khurana, R.; Labadie, B.; Hirz, T.; Mei, S.; Hasan, R.; Shafran, J.; Kelley, A.; Apostolov, E.; et al. Reversal of Lactate and PD-1–mediated Macrophage Immunosuppression Controls Growth of PTEN/p53-deficient Prostate Cancer. Clin. Cancer Res. 2023, 29, 1952–1968. [Google Scholar] [CrossRef]
- He, Y.; Wang, L.; Wei, T.; Xiao, Y.-T.; Sheng, H.; Su, H.; Hollern, D.P.; Zhang, X.; Ma, J.; Wen, S.; et al. FOXA1 overexpression suppresses interferon signaling and immune response in cancer. J. Clin. Investig. 2021, 131, e147025. [Google Scholar] [CrossRef]
- Jin, H.-J.; Zhao, J.C.; Wu, L.; Kim, J.; Yu, J. Cooperativity and equilibrium with FOXA1 define the androgen receptor transcriptional program. Nat. Commun. 2014, 5, 3972. [Google Scholar] [CrossRef]
- Brea, L.T.; Wang, X.; Yu, J. Epithelial Transcription Factor FOXA1 Regulates Prostate Cancer Immune Response. J. Endocr. Soc. 2021, 5, A1017. [Google Scholar] [CrossRef]
- Ennishi, D.; Takata, K.; Béguelin, W.; Duns, G.; Mottok, A.; Farinha, P.; Bashashati, A.; Saberi, S.; Boyle, M.; Meissner, B.; et al. Molecular and Genetic Characterization of MHC Deficiency Identifies EZH2 as Therapeutic Target for Enhancing Immune Recognition. Cancer Discov. 2019, 9, 546–563. [Google Scholar] [CrossRef]
- Varambally, S.; Dhanasekaran, S.M.; Zhou, M.; Barrette, T.R.; Kumar-Sinha, C.; Sanda, M.G.; Ghosh, D.; Pienta, K.J.; Sewalt, R.G.A.B.; Otte, A.P.; et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 2002, 419, 624–629. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, W.; Xie, L.; Wang, J.; Deng, Y.; Peng, Q.; Zhai, L.; Li, S.; Qin, X. Prognostic significance of dickkopf-1 overexpression in solid tumors: A meta-analysis. Tumor Biol. 2014, 35, 3145–3154. [Google Scholar] [CrossRef]
- D’amico, L.; Mahajan, S.; Capietto, A.-H.; Yang, Z.; Zamani, A.; Ricci, B.; Bumpass, D.B.; Meyer, M.; Su, X.; Wang-Gillam, A.; et al. Dickkopf-related protein 1 (Dkk1) regulates the accumulation and function of myeloid derived suppressor cells in cancer. J. Exp. Med. 2016, 213, 827–840. [Google Scholar] [CrossRef]
- Thudi, N.K.; Martin, C.K.; Murahari, S.; Shu, S.T.; Lanigan, L.G.; Werbeck, J.L.; Keller, E.T.; McCauley, L.K.; Pinzone, J.J.; Rosol, T.J. Dickkopf-1 (DKK-1) stimulated prostate cancer growth and metastasis and inhibited bone formation in osteoblastic bone metastases. Prostate 2011, 71, 615–625. [Google Scholar] [CrossRef]
- Hall, C.L.; Zhang, H.; Baile, S.; Ljungman, M.; Kuhstoss, S.; Keller, E.T. p21CIP-1/WAF-1 induction is required to inhibit prostate cancer growth elicited by deficient expression of the Wnt inhibitor Dickkopf-1. Cancer Res. 2010, 70, 9916–9926. [Google Scholar] [CrossRef]
- Rachner, T.D.; Thiele, S.; Göbel, A.; Browne, A.; Fuessel, S.; Erdmann, K.; Wirth, M.P.; Fröhner, M.; Todenhöfer, T.W.; Muders, M.H.; et al. High serum levels of Dickkopf-1 are associated with a poor prognosis in prostate cancer patients. BMC Cancer 2014, 14, 649. [Google Scholar] [CrossRef]
- Wise, D.R.; Schneider, J.A.; Armenia, J.; Febles, V.A.; McLaughlin, B.; Brennan, R.; Thoren, K.L.; Abida, W.; Sfanos, K.S.; De Marzo, A.M.; et al. Dickkopf-1 Can Lead to Immune Evasion in Metastatic Castration-Resistant Prostate Cancer. JCO Precis. Oncol. 2020, 4, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Morishita, M.; di Luccio, E. Cancers and the NSD family of histone lysine methyltransferases. Biochim. Biophys. Acta 2011, 1816, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Guo, L.; Duan, Z.J.; Tepper, C.G.; Xue, L.; Chen, X.; Kung, H.J.; Gao, A.C.; Zou, J.X.; Chen, H.W. Histone methyltransferase NSD2/MMSET mediates constitutive NF-kappaB signaling for cancer cell proliferation, survival, and tumor growth via a feedforward loop. Mol. Cell Biol. 2012, 32, 3121–3131. [Google Scholar] [CrossRef]
- Want, M.Y.; Tsuji, T.; Singh, P.K.; Thorne, J.L.; Matsuzaki, J.; Karasik, E.; Gillard, B.; Cortes Gomez, E.; Koya, R.C.; Lugade, A.; et al. WHSC1/NSD2 regulates immune infiltration in prostate cancer. J. Immunother. Cancer 2021, 9, e001374. [Google Scholar] [CrossRef] [PubMed]
- Want, M.Y.; Karasik, E.; Gillard, B.; McGray, A.J.R.; Battaglia, S. Inhibition of WHSC1 Allows for Reprogramming of the Immune Compartment in Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 8742. [Google Scholar] [CrossRef]
- Fuertes, M.B.; Domaica, C.I.; Zwirner, N.W. Leveraging NKG2D Ligands in Immuno-Oncology. Front. Immunol. 2021, 12, 713158. [Google Scholar] [CrossRef]
- Shimasaki, N.; Jain, A.; Campana, D. NK cells for cancer immunotherapy. Nat. Rev. Drug Discov. 2020, 19, 200–218. [Google Scholar] [CrossRef]
- Pasero, C.; Gravis, G.; Granjeaud, S.; Guerin, M.; Thomassin-Piana, J.; Rocchi, P.; Salem, N.; Walz, J.; Moretta, A.; Olive, D. Highly effective NK cells are associated with good prognosis in patients with metastatic prostate cancer. Oncotarget 2015, 6, 14360–14373. [Google Scholar] [CrossRef]
- Pasero, C.; Gravis, G.; Guerin, M.; Granjeaud, S.; Thomassin-Piana, J.; Rocchi, P.; Paciencia-Gros, M.; Poizat, F.; Bentobji, M.; Azario-Cheillan, F.; et al. Inherent and Tumor-Driven Immune Tolerance in the Prostate Microenvironment Impairs Natural Killer Cell Antitumor Activity. Cancer Res. 2016, 76, 2153–2165. [Google Scholar] [CrossRef]
- Lundholm, M.; Schröder, M.; Nagaeva, O.; Baranov, V.; Widmark, A.; Mincheva-Nilsson, L.; Wikström, P. Prostate Tumor-Derived Exosomes Down-Regulate NKG2D Expression on Natural Killer Cells and CD8+ T Cells: Mechanism of Immune Evasion. PLoS ONE 2014, 9, e108925. [Google Scholar] [CrossRef]
- Horenstein, A.L.; Chillemi, A.; Zaccarello, G.; Bruzzone, S.; Quarona, V.; Zito, A.; Serra, S.; Malavasi, F. A CD38/CD203a/CD73 ectoenzymatic pathway independent of CD39 drives a novel adenosinergic loop in human T lymphocytes. Oncoimmunology 2013, 2, e26246. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Crespo, M.; Gurel, B.; Dolling, D.; Rekowski, J.; Sharp, A.; Petremolo, A.; Sumanasuriya, S.; Rodrigues, D.N.; Ferreira, A.; et al. CD38 in Advanced Prostate Cancers. Eur. Urol. 2021, 79, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Daenthanasanmak, A.; Chakraborty, P.; Wyatt, M.; Dhar, P.; Selvam, S.P.; Fu, J.; Zhang, J.; Nguyen, H.; Kang, I.; et al. CD38-NAD+Axis Regulates Immunotherapeutic Anti-Tumor T Cell Response. Cell Metab. 2018, 27, 85–100.e8. [Google Scholar] [CrossRef] [PubMed]
- Zucali, P.A.; Lin, C.-C.; Carthon, B.C.; Bauer, T.M.; Tucci, M.; Italiano, A.; Iacovelli, R.; Su, W.-C.; Massard, C.; Saleh, M.; et al. Targeting CD38 and PD-1 with isatuximab plus cemiplimab in patients with advanced solid malignancies: Results from a phase I/II open-label, multicenter study. J. Immunother. Cancer 2022, 10, e003697. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Han, H.H.; Wang, Y.; Zhang, B.; Zhou, B.; Cheng, Y.; Rumandla, A.; Gurrapu, S.; Chakraborty, G.; Su, J.; et al. The Polycomb Repressor Complex 1 Drives Double-Negative Prostate Cancer Metastasis by Coordinating Stemness and Immune Suppression. Cancer Cell 2019, 36, 139–155.e10. [Google Scholar] [CrossRef]
- Cai, X.; Xu, Y.; Cheung, A.K.; Tomlinson, R.C.; Alcazar-Roman, A.; Murphy, L.; Billich, A.; Zhang, B.; Feng, Y.; Klumpp, M.; et al. PIKfyve, a class III PI kinase, is the target of the small molecular IL-12/IL-23 inhibitor apilimod and a player in Toll-like receptor signaling. Chem. Biol. 2013, 20, 912–921. [Google Scholar] [CrossRef]
- Qiao, Y.; Choi, J.E.; Tien, J.C.; Simko, S.A.; Rajendiran, T.; Vo, J.N.; Delekta, A.D.; Wang, L.; Xiao, L.; Hodge, N.B.; et al. Autophagy inhibition by targeting PIKfyve potentiates response to immune checkpoint blockade in prostate cancer. Nat. Cancer 2021, 2, 978–993. [Google Scholar] [CrossRef]
- Laforgia, M.; Calabrò, C.; Scattone, A.; Laface, C.; Porcelli, M.; Gadaleta, C.D.; Nardulli, P.; Ranieri, G. Pharmacotherapy in Mast Cell Leukemia. Expert Opin. Pharmacother. 2020, 21, 1059–1069. [Google Scholar] [CrossRef]
- Laface, C.; Ranieri, G.; Maselli, F.M.; Ambrogio, F.; Foti, C.; Ammendola, M.; Laterza, M.; Cazzato, G.; Memeo, R.; Mastrandrea, G.; et al. Immunotherapy and the Combination with Targeted Therapies for Advanced Hepatocellular Carcinoma. Cancers 2023, 15, 654. [Google Scholar] [CrossRef]
- Rebuzzi, S.E.; Rescigno, P.; Catalano, F.; Mollica, V.; Vogl, U.M.; Marandino, L.; Massari, F.; Mestre, R.P.; Zanardi, E.; Signori, A.; et al. Immune Checkpoint Inhibitors in Advanced Prostate Cancer: Current Data and Future Perspectives. Cancers 2022, 14, 1245. [Google Scholar] [CrossRef]
- Rizzo, A.; Mollica, V.; Cimadamore, A.; Santoni, M.; Scarpelli, M.; Giunchi, F.; Cheng, L.; Lopez-Beltran, A.; Fiorentino, M.; Montironi, R.; et al. Is There a Role for Immunotherapy in Prostate Cancer? Cells 2020, 9, 2051. [Google Scholar] [CrossRef] [PubMed]
- Small, E.J.; Tchekmedyian, N.S.; Rini, B.I.; Fong, L.; Lowy, I.; Allison, J.P. A Pilot Trial of CTLA-4 Blockade with Human Anti-CTLA-4 in Patients with Hormone-Refractory Prostate Cancer. Clin. Cancer Res. 2007, 13, 1810–1815. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Kwon, E.D.; Drake, C.G.; Fizazi, K.; Logothetis, C.; Gravis, G.; Ganju, V.; Polikoff, J.; Saad, F.; Humanski, P.; et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients with Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2017, 35, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.D.; Drake, C.G.; Scher, H.I.; Fizazi, K.; Bossi, A.; Van den Eertwegh, A.J.M.; Krainer, M.; Houede, N.; Santos, R.; Mahammedi, H.; et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014, 15, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.R.; Massard, C.; Ott, P.A.; Haas, N.B.; Lopez, J.S.; Ejadi, S.; Wallmark, J.M.; Keam, B.; Delord, J.-P.; Aggarwal, R.; et al. Pembrolizumab for advanced prostate adenocarcinoma: Findings of the KEYNOTE-028 study. Ann. Oncol. 2018, 29, 1807–1813. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Piulats, J.M.; Gross-Goupil, M.; Goh, J.; Ojamaa, K.; Hoimes, C.J.; Vaishampayan, U.; Berger, R.; Sezer, A.; Alanko, T.; et al. Pembrolizumab for Treatment-Refractory Metastatic Castration-Resistant Prostate Cancer: Multicohort, Open-Label Phase II KEYNOTE-199 Study. J. Clin. Oncol. 2020, 38, 395–405. [Google Scholar] [CrossRef]
- Robinson, D.; Van Allen, E.M.; Wu, Y.-M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.-M.; Montgomery, B.; Taplin, M.-E.; Pritchard, C.C.; Attard, G.; et al. Integrative Clinical Genomics of Advanced Prostate Cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef]
- Dias, A.; Kote-Jarai, Z.; Mikropoulos, C.; Eeles, R. Prostate Cancer Germline Variations and Implications for Screening and Treatment. Cold Spring Harb. Perspect. Med. 2017, 8, a030379. [Google Scholar] [CrossRef]
- Mouw, K.W.; Goldberg, M.S.; Konstantinopoulos, P.A.; D’Andrea, A.D. DNA Damage and Repair Biomarkers of Immunotherapy Response. Cancer Discov. 2017, 7, 675–693. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Loriot, Y.; Shaffer, D.R.; Braiteh, F.; Powderly, J.; Harshman, L.C.; Conkling, P.; Delord, J.-P.; Gordon, M.; Kim, J.W.; et al. Safety and Clinical Activity of Atezolizumab in Patients with Metastatic Castration-Resistant Prostate Cancer: A Phase I Study. Clin. Cancer Res. 2021, 27, 3360–3369. [Google Scholar] [CrossRef]
- Heery, C.R.; O’Sullivan-Coyne, G.; Madan, R.A.; Cordes, L.; Rajan, A.; Rauckhorst, M.; Lamping, E.; Oyelakin, I.; Marté, J.L.; Lepone, L.M.; et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): A phase 1a, multicohort, dose-escalation trial. Lancet Oncol. 2017, 18, 587–598. [Google Scholar] [CrossRef]
- Fakhrejahani, F.; Amrit Madan, R.; Dahut, W.L.; Karzai, F.; Cordes, L.M.; Schlom, J.; Gulley, J.L. Avelumab in metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2017, 35 (Suppl. S6), 159. [Google Scholar] [CrossRef]
- Venkatachalam, S.; McFarland, T.R.; Agarwal, N.; Swami, U. Immune Checkpoint Inhibitors in Prostate Cancer. Cancers 2021, 13, 2187. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef]
- Kobiyama, K.; Jounai, N.; Aoshi, T.; Tozuka, M.; Takeshita, F.; Coban, C.; Ishiii, K.J. Innate immune signaling by, and genetic adjuvants for DNA vaccination. Vaccines 2013, 1, 278–292. [Google Scholar] [CrossRef]
- Hafron, J.M.; Wilfehrt, H.M.; Ferro, C.; Harmon, M.; Flanders, S.C.; McKay, R.R. Real-World Effectiveness of Sipuleucel-T on Overall Survival in Men with Advanced Prostate Cancer Treated with Androgen Receptor-Targeting Agents. Adv. Ther. 2022, 39, 2515–2532. [Google Scholar] [CrossRef]
- Dorff, T.; Hirasawa, Y.; Acoba, J.; Pagano, I.; Tamura, D.; Pal, S.; Zhang, M.; Waitz, R.; Dhal, A.; Haynes, W.; et al. Phase Ib study of patients with metastatic castrate-resistant prostate cancer treated with different sequencing regimens of atezolizumab and sipuleucel-T. J. Immunother. Cancer 2021, 9, e002931. [Google Scholar] [CrossRef]
- Marshall, C.H.; Fu, W.; Wang, H.; Park, J.C.; DeWeese, T.L.; Tran, P.T.; Song, D.Y.; King, S.; Afful, M.; Hurrelbrink, J.; et al. Randomized Phase II Trial of Sipuleucel-T with or without Radium-223 in Men with Bone-metastatic Castration-resistant Prostate Cancer. Clin. Cancer Res. 2021, 27, 1623–1630. [Google Scholar] [CrossRef]
- Simons, J.; Sacks, N. Granulocyte-macrophage colony-stimulating factor−transduced allogeneic cancer cellular immunotherapy: The GVAX® vaccine for prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2006, 24, 419–424. [Google Scholar] [CrossRef]
- Gamat-Huber, M.; Jeon, D.; Johnson, L.E.; Moseman, J.E.; Muralidhar, A.; Potluri, H.K.; Rastogi, I.; Wargowski, E.; Zahm, C.D.; McNeel, D.G. Treatment Combinations with DNA Vaccines for the Treatment of Metastatic Castration-Resistant Prostate Cancer (mCRPC). Cancers 2020, 12, 2831. [Google Scholar] [CrossRef]
- Obradovic, A.Z.; Dallos, M.C.; Zahurak, M.L.; Partin, A.W.; Schaeffer, E.M.; Ross, A.E.; Allaf, M.E.; Nirschl, T.R.; Liu, D.; Chapman, C.G.; et al. T-Cell Infiltration and Adaptive Treg Resistance in Response to Androgen Deprivation with or Without Vaccination in Localized Prostate Cancer. Clin. Cancer Res. 2020, 26, 3182–3192. [Google Scholar] [CrossRef] [PubMed]
- Gulley, J.L.; Madan, R.A.; Tsang, K.Y.; Jochems, C.; Marté, J.L.; Farsaci, B.; Tucker, J.A.; Hodge, J.W.; Liewehr, D.J.; Steinberg, S.M.; et al. Immune Impact Induced by PROSTVAC (PSA-TRICOM), a Therapeutic Vaccine for Prostate Cancer. Cancer Immunol. Res. 2014, 2, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Kantoff, P.W.; Gulley, J.L.; Pico-Navarro, C. Revised overall survival analysis of a phase II, randomized, double-blind, controlled study of PROSTVAC in men with metastatic castrationresistant prostate cancer. Physiol. Behav. 2016, 176, 100–106. [Google Scholar]
- Gulley, J.L.; Borre, M.; Vogelzang, N.J.; Ng, S.; Agarwal, N.; Parker, C.C.; Pook, D.; Rathenborg, P.; Flaig, T.W.; Carles, J.; et al. Phase III Trial of PROSTVAC in Asymptomatic or Minimally Symptomatic Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2019, 37, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Stenzl, A. Re: Phase III Trial of PROSTVAC in Asymptomatic or Minimally Symptomatic Metastatic Castration-resistant Prostate Cancer. Eur. Urol. 2020, 77, 131–132. [Google Scholar] [CrossRef]
- Mukherjee, A.G.; Wanjari, U.R.; Prabakaran, D.S.; Ganesan, R.; Renu, K.; Dey, A.; Vellingiri, B.; Kandasamy, S.; Ramesh, T.; Gopalakrishnan, A.V. The Cellular and Molecular Immunotherapy in Prostate Cancer. Vaccines 2022, 10, 1370. [Google Scholar] [CrossRef]
- Kyriakopoulos, C.E.; Eickhoff, J.C.; Ferrari, A.C.; Schweizer, M.T.; Wargowski, E.; Olson, B.M.; McNeel, D.G. Multicenter phase i trial of a DNA vaccine encoding the androgen receptor ligand-binding domain (pTVG-AR, MVI-118) in Patients with metastatic prostate cancer. Clin. Cancer Res. 2020, 26, 5162–5171. [Google Scholar] [CrossRef]
- Shore, N.D.; Morrow, M.P.; McMullan, T.; Kraynyak, K.A.; Sylvester, A.; Bhatt, K.; Cheung, J.; Boyer, J.D.; Liu, L.; Sacchetta, B.; et al. CD8+ T Cells Impact Rising PSA in Biochemically Relapsed Cancer Patients Using Immunotherapy Targeting Tumor-Associated Antigens. Mol. Ther. 2020, 28, 1238–1250. [Google Scholar] [CrossRef]
- Mehrabadi, A.Z.; Ranjbar, R.; Farzanehpour, M.; Shahriary, A.; Dorostkar, R.; Hamidinejad, M.A.; Ghaleh, H.E.G. Therapeutic potential of CAR T cell in malignancies: A scoping review. Biomed. Pharmacother. 2021, 146, 112512. [Google Scholar] [CrossRef]
- Bębnowska, D.; Grywalska, E.; Niedźwiedzka-Rystwej, P.; Sosnowska-Pasiarska, B.; Smok-Kalwat, J.; Pasiarski, M.; Góźdź, S.; Roliński, J.; Polkowski, W. CAR-T Cell Therapy—An Overview of Targets in Gastric Cancer. J. Clin. Med. 2020, 9, 1894. [Google Scholar] [CrossRef]
- Narayan, V.; Barber-Rotenberg, J.S.; Jung, I.-Y.; Lacey, S.F.; Rech, A.J.; Davis, M.M.; Hwang, W.-T.; Lal, P.; Carpenter, E.L.; Maude, S.L.; et al. PSMA-targeting TGFβ-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: A phase 1 trial. Nat. Med. 2022, 28, 724–734. [Google Scholar] [CrossRef]
- Alkhouli, M.A.; Bazargan, S.; Pilon-Thomas, S.; Poch, M.; Chahoud, J. Current State of Cell Therapies for Genitourinary Malignancies. Cancer J. 2022, 28, 294–300. [Google Scholar] [CrossRef]
- Hummel, H.D.; Kufer, P.; Grüllich, C.; SeggewissBernhardt, R.; Deschler-Baier, B.; Chatterjee, M.; Goebeler, M.E.; Miller, K.; de Santis, M.; Loidl, W.; et al. Pasotuxizumab, a BiTE® immune therapy for castration-resistant prostate cancer: Phase I, dose-escalation study findings. Immunotherapy 2021, 13, 125–141. [Google Scholar] [CrossRef]
- Deegen, P.; Thomas, O.; Nolan-Stevaux, O.; Li, S.; Wahl, J.; Bogner, P.; Aeffner, F.; Friedrich, M.; Liao, M.Z.; Matthes, K.; et al. The PSMA-targeting Half-life Extended BiTE Therapy AMG 160 has Potent Antitumor Activity in Preclinical Models of Metastatic Castration-resistant Prostate Cancer. Clin. Cancer Res. 2021, 27, 2928–2937. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Park, J.A.; Long, A.; Guo, H.-F.; Cheung, N.-K.V. Novel potent anti-STEAP1 bispecific antibody to redirect T cells for cancer immunotherapy. J. Immunother. Cancer 2021, 9, e003114. [Google Scholar] [CrossRef]
- Dorff, T.B.; Narayan, V.; Forman, S.J.; Zang, P.D.; Fraietta, J.A.; June, C.H.; Haas, N.B.; Priceman, S.J. Novel Redirected T–Cell Immunotherapies for Advanced Prostate Cancer. Clin. Cancer Res. 2022, 28, 576–584. [Google Scholar] [CrossRef]
- McNeel, D.G.; Smith, H.A.; Eickhoff, J.C.; Lang, J.M.; Staab, M.J.; Wilding, G.; Liu, G. Phase I trial of tremelimumab in combination with short-term androgen deprivation in patients with PSA-recurrent prostate cancer. Cancer Immunol. Immunother. 2011, 61, 1137–1147. [Google Scholar] [CrossRef]
- Graff, J.N.; Beer, T.M.; Alumkal, J.J.; Slottke, R.E.; Redmond, W.L.; Thomas, G.V.; Thompson, R.F.; Wood, M.A.; Koguchi, Y.; Chen, Y.; et al. A phase II single-arm study of pembrolizumab with enzalutamide in men with metastatic castration-resistant prostate cancer progressing on enzalutamide alone. J. Immunother. Cancer 2020, 8, e000642. [Google Scholar] [CrossRef]
- Powles, T.; Yuen, K.C.; Gillessen, S.; Kadel, E.E., 3rd; Rathkopf, D.; Matsubara, N.; Drake, C.G.; Fizazi, K.; Piulats, J.M.; Wysocki, P.J.; et al. Atezolizumab with enzalutamide versus enzalutamide alone in metastatic castration-resistant prostate cancer: A randomized phase 3 trial. Nat. Med. 2022, 28, 144–153. [Google Scholar] [CrossRef]
- Graff, J.N.; Liang, L.W.; Kim, J.; Stenzl, A. KEYNOTE-641: A Phase III study of pembrolizumab plus enzalutamide for metastatic castration-resistant prostate cancer. Futur. Oncol. 2021, 17, 3017–3026. [Google Scholar] [CrossRef]
- Ross, A.E.; Hurley, P.J.; Tran, P.T.; Rowe, S.P.; Benzon, B.; Neal, T.O.; Chapman, C.; Harb, R.; Milman, Y.; Trock, B.J.; et al. A pilot trial of pembrolizumab plus prostatic cryotherapy for men with newly diagnosed oligometastatic hormone-sensitive prostate cancer. Prostate Cancer Prostatic Dis. 2020, 23, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Slovin, S.; Higano, C.; Hamid, O.; Tejwani, S.; Harzstark, A.; Alumkal, J.; Scher, H.; Chin, K.; Gagnier, P.; McHenry, M.; et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: Results from an open-label, multicenter phase I/II study. Ann. Oncol. 2013, 24, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Fong, L.; Morris, M.J.; Sartor, O.; Higano, C.S.; Pagliaro, L.; Alva, A.; Appleman, L.J.; Tan, W.; Vaishampayan, U.; Porcu, R.; et al. A Phase Ib Study of Atezolizumab with Radium-223 Dichloride in Men with Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2021, 27, 4746–4756. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Drake, C.G.; Beer, T.M.; Kwon, E.D.; Scher, H.I.; Gerritsen, W.R.; Bossi, A.; Eertwegh, A.J.V.D.; Krainer, M.; Houede, N.; et al. Final Analysis of the Ipilimumab Versus Placebo Following Radiotherapy Phase III Trial in Postdocetaxel Metastatic Castration-resistant Prostate Cancer Identifies an Excess of Long-term Survivors. Eur. Urol. 2020, 78, 822–830. [Google Scholar] [CrossRef]
- Yu, E.Y.; Kolinsky, M.P.; Berry, W.R.; Retz, M.; Mourey, L.; Piulats, J.M.; Appleman, L.J.; Romano, E.; Gravis, G.; Gurney, H.; et al. Pembrolizumab Plus Docetaxel and Prednisone in Patients with Metastatic Castration-resistant Prostate Cancer: Long-term Results from the Phase 1b/2 KEYNOTE-365 Cohort B Study. Eur. Urol. 2022, 82, 22–30. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Ratta, R.; Gafanov, R.; Facchini, G.; Piulats, J.M.; Kramer, G.; Flaig, T.W.; Chandana, S.R.; Li, B.; Burgents, J.; et al. KEYNOTE-921: Phase III study of pembrolizumab plus docetaxel for metastatic castration-resistant prostate cancer. Futur. Oncol. 2021, 17, 3291–3299. [Google Scholar] [CrossRef]
- Palicelli, A.; Croci, S.; Bisagni, A.; Zanetti, E.; De Biase, D.; Melli, B.; Sanguedolce, F.; Ragazzi, M.; Zanelli, M.; Chaux, A.; et al. What Do We Have to Know about PD-L1 Expression in Prostate Cancer? A Systematic Literature Review (Part 6): Correlation of PD-L1 Expression with the Status of Mismatch Repair System, BRCA, PTEN, and Other Genes. Biomedicines 2022, 10, 236. [Google Scholar] [CrossRef]
- Reisländer, T.; Lombardi, E.P.; Groelly, F.J.; Miar, A.; Porru, M.; Di Vito, S.; Wright, B.; Lockstone, H.; Biroccio, A.; Harris, A.; et al. BRCA2 abrogation triggers innate immune responses potentiated by treatment with PARP inhibitors. Nat. Commun. 2019, 10, 3143. [Google Scholar] [CrossRef]
- Shen, J.; Zhao, W.; Ju, Z.; Wang, L.; Peng, Y.; Labrie, M.; Yap, T.A.; Mills, G.B.; Peng, G. PARPi Triggers the STING-Dependent Immune Response and Enhances the Therapeutic Efficacy of Immune Checkpoint Blockade Independent of BRCAness. Cancer Res. 2019, 79, 311–319. [Google Scholar] [CrossRef]
- Chabanon, R.M.; Muirhead, G.; Krastev, D.B.; Adam, J.; Morel, D.; Garrido, M.; Lamb, A.; Hénon, C.; Dorvault, N.; Rouanne, M.; et al. PARP inhibition enhances tumor cell–intrinsic immunity in ERCC1-deficient non–small cell lung cancer. J. Clin. Investig. 2019, 129, 1211–1228. [Google Scholar] [CrossRef]
- Jiao, S.; Xia, W.; Yamaguchi, H.; Wei, Y.; Chen, M.K.; Hsu, J.M.; Hsu, J.L.; Yu, W.H.; Du, Y.; Lee, H.H.; et al. PARP Inhibitor Upregulates PD-L1 Expression and Enhances Cancer-Associated Immunosuppression. Clin. Cancer Res. 2017, 23, 3711–3720. [Google Scholar] [CrossRef]
- Sato, H.; Niimi, A.; Yasuhara, T.; Permata, T.B.M.; Hagiwara, Y.; Isono, M.; Nuryadi, E.; Sekine, R.; Oike, T.; Kakoti, S.; et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat. Commun. 2017, 8, 1751. [Google Scholar] [CrossRef]
- Fay, A.P.; Antonarakis, E.S. Blocking the PD-1/PD-L1 axis in advanced prostate cancer: Are we moving in the right direction? Ann. Transl. Med. 2019, 7, S7. [Google Scholar] [CrossRef]
- Gallyas, F., Jr.; Sumegi, B.; Szabo, C. Role of Akt Activation in PARP Inhibitor Resistance in Cancer. Cancers 2020, 12, 532. [Google Scholar] [CrossRef]
- Juvekar, A.; Burga, L.N.; Hu, H.; Lunsford, E.P.; Ibrahim, Y.H.; Balmaña, J.; Rajendran, A.; Papa, A.; Spencer, K.; Lyssiotis, C.A.; et al. Combining a PI3K Inhibitor with a PARP Inhibitor Provides an Effective Therapy for BRCA1-Related Breast Cancer. Cancer Discov. 2012, 2, 1048–1063. [Google Scholar] [CrossRef]
- de Porras, V.R.; Pardo, J.C.; Notario, L.; Etxaniz, O.; Font, A. Immune Checkpoint Inhibitors: A Promising Treatment Option for Metastatic Castration-Resistant Prostate Cancer? Int. J. Mol. Sci. 2021, 22, 4712. [Google Scholar] [CrossRef]
- Yu, E.Y.; Massard, C.; Retz, M.; Tafreshi, A.; Galceran, J.C.; Hammerer, P.; Fong, P.C.C.; Shore, N.D.; Joshua, A.; Linch, M.D.; et al. Keynote-365 cohort a: Pembrolizumab (pembro) plus olaparib in docetaxel-pretreated patients (pts) with metastatic castrate-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2019, 37, 145. [Google Scholar] [CrossRef]
- Yu, E.Y.; Piulats, J.M.; Gravis, G.; Laguerre, B.; Arija, J.A.A.; Oudard, S.; Fong, P.C.; Kolinsky, M.P.; Augustin, M.; Feyerabend, S.; et al. KEYNOTE-365 cohort A updated results: Pembrolizumab (pembro) plus olaparib in docetaxel-pretreated patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2020, 38, 100. [Google Scholar] [CrossRef]
- Karzai, F.; VanderWeele, D.; Madan, R.A.; Owens, H.; Cordes, L.M.; Hankin, A.; Couvillon, A.; Nichols, E.; Bilusic, M.; Beshiri, M.; et al. Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations. J. Immunother. Cancer 2018, 6, 141. [Google Scholar] [CrossRef]
- Durvalumab and Olaparib for the Treatment of Prostate Cancer in Men Predicted to Have a High Neoantigen Load. (NCT04336943). Available online: https://clinicaltrials.gov/ct2/show/NCT04336943 (accessed on 5 March 2023).
- Pachynski, R.K.; Retz, M.; Goh, J.C.; Burotto, M.; Gravis, G.; Castellano, D.; Flechon, A.; Zschaebitz, S.; Shaffer, D.R.; Limon, J.C.V.; et al. CheckMate 9KD cohort A1 final analysis: Nivolumab (NIVO) + rucaparib for post-chemotherapy (CT) metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2021, 39, 5044. [Google Scholar] [CrossRef]
- Laface, C.; Fedele, P.; Maselli, F.M.; Ambrogio, F.; Foti, C.; Molinari, P.; Ammendola, M.; Lioce, M.; Ranieri, G. Targeted Therapy for Hepatocellular Carcinoma: Old and New Opportunities. Cancers 2022, 14, 4028. [Google Scholar] [CrossRef] [PubMed]
- Cabazitaxel, Carboplatin, and Cetrelimab Followed by Niraparib with or without Cetrelimab for the Treatment of Aggressive Variant Metastatic Prostate Cancer. (NCT04592237). Available online: https://clinicaltrials.gov/ct2/show/NCT04592237 (accessed on 6 March 2023).
- Caruso, C. Anti-PD-1-CTLA4 Combo Hits Prostate Cancer. Cancer Discov. 2019, 9, 569–570. [Google Scholar] [CrossRef]
- Shenderov, E.; Boudadi, K.; Fu, W.; Wang, H.; Sullivan, R.; Jordan, A.; Dowling, D.; Harb, R.; Schonhoft, J.; Jendrisak, A.; et al. Nivolumab plus ipilimumab, with or without enzalutamide, in AR-V7-expressing metastatic castration-resistant prostate cancer: A phase-2 nonrandomized clinical trial. Prostate 2021, 81, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Coyne, G.O.; Jochems, C.; Heery, C.; Singh, H.; Surolia, I.; Riley, R.; Madan, R.; Dahut, W.; Steinberg, S.; Gulley, J.; et al. A combination trial of vaccine plus ipilimumab in metastatic castration-resistant prostate cancer patients: Immune correlates. Cancer Immunol. Immunother. 2014, 63, 407–418. [Google Scholar] [CrossRef]
- McNeel, D.G.; Eickhoff, J.C.; Wargowski, E.; Johnson, L.E.; Kyriakopoulos, C.E.; Emamekhoo, H.; Lang, J.M.; Brennan, M.J.; Liu, G. Phase 2 trial of T-cell activation using MVI-816 and pembrolizumab in patients with metastatic, castration-resistant prostate cancer (mCRPC). J. Immunother. Cancer 2022, 10, e004198. [Google Scholar] [CrossRef]
- McNeel, D.G.; Eickhoff, J.C.; Johnson, L.E.; Roth, A.R.; Perk, T.G.; Fong, L.; Antonarakis, E.S.; Wargowski, E.; Jeraj, R.; Liu, G. Phase II trial of a DNA vaccine encoding prostatic acid phosphatase (pTVG-HP [MVI-816]) in patients with progressive, nonmetastatic, castration-sensitive prostate cancer. J. Clin. Oncol. 2019, 37, 3507–3517. [Google Scholar] [CrossRef]
- Stein, M.N.; Fong, L.; Tutrone, R.; Mega, A.; Lam, E.T.; Parsi, M.; Vangala, S.; Gutierrez, A.A.; Haas, N.B. ADXS31142 Immunotherapy ± Pembrolizumab Treatment for Metastatic Castration-Resistant Prostate Cancer: Open-Label Phase I/II KEYNOTE-046 Study. Oncologist 2022, 27, 453–461. [Google Scholar] [CrossRef]
- Xu, P.; Wasielewski, L.J.; Yang, J.C.; Cai, D.; Evans, C.P.; Murphy, W.J.; Liu, C. The Immunotherapy and Immunosuppressive Signaling in Therapy-Resistant Prostate Cancer. Biomedicines 2022, 10, 1778. [Google Scholar] [CrossRef]
- Fong, L.; Kwek, S.S.; O’Brien, S.; Kavanagh, B.; McNeel, D.G.; Weinberg, V.; Lin, A.M.; Rosenberg, J.; Ryan, C.J.; Rini, B.I.; et al. Potentiating Endogenous Antitumor Immunity to Prostate Cancer through Combination Immunotherapy with CTLA4 Blockade and GM-CSF. Cancer Res. 2009, 69, 609–615. [Google Scholar] [CrossRef]
- Hegde, A.; Jayaprakash, P.; Couillault, C.A.; Piha-Paul, S.; Karp, D.; Rodon, J.; Pant, S.; Fu, S.; Dumbrava, E.E.; Yap, T.A.; et al. A Phase I Dose-Escalation Study to Evaluate the Safety and Tolerability of Evofosfamide in Combination with Ipilimumab in Advanced Solid Malignancies. Clin. Cancer Res. 2021, 27, 3050–3060. [Google Scholar] [CrossRef]
- Majidpoor, J.; Mortezaee, K. The efficacy of PD-1/PD-L1 blockade in cold cancers and future perspectives. Clin. Immunol. 2021, 226, 108707. [Google Scholar] [CrossRef]
- Jafari, S.; Molavi, O.; Kahroba, H.; Hejazi, M.S.; Maleki-Dizaji, N.; Barghi, S.; Kiaie, S.H.; Jadidi-Niaragh, F. Clinical application of immune checkpoints in targeted immunotherapy of prostate cancer. Cell. Mol. Life Sci. 2020, 77, 3693–3710. [Google Scholar] [CrossRef]
- Schöffski, P.; Tan, D.S.W.; Martín, M.; Ochoa-De-Olza, M.; Sarantopoulos, J.; Carvajal, R.D.; Kyi, C.; Esaki, T.; Prawira, A.; Akerley, W.; et al. Phase I/II study of the LAG-3 inhibitor ieramilimab (LAG525) ± anti-PD-1 spartalizumab (PDR001) in patients with advanced malignancies. J. Immunother. Cancer 2022, 10, e003776. [Google Scholar] [CrossRef]
- Schuhmacher, J.; Heidu, S.; Balchen, T.; Richardson, J.R.; Schmeltz, C.; Sonne, J.; Schweiker, J.; Rammensee, H.G.; Thor Straten, P.; Røder, M.A.; et al. Vaccination against RhoC induces long-lasting immune responses in patients with prostate cancer: Results from a phase I/II clinical trial. J. Immuno Ther. Cancer 2020, 8, e001157. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, M.; Du, Y.; Liu, X.; Weng, X.; Li, C. 4-1BBL has a Possible Role in Mediating Castration-Resistant Conversion of Prostate Cancer via Up-Regulation of Androgen Receptor. J. Cancer 2019, 10, 2464–2471. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, L.; Zhang, H.; Chen, S.; Xiao, Y. CAR-T Cell Therapy in Hematological Malignancies: Current Opportunities and Challenges. Front. Immunol. 2022, 13, 927153. [Google Scholar] [CrossRef]
- Laforgia, M.; Laface, C.; Calabrò, C.; Ferraiuolo, S.; Ungaro, V.; Tricarico, D.; Gadaleta, C.D.; Nardulli, P.; Ranieri, G. Peripheral Neuropathy under Oncologic Therapies: A Literature Review on Pathogenetic Mechanisms. Int. J. Mol. Sci. 2021, 22, 1980. [Google Scholar] [CrossRef]
- Holko, P.; Kawalec, P. Economic evaluation of sipuleucel-T immunotherapy in castration-resistant prostate cancer. Expert Rev. Anticancer. Ther. 2014, 14, 63–73. [Google Scholar] [CrossRef]
- Di Bello, F.; Di Mauro, E.; Ruvolo, C.C.; Creta, M.; La Rocca, R.; Celentano, G.; Capece, M.; Napolitano, L.; Fraia, A.; Pezone, G.; et al. Immunotherapy for Urological Tumors on YouTubeTM: An Information-Quality Analysis. Vaccines 2022, 11, 92. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).