High TLR6 Expression Status Predicts a More Favorable Prognosis after Esophagectomy for Locally Advanced Thoracic Esophageal Squamous Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Esophagectomy
2.3. ESCC Tissue Microarray
2.4. Immunohistochemistry (IHC)
2.5. Cell Lines
2.6. Cell Proliferation Assay
2.7. Statistical Analysis
3. Results
3.1. Immunohistochemical Analysis of TLR6 Expression
3.2. TLR6 Expression Status and 5-Year OS and SDD in 177 ESCC Patients
3.3. TLR6 Expression Status Is a Prognostic Factor Affecting 5-Year OS
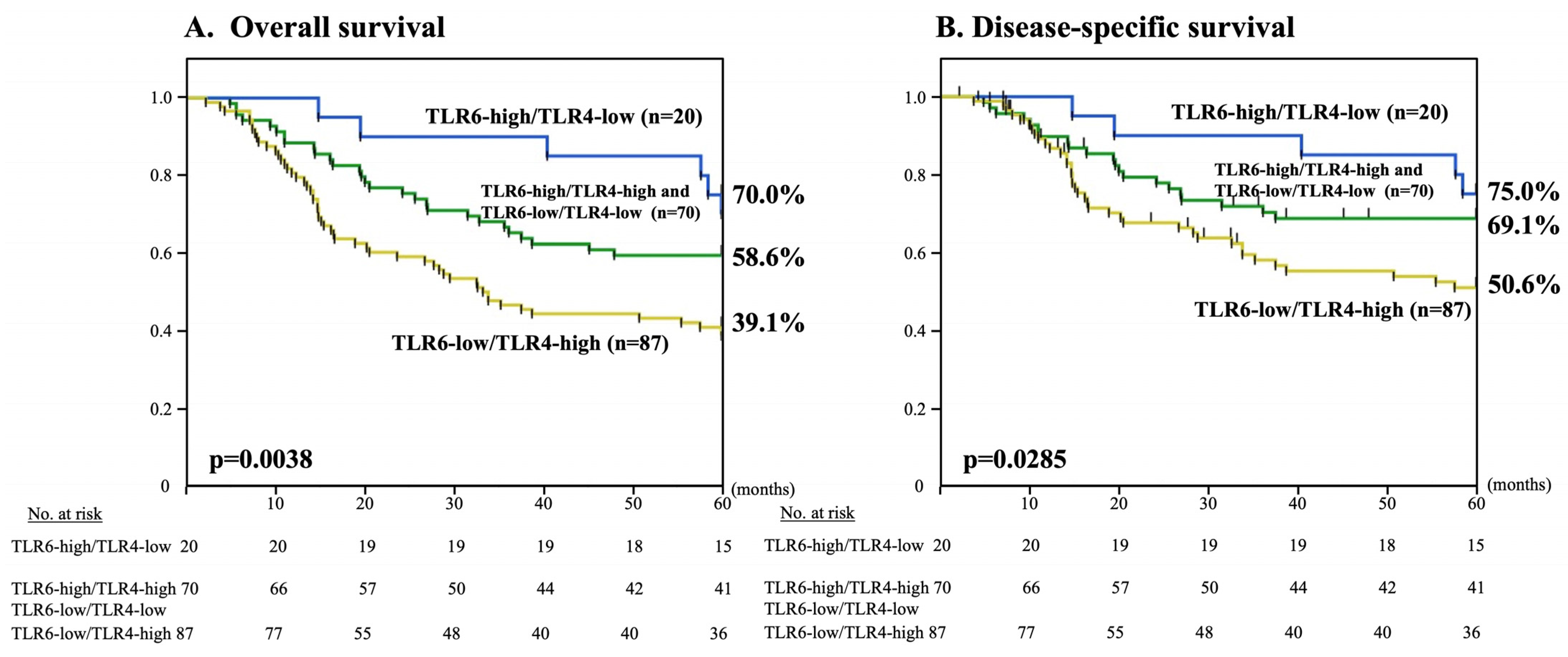
3.4. Combined TLR6 and TLR4 Expression Statuses and 5-Year OS and DSS
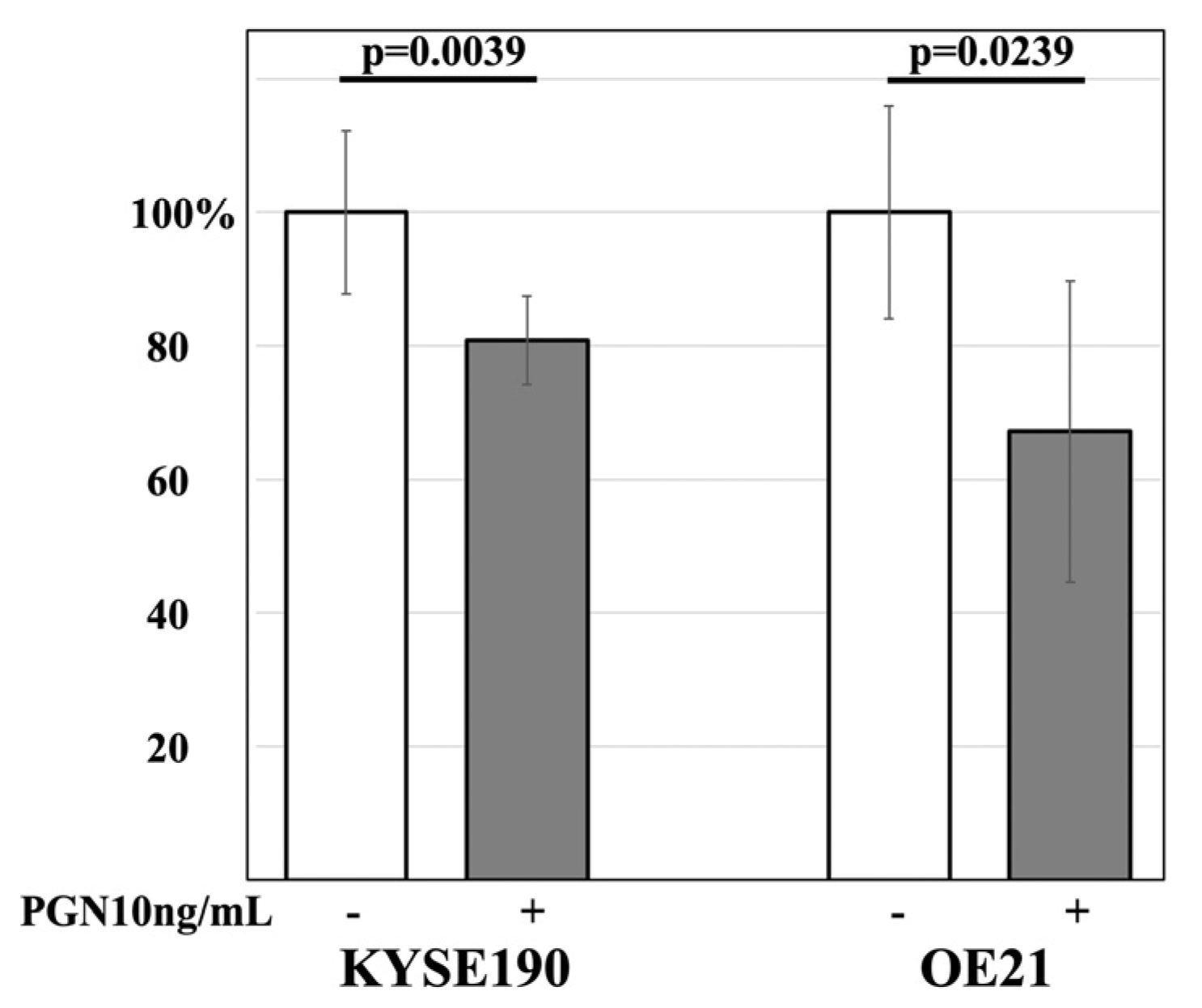
3.5. The Effect of PGN on the Cell Proliferation Activity of ESCC Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer K Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Rustgi, A.K.; El-Serag, H.B. Esophageal carcinoma. N. Engl. J. Med. 2014, 371, 2499–2509. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Soerjomataram, I.; Ferlay, J.; Forman, D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015, 64, 381–387. [Google Scholar] [CrossRef] [PubMed]
- McCormack, V.A.; Menya, D.; Munishi, M.O.; Dzamalala, C.; Gasmelseed, N.; Leon Roux, M.; Assefa, M.; Osano, O.; Watts, M.; Mwasamwaja, A.O.; et al. Informing etiologic research priorities for squamous cell esophageal cancer in Africa: A review of setting-specific exposures to known and putative risk factors. Int. J. Cancer 2017, 140, 259–271. [Google Scholar] [CrossRef]
- Ahrens, W.; Pohlabeln, H.; Foraita, R.; Nelis, M.; Lagiou, P.; Lagiou, A.; Bouchardy, C.; Slamova, A.; Schejbalova, M.; Merletti, F.; et al. Oral health, dental care and mouthwash associated with upper aerodigestive tract cancer risk in Europe: The ARCAGE study. Oral Oncol. 2014, 50, 616–625. [Google Scholar] [CrossRef]
- Baba, Y.; Iwatsuki, M.; Yoshida, N.; Watanabe, M.; Baba, H. Review of the gut microbiome and esophageal cancer: Pathogenesis and potential clinical implications. Ann. Gastroenterol. Surg. 2017, 1, 99–104. [Google Scholar] [CrossRef]
- Gao, S.; Li, S.; Ma, Z.; Liang, S.; Shan, T.; Zhang, M.; Zhu, X.; Zhang, P.; Liu, G.; Zhou, F.; et al. Presence of Porphyromonas gingivalis in esophagus and its association with the clinicopathological characteristics and survival in patients with esophageal cancer. Infect. Agents Cancer 2016, 11, 3. [Google Scholar] [CrossRef]
- Yamamura, K.; Baba, Y.; Nakagawa, S.; Mima, K.; Miyake, K.; Nakamura, K.; Sawayama, H.; Kinoshita, K.; Ishimoto, T.; Iwatsuki, M.; et al. Human microbiome Fusobacterium nucleatum in esophageal cancer tissue is associated with prognosis. Clin. Cancer Res. 2016, 22, 5574–5581. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Sato, Y.; Goto, Y.; Narita, N.; Hoon, D.S. Cancer Cells Expressing Toll-like Receptors and the Tumor Microenvironment. Cancer Microenviron. 2009, 2 (Suppl. S1), 205–214. [Google Scholar] [CrossRef]
- Holt, S.C.; Ebersole, J.; Felton, J.; Brunsvold, M.; Kornman, K.S. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science 1988, 239, 55–57. [Google Scholar] [CrossRef]
- Socransky, S.S. Microbiology of periodontal disease—Present status and future considerations. J. Periodontol. 1977, 48, 497–504. [Google Scholar] [CrossRef]
- Sato, Y.; Motoyama, S.; Wakita, A.; Kawakita, Y.; Liu, J.; Nagaki, Y.; Nanjo, H.; Ito, S.; Terata, K.; Imai, K.; et al. High TLR4 expression predicts a poor prognosis after esophagectomy for advanced thoracic esophageal squamous cell carcinoma. Esophagus 2020, 17, 408–416. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, S.; Shi, J.; Xie, Q.; Li, N.; Guan, J.; Evivie, S.E.; Liu, F.; Li, B.; Huo, G. Effects of Lactobacillus acidophilus KLDS1.0901 on Proliferation and Apoptosis of Colon Cancer Cells. Front. Microbiol. 2022, 12, 788040. [Google Scholar] [CrossRef]
- Botta, C.; Spyridopoulou, K.; Bertolino, M.; Rantsiou, K.; Chlichlia, K.; Cocolin, L. Lactiplantibacillus plantarum inhibits colon cancer cell proliferation as function of its butyrogenic capability. Biomed. Pharmacother. 2022, 149, 112755. [Google Scholar] [CrossRef]
- Zhang, L.; Yi, H. Potential antitumor and anti-inflammatory activities of an extracellular polymeric substance (EPS) from Bacillus subtilis isolated from a housefly. Sci. Rep. 2022, 12, 1383. [Google Scholar] [CrossRef]
- Huhta, H.; Helminen, O.; Lehenkari, P.P.; Saarnio, J.; Karttunen, T.J.; Kauppila, J.H. Toll-like receptors 1, 2, 4 and 6 in esophageal epithelium, Barrett’s esophagus, dysplasia and adenocarcinoma. Oncotarget 2016, 7, 23658–23667. [Google Scholar] [CrossRef]
- Sato, Y.; Motoyama, S.; Nanjo, H.; Ito, S.; Yoshino, K.; Sasaki, T.; Kuribayashi, K.; Nagaki, Y.; Imai, K.; Saito, H.; et al. REG1A Expression Status Suggests Chemosensitivity among Advanced Thoracic Esophageal Squamous Cell Carcinoma Patients Treated with Esophagectomy Followed by Adjuvant Chemotherapy. Ann. Surg. Oncol. 2013, 20, 3044–3051. [Google Scholar] [CrossRef]
- Sato, Y.; Motoyama, S.; Nanjo, H.; Wakita, A.; Yoshino, K.; Sasaki, T.; Nagaki, Y.; Liu, J.; Imai, K.; Saito, H.; et al. CXCL10 Expression Status is Prognostic in Patients with Advanced Thoracic Esophageal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2016, 23, 936–942. [Google Scholar] [CrossRef]
- Sato, Y.; Motoyama, S.; Wakita, A.; Kawakita, Y.; Liu, J.; Nagaki, Y.; Nanjo, H.; Terata, K.; Imai, K.; Saito, H.; et al. TLR3 expression status predicts prognosis in patients with advanced thoracic esophageal squamous cell carcinoma after esophagectomy. Am. J. Surg. 2018, 216, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Camp, R.L.; Neumeister, V.; Rimm, D.L. A decade of tissue microarrays: Progress in the discovery and validation of cancer biomarkers. J. Clin. Oncol. 2008, 26, 5630–5637. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.H.; Schwartz, J.N.; Hagerty, K.L.; Allred, D.C.; Cote, R.J.; Dowsett, M.; Fitzgibbons, P.L.; Hanna, W.M.; Langer, A.; et al. American Society of Clinical Oncology/ College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 2007, 25, 118–145. [Google Scholar] [CrossRef] [PubMed]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br. J. Cancer 2005, 93, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Semlali, A.; Almutairi, M.; Pathan, A.A.K.; Azzi, A.; Parine, N.R.; AlAmri, A.; Arafah, M.; Aljebreen, A.M.; Almadi, M.A.; Azzam, N.A.; et al. Toll-like receptor 6 expression, sequence variants, and their association with colorectal cancer risk. J. Cancer 2019, 10, 2969–2981. [Google Scholar] [CrossRef]
- Kim, J.H.; Kordahi, M.C.; Chac, D.; DePaolo, R.W. Toll-like Receptor-6 Signaling Prevents Inflammation and Impacts Composition of the Microbiota during Inflammation-Induced Colorectal Cancer. Cancer Prev. Res. 2020, 13, 25–40. [Google Scholar] [CrossRef]
- Chen, Y.L.; Huang, K.C.; Wu, J.H.; Liu, T.; Chen, J.W.; Xie, J.Y.; Chen, M.Y.; Wu, L.W.; Tung, C.L. Microbiome dysbiosis inhibits carcinogen-induced murine oral tumorigenesis. J. Cancer 2022, 13, 3051–3060. [Google Scholar] [CrossRef]
- Semlali, A.; Almutairi, M.; Rouabhia, M.; Reddy Parine, N.; Al Amri, A.; Al-Numair, S.N.; Hawsawi, M.Y.; Saud Alanazi, M. Novel sequence variants in the TLR6 gene associated with advanced breast cancer risk in the Saudi Arabian population. PLoS ONE 2018, 13, e0203376. [Google Scholar] [CrossRef]
- Gebrayel, P.; Nicco, C.; Al Khodor, S.; Bilinski, J.; Caselli, E.; Comelli, E.M.; Egert, M.; Giaroni, C.; Karpinski, T.M.; Loniewski, I.; et al. Microbiota medicine: Towards clinical revolution. J. Transl. Med. 2022, 20, 111. [Google Scholar] [CrossRef]
- Sun, J.; Tang, Q.; Yu, S.; Xie, M.; Xie, Y.; Chen, G.; Chen, L. Role of the oral microbiota in cancer evolution and progression. Cancer Med. 2020, 9, 6306–6321. [Google Scholar] [CrossRef]
- Sato, Y.; Motoyama, S.; Takano, H.; Nakata, A.; Liu, J.; Harimaya, D.; Todo, N.; Yoshino, K.; Sasaki, T.; Wakita, A.; et al. Esophageal Cancer Patients Have a High Incidence of Severe Periodontitis and Preoperative Dental Care Reduces the Likelihood of Severe Pneumonia after Esophagectomy. Dig. Surg. 2016, 33, 495–502. [Google Scholar] [CrossRef]
- Carr, E.; Aslam-Pervez, B. Does the use of alcohol mouthwash increase the risk of developing oral cancer? Evid. Based Dent. 2022, 23, 28–29. [Google Scholar] [CrossRef]

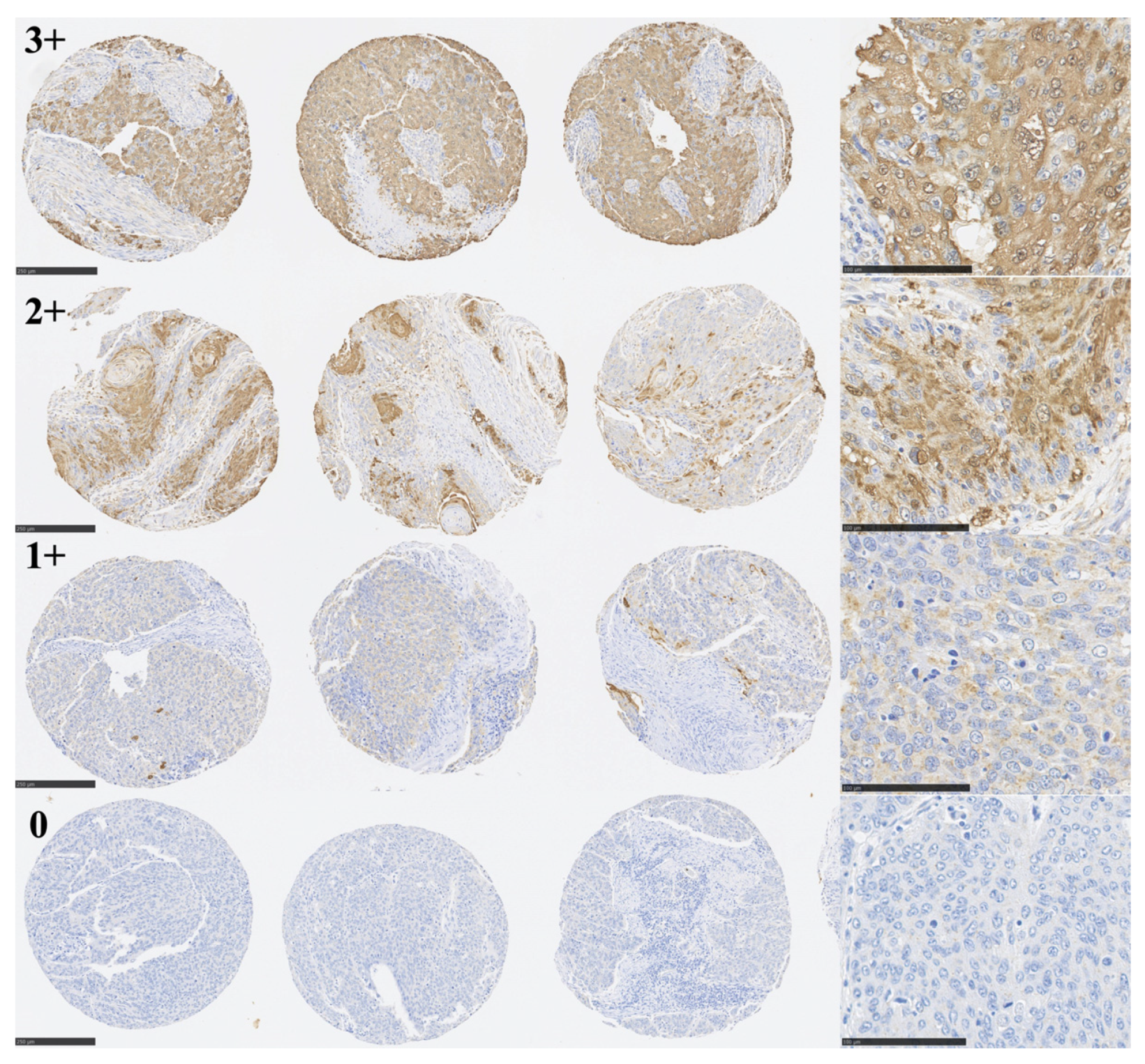

| Characteristics | TLR6-High n = 65 (36.7%) | TLR6-Low n = 112 (63.3%) | p Value |
|---|---|---|---|
| Sex Female Male | 0.082 | ||
| 5 (7.7%) | 19 (17.0%) | ||
| 60 (92.3%) | 93 (83.0%) | ||
| Age at surgery | 65 (50–76) | 67 (38–82) | 0.052 |
| Smoking history (pack/day × year) | 40 (0–120) | 40 (0–250) | 0.327 |
| Habitual smoking Current | 0.804 | ||
| 36 (55.4%) | 64 (57.1%) | ||
| Past Never | 16 (24.6%) 13 (20.0%) | 23 (20.6%) 25 (22.3%) | |
| Habitual alcohol consumption Current Past Never | 48 (73.9%) 9 (13.9%) 8 (12.2%) | 84 (75.0%) 14 (12.5%) 14 (12.5%) | 0.968 |
| Tumor location Upper Middle Lower | 2 (3.1%) 47 (72.3%) 16 (24.6%) | 4 (3.6%) 69 (61.6%) 39 (34.8%) | 0.345 |
| Depth of invasion (pT) pT2 pT3 pT4a | 10 (15.4%) 48 (73.8%) 7 (10.8%) | 21 (18.7%) 89 (79.5%) 2 (1.8%) | 0.031 * |
| Lymph node metastasis (pN) pN0 pN1 pN2 pN3 M1 Lymph (supraclavicular) | 26 (40.0%) 18 (27.7%) 8 (12.3%) 8 (12.3%) 5 (7.7%) | 23 (20.6%) 37 (33.0%) 27 (24.1%) 12 (10.7%) 13 (11.6%) | 0.047 * |
| Pathological stage pStage IIA pStage IIB pStage IIIA pStage IIIB pStage IVA pStage IVB (M1 Lymph) | 9 (13.9%) 15 (23.1%) 1 (1.5%) 25 (38.4%) 10 (15.4%) 5 (7.7%) | 11 (9.8%) 12 (10.7%) 6 (5.4%) 57 (50.9%) 13 (11.6%) 13 (11.6%) | 0.123 |
| Tumor differentiation Well Moderate Poor | 21 (32.3%) 32 (49.2%) 12 (18.5%) | 8 (7.1%) 59 (52.7%) 45 (40.2%) | <0.001 * |
| Adjuvant chemotherapy Positive Negative | 42 (64.6%) 23 (35.4%) | 63 (56.3%) 49 (43.7%) | 0.275 |
| Recurrence of ESCC Positive Negative | 28 (43.1%) 37 (56.9%) | 52 (46.4%) 60 (53.6%) | 0.666 |
| Prognosis Alive Deceased from ESCC Deceased from other cancer Deceased from other diseases | 38 (58.4%) 20 (30.8%) 0 7 (10.8%) | 38 (33.9%) 47 (42.0%) 5 (4.5%) 22 (19.6%) | 0.007 * |
| (A) | |||
|---|---|---|---|
| Variable | p Value | Hazard Ratio | 95% CI |
| TLR6 expression: low (n = 112) vs. high (n = 65) | 0.0052 * | 1.955 | 1.222–3.128 |
| Age: 65 and older (n = 104) vs. younger (n = 73) | 0.1174 | 1.415 | 0.916–2.185 |
| Sex: male (n = 153) vs. Female (n = 24) | 0.1047 | 1.825 | 0.882–3.776 |
| Smoking history: 40 over (n = 93) vs. under 40 (n = 84) | 0.067 | 1.486 | 0.973–2.269 |
| Habitual smoking: current (n = 100) vs. others (n = 77) | 0.8729 | 1.035 | 0.678–1.581 |
| Habitual alcohol consumption: current (n = 132) vs. others (n = 45) | 0.8906 | 1.035 | 0.634–1.690 |
| pT: pT3-4 (n = 146) vs. T2 (n-31) | 0.4325 | 1.257 | 0.710–2.227 |
| pN: pN1-3 (n = 128) vs. pN0 (n = 49) | <0.0001 * | 5.77 | 2.785–11.95 |
| pStage: IIIB over (n = 123) vs. under IIIA (n = 54) | <0.0001 * | 5.162 | 2.667–9.991 |
| Tumor differentiation: poor (n = 57) vs. others (n = 120) | 0.0032 * | 1.9 | 1.240–2.911 |
| Adjuvant chemotherapy: negative (n = 72) vs. positive (n = 105) | 0.4269 | 1.187 | 0.778–1.809 |
| (B) | |||
| Variable | p Value | Hazard Ratio | 95% CI |
| TLR6 expression (crude) | 0.0052 * | 1.955 | 1.222–3.128 |
| Adjusted for age and sex | 0.0026 * | 2.07 | 1.289–3.322 |
| Adjusted for age, sex, pT, pN, pStage, and tumor differentiation | 0.0277 * | 1.745 | 1.062–2.864 |
| n (%) | Alive | Deceased from ESCC | Deceased from Other Cancers | Deceased from Other Diseases | |
|---|---|---|---|---|---|
| TLR6-high/TLR4-low | 20 (11.3%) | 12 (60.0%) | 6 (30.0%) | 0 | 2 (10.0%) |
| TLR6-high/TLR4-high and TLR6-low/TLR4-low | 70 (39.5%) | 35 (50.0%) | 23 (32.9%) | 0 | 12 (17.1%) |
| TLR6-low/TLR4-high | 87 (49.2%) | 29 (33.3%) | 38 (43.7%) | 5 (5.8%) | 15 (17.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, Y.; Wakita, A.; Maeda, E.; Nagaki, Y.; Sasamori, R.; Kemuriyama, K.; Nozaki, S.; Ito, S.; Terata, K.; Imai, K.; et al. High TLR6 Expression Status Predicts a More Favorable Prognosis after Esophagectomy for Locally Advanced Thoracic Esophageal Squamous Cell Carcinoma. Curr. Oncol. 2023, 30, 4724-4735. https://doi.org/10.3390/curroncol30050356
Sato Y, Wakita A, Maeda E, Nagaki Y, Sasamori R, Kemuriyama K, Nozaki S, Ito S, Terata K, Imai K, et al. High TLR6 Expression Status Predicts a More Favorable Prognosis after Esophagectomy for Locally Advanced Thoracic Esophageal Squamous Cell Carcinoma. Current Oncology. 2023; 30(5):4724-4735. https://doi.org/10.3390/curroncol30050356
Chicago/Turabian StyleSato, Yusuke, Akiyuki Wakita, Eri Maeda, Yushi Nagaki, Ryohei Sasamori, Kohei Kemuriyama, Shu Nozaki, Satoru Ito, Kaori Terata, Kazuhiro Imai, and et al. 2023. "High TLR6 Expression Status Predicts a More Favorable Prognosis after Esophagectomy for Locally Advanced Thoracic Esophageal Squamous Cell Carcinoma" Current Oncology 30, no. 5: 4724-4735. https://doi.org/10.3390/curroncol30050356
APA StyleSato, Y., Wakita, A., Maeda, E., Nagaki, Y., Sasamori, R., Kemuriyama, K., Nozaki, S., Ito, S., Terata, K., Imai, K., Nanjo, H., Nomura, K., & Minamiya, Y. (2023). High TLR6 Expression Status Predicts a More Favorable Prognosis after Esophagectomy for Locally Advanced Thoracic Esophageal Squamous Cell Carcinoma. Current Oncology, 30(5), 4724-4735. https://doi.org/10.3390/curroncol30050356






