Triplet Therapy in Metastatic Castrate Sensitive Prostate Cancer (mCSPC)—A Potential New Standard of Care
Abstract
1. Introduction
2. Evolution of Systemic Therapy of mCSPC Prior to Triplet Therapy
3. Triplet Therapy in mCSPC—Rationale and Summary of Clinical Data
4. Putting the Evidence into Perspective
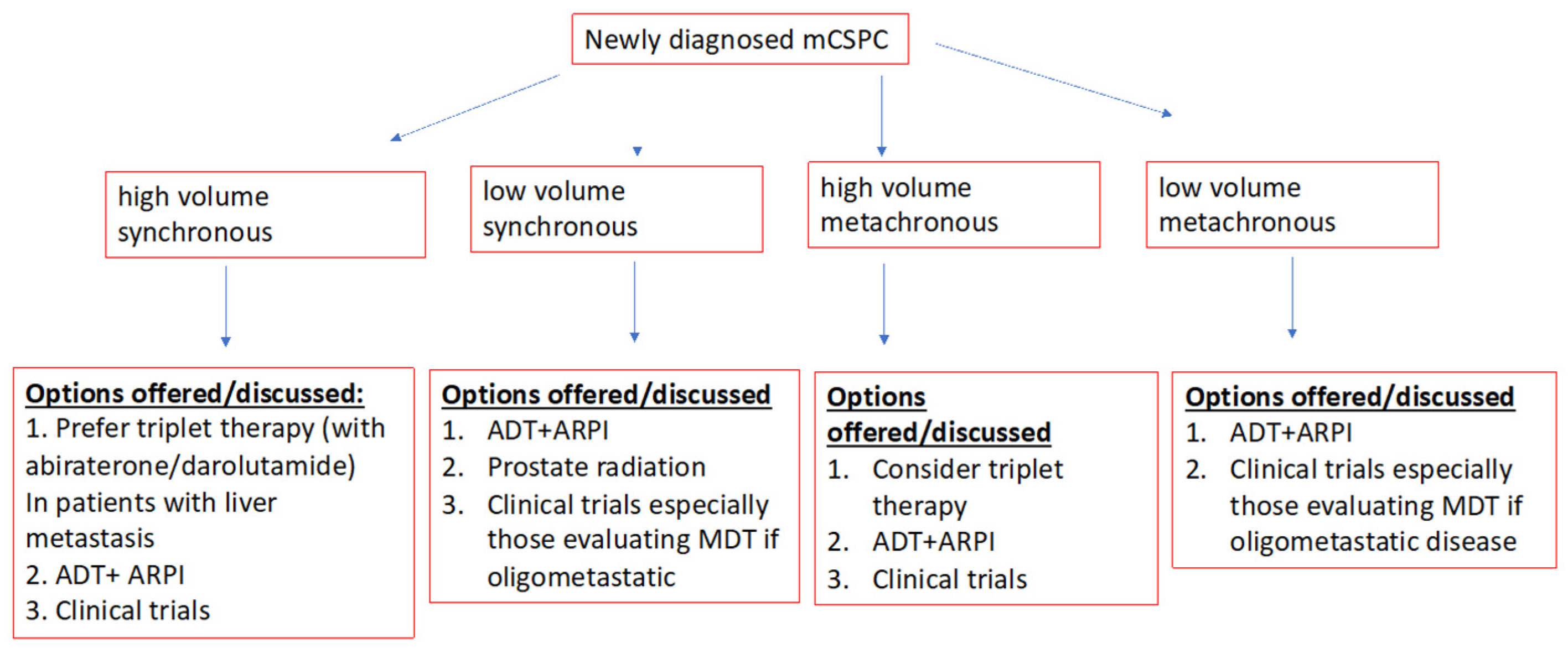
5. Clinical Practise Points
6. Future Directions
| Keynote 991 [58] | PSMAddition [59] | AMPLITUDE [54] | TALAPRO-3 [55] | CAPITELLO-281 [56] | CYCLONE-3 [57] | SPARKLE [64] | |
|---|---|---|---|---|---|---|---|
| NCT number | NCT04191096 | NCT04720157 | NCT04497844 | NCT04821622 | NCT04493853 | NCT05288166 | NCT05352178 |
| Experimental arm | Pembrolizumab plus Enzalutamide plus ADT | Lu-177 plus SOC | Niraparib plus AAP plus ADT | Talazoparib plus enzalutamide plus ADT | Capivasertib plus AAP plus ADT | Abemaciclib plus AAP plus ADT | 1 = MDT plus 1 month ADT 2 = MDT plus 6 months ADT + enzalutamide |
| Control arm | Enzalutamide plus ADT | SOC alone | AAP plus ADT | Enzalutamide plus ADT | AAP plus ADT | AAP plus ADT | MDT alone |
| Design | Randomised phase III double blind | Randomised phase III with cross over allowed | Randomised phase III double blind | Randomised phase III double blind | Randomised phase III double blind | Randomised phase III double blind | Randomised phase III open label |
| Number of patients | 1232 | 1126 | 788 | 550 | 1000 | 900 | 873 |
| Primary end point | rPFS and OS | rPFS | rPFS | rPFS | rPFS | rPFS | Poly metastatic free survival (PMFS) |
| Current status | Active, not recruiting | Recruiting | recruiting | Completed recruiting | Recruiting | recruiting | Recruiting |
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Prostate Cancer Statistics|World Cancer Research Fund International. Available online: https://www.wcrf.org/cancer-trends/prostate-cancer-statistics/ (accessed on 16 January 2023).
- Prostate Cancer Incidence and Survival, by Stage and Race/Ethnicity—United States, 2001–2017. Available online: https://www.cdc.gov/mmwr/volumes/69/wr/mm6941a1.htm (accessed on 21 January 2023).
- Leigh, J.; Qureshi, D.; Sucha, E.; Mahdavi, R.; Kushnir, I.; Lavallée, L.T.; Bosse, D.; Webber, C.; Tanuseputro, P.; Ong, M. A population-based study of factors associated with systemic treatment in advanced prostate cancer decedents. Cancer Med. 2022, 12, 5569–5579. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Chen, Y.H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hor-mone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Vale, C.L.; Fisher, D.; Godolphin, P.; Rydzewska, L.H.; Boher, J.-M.; Burdett, S.; Chen, Y.-H.; Gravis, G.; James, N.D.; Liu, G.; et al. Defining more precisely the effects of docetaxel plus ADT for men with mHSPC: Meta-analysis of individual participant data from randomized trials. J. Clin. Oncol. 2022, 40, 5070. [Google Scholar] [CrossRef]
- Mar, N.; Kalebasty, A.R.; Uchio, E.M. Management of Advanced Prostate Cancer in Clinical Practice: Real-World Answers to Challenging Dilemmas. JCO Oncol. Pract. 2020, 16, 783–789. [Google Scholar] [CrossRef]
- Fizazi, K.; Foulon, S.; Carles, J.; Roubaud, G.; McDermott, R.; Fléchon, A.; Tombal, B.; Supiot, S.; Berthold, D.; Ronchin, P.; et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): A multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet 2022, 399, 1695–1707. [Google Scholar] [CrossRef]
- Smith, M.R.; Hussain, M.; Saad, F.; Fizazi, K.; Sternberg, C.N.; Crawford, E.D.; Kopyltsov, E.; Park, C.H.; Alekseev, B.; Montesa-Pino, Á.; et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2022, 386, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Davis, I.D. Combination therapy in metastatic hormone-sensitive prostate cancer: Is three a crowd? Ther. Adv. Med. Oncol. 2022, 14, 17588359221086828. [Google Scholar] [CrossRef]
- Cook, A.; Beesley, S.; O’Sullivan, J.M.; Birtle, A.J.; Thalmann, G.; Graham, J.D.; Spears, M.R.; Brock, S.; Srinivasan, R.; Protheroe, A.; et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016, 387, 1163–1177. [Google Scholar] [CrossRef]
- Kyriakopoulos, C.E.; Chen, Y.-H.; Carducci, M.A.; Liu, G.; Jarrard, D.F.; Hahn, N.M.; Shevrin, D.H.; Dreicer, R.; Hussain, M.; Eisenberger, M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J. Clin. Oncol. 2018, 36, 1080–1087. [Google Scholar] [CrossRef]
- Gravis, G.; Boher, J.-M.; Joly, F.; Soulié, M.; Albiges, L.; Priou, F.; Latorzeff, I.; Delva, R.; Krakowski, I.; Laguerre, B.; et al. Androgen Deprivation Therapy (ADT) Plus Docetaxel Versus ADT Alone in Metastatic Non castrate Prostate Cancer: Impact of Metastatic Burden and Long-term Survival Analysis of the Randomized Phase 3 GETUG-AFU15 Trial. Eur. Urol. 2016, 70, 256–262. [Google Scholar] [CrossRef]
- Gravis, G.; Fizazi, K.; Joly, F.; Oudard, S.; Priou, F.; Esterni, B.; Latorzeff, I.; Delva, R.; Krakowski, I.; Laguerre, B.; et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013, 14, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Clarke, N.W.; Ali, A.; Ingleby, F.C.; Hoyle, A.; Amos, C.L.; Attard, G.; Brawley, C.D.; Calvert, J.; Chowdhury, S.; Cook, A.; et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: Long-term survival results from the STAMPEDE trial. Ann. Oncol. 2019, 30, 1992–2003. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef] [PubMed]
- James, N.D.; de Bono, J.S.; Spears, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Ritchie, A.W.S.; Amos, C.L.; Gilson, C.; Jones, R.J.; et al. Abiraterone for Prostate Cancer Not Previ-ously Treated with Hormone Therapy. N. Engl. J. Med. 2017, 377, 338–351. [Google Scholar] [CrossRef]
- Hoyle, A.P.; Ali, A.; James, N.D.; Cook, A.; Parker, C.C.; de Bono, J.S.; Attard, G.; Chowdhury, S.; Cross, W.R.; Dearnaley, D.P.; et al. Abiraterone in “High-” and “Low-risk” Metastatic Hor-mone-sensitive Prostate Cancer. Eur. Urol. 2019, 76, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Agarwal, N.; Bjartell, A.; Chung, B.H.; Pereira de Santana Gomes, A.J.; Given, R.; Juárez Soto, Á.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide for Metastatic, Cas-tration-Sensitive Prostate Cancer. N. Engl. J. Med. 2019, 381, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Azad, A.; Alcaraz, A.; Alekseev, B.; Iguchi, T.; Shore, N.D.; et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy with Enzalutamide or Placebo in Men with Metastatic Hormone-Sensitive Prostate Cancer. J. Clin. Oncol. 2019, 37, 2974–2986. [Google Scholar] [CrossRef]
- ESMO 2021: Final Overall Survival Analysis from ARCHES: A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study of Enzalutamide + ADT in Men with mHSPC. Available online: https://www.urotoday.com/conference-highlights/esmo-2021/esmo-2021-prostate-cancer/132209-esmo-2021-lba25-final-overall-survival-os-analysis-from-arches-a-phase-3-randomized-double-blind-placebo-pbo-controlled-study-of-enzalutamide-enza-androgen-deprivation-therapy-adt-in-men-with-metastatic-hormone-sensitive-prostate-cancer-mhspc.html (accessed on 18 December 2022).
- Davis, I.D.; Martin, A.J.; Zielinski, R.R.; Thomson, A.; Tan, T.H.; Sandhu, S.; Reaume, M.N.; Pook, D.W.; Parnis, F.; North, S.A.; et al. Updated overall survival outcomes in ENZAMET (ANZUP 1304), an international, cooperative group trial of enzalutamide in metastatic hormone-sensitive prostate cancer (mHSPC). J. Clin. Oncol. 2022, 40, LBA5004. [Google Scholar] [CrossRef]
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.G.; et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 121–131. [Google Scholar] [CrossRef]
- Hird, A.E.; Magee, D.E.; Cheung, D.C.; Matta, R.; Kulkarni, G.S.; Nam, R.K. Abiraterone vs. docetaxel for metastatic hor-mone-sensitive prostate cancer: A microsimulation model. Can. Urol. Assoc. J. 2020, 14, E418–E427. [Google Scholar]
- Wallis, C.J.; Klaassen, Z.; Bhindi, B.; Goldberg, H.; Chandrasekar, T.; Farrell, A.M.; Boorjian, S.A.; Kulkarni, G.S.; Karnes, R.J.; Satkunasivam, R. Comparison of Abiraterone Acetate and Docetaxel with Androgen Deprivation Therapy in High-risk and Metastatic Hormone-naïve Prostate Cancer: A Systematic Review and Network Meta-analysis. Eur. Urol. 2018, 73, 834–844. [Google Scholar] [CrossRef]
- Sydes, M.; Spears, M.; Mason, M.; Clarke, N.; Dearnaley, D.; de Bono, J.; Attard, G.; Chowdhury, S.; Cross, W.; Gillessen, S.; et al. Adding abiraterone or docetaxel to long-term hormone therapy for prostate cancer: Directly randomised data from the STAMPEDE multi-arm, multi-stage platform protocol. Ann. Oncol. 2018, 29, 1235–1248. [Google Scholar] [CrossRef]
- Stecca, C.E.; Jiang, D.M.; Veitch, Z.; Hotte, S.J.; Alimohamed, N.; Wood, L.; Sridhar, S.S. Evaluation of Trends in Treatment of Metastatic Hormone Sensitive Prostate Cancer (mHSPC) Across Canada During the COVID-19 Pandemic. Clin. Genitourin. Cancer 2022, 21, 273–277. [Google Scholar] [CrossRef]
- George, D.J.; Agarwal, N.; Rider, J.R.; Li, B.; Shirali, R.; Sandin, R.; Hong, A.; Russell, D.; Ramaswamy, K.; Freedland, S.J. Real-world treatment patterns among patients diagnosed with metastatic castration-sensitive prostate cancer (mCSPC) in community oncology settings. J. Clin. Oncol. 2021, 39, 5074. [Google Scholar] [CrossRef]
- Swami, U.; Hong, A.; El-Chaar, N.N.; Nimke, D.; Ramaswamy, K.; Bell, E.J.; Sandin, R.; Agarwal, N. Real-world first-line (1L) treatment patterns in patients (pts) with metastatic castration-sensitive prostate cancer (mCSPC) in a U.S. health insurance database. J. Clin. Oncol. 2021, 39, 5072. [Google Scholar] [CrossRef]
- Tripathi, A.; Chen, Y.-H.; Jarrard, D.F.; Hahn, N.M.; Garcia, J.A.; Dreicer, R.; Liu, G.; Hussain, M.H.A.; Shevrin, D.H.; Cooney, M.M.; et al. Eight-year survival rates by baseline prognostic groups in patients with metastatic hormone-sensitive prostate cancer (mHSPC): An analysis from the ECOG-ACRIN 3805 (CHAARTED) trial. J. Clin. Oncol. 2022, 40, 5081. [Google Scholar] [CrossRef]
- Leith, A.; Kim, J.; Ribbands, A.; Clayton, E.; Yang, L.; Ghate, S.R. Real-World Treatment Patterns in Metastatic Castration-Resistant Prostate Cancer Across Europe (France, Germany, Italy, Spain, and the United Kingdom) and Japan. Adv. Ther. 2022, 39, 2236–2255. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Bjartell, A.; Agarwal, N.; Chung, B.; Given, R.; Gomes, A.P.d.S.; Merseburger, A.; Özgüroğlu, M.; Soto, J.; Uemura, H.; et al. Deep, rapid, and durable prostate-specific antigen decline with apalutamide plus androgen deprivation therapy is associated with longer survival and improved clinical outcomes in TITAN patients with metastatic castration-sensitive prostate cancer. Ann. Oncol. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Matsubara, N.; Chi, K.N.; Özgüroğlu, M.; Rodriguez-Antolin, A.; Feyerabend, S.; Fein, L.; Alekseev, B.Y.; Sulur, G.; Protheroe, A.; Li, S.; et al. Correlation of Prostate-specific Antigen Kinetics with Overall Survival and Radiological Progression-free Survival in Metastatic Castration-sensitive Prostate Cancer Treated with Abiraterone Acetate plus Prednisone or Placebos Added to Androgen Deprivation Therapy: Post Hoc Analysis of Phase 3 LATITUDE Study. Eur. Urol. 2020, 77, 494–500. [Google Scholar] [PubMed]
- Sayegh, N.; Tripathi, N.; Chigarira, B.; Jo, Y.; McFarland, T.R.; Kessel, A.; Nussenzveig, R.; Li, H.; Tander, C.; Goel, H.; et al. Survival outcomes and characterization of patients (pts) with metastatic castration-sensitive prostate cancer (mCSPC) undergoing intensified androgen deprivation therapy (ADT) who do not achieve an optimal PSA response (PSA ≤ 0.2 ng/mL). J. Clin. Oncol. 2022, 40, 123. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Cheung, L.; Chi, K.N.; Chowdhury, S.; Frydenberg, M.; Horvath, L.G.; Joshua, A.M.; et al. Testosterone suppression plus enzalutamide versus testosterone suppression plus standard antiandrogen therapy for metastatic hormone-sensitive prostate cancer (ENZAMET): An international, open-label, randomised, phase 3 trial. Lancet Oncol. 2023, 24, 323–334. [Google Scholar] [CrossRef]
- Hussain, M.; Tombal, B.; Saad, F.; Fizazi, K.; Sternberg, C.N.; Crawford, E.D.; Shore, N.; Kopyltsov, E.; Kalebasty, A.R.; Bögemann, M.; et al. Darolutamide Plus Androgen-Deprivation Therapy and Docetaxel in Metastatic Hormone-Sensitive Prostate Cancer by Disease Volume and Risk Subgroups in the Phase III ARASENS Trial. J. Clin. Oncol. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Roy, S.; Sayyid, R.; Saad, F.; Sun, Y.; Lajkosz, K.; Ong, M.; Klaassen, Z.; Malone, S.; Spratt, D.E.; Wallis, C.J.; et al. Addition of Docetaxel to Androgen Receptor Axis–targeted Therapy and Androgen Deprivation Therapy in Metastatic Hormone-sensitive Prostate Cancer: A Network Meta-analysis. Eur. Urol. Oncol. 2022, 5, 494–502. [Google Scholar] [CrossRef]
- Mandel, P.; Hoeh, B.; Wenzel, M.; Preisser, F.; Tian, Z.; Tilki, D.; Steuber, T.; Karakiewicz, P.I.; Chun, F.K. Triplet or Doublet Therapy in Metastatic Hormone-sensitive Prostate Cancer Patients: A Systematic Review and Network Meta-analysis. Eur. Urol. Focus 2022, 9, 96–105. [Google Scholar] [CrossRef]
- Naqvi, S.A.A.; Bin Riaz, Z.; Riaz, A.; Islam, M.; Siddiqi, R.; Ikram, W.; Jafar, M.A.; Singh, P.; Ravi, P.K.; Bin Riaz, I.; et al. Triplet therapy in metastatic castration-sensitive prostate cancer: A systematic review and meta-analysis. J. Clin. Oncol. 2022, 40, 136. [Google Scholar] [CrossRef]
- Ciccarese, C.; Iacovelli, R.; Sternberg, C.N.; Gillessen, S.; Tortora, G.; Fizazi, K. Triplet therapy with androgen deprivation, docetaxel, and androgen receptor signalling inhibitors in metastatic castration-sensitive prostate cancer: A meta-analysis. Eur. J. Cancer 2022, 173, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Emergence of Triplet Therapy for Metastatic Cas-Tration-Sensitive Prostate Cancer: An Updated Systematic Review and Network Meta-Analysis. Available online: https://www.sciencedirect.com/science/article/pii/S107814392200391X (accessed on 18 December 2022).
- Riaz, I.B.; Naqvi, S.A.A.; He, H.; Asghar, N.; Siddiqi, R.; Liu, H.; Singh, P.; Childs, D.S.; Ravi, P.; Hussain, S.A.; et al. First-line Systemic Treatment Options for Metastatic Castration-Sensitive Prostate Cancer: A Living Systematic Review and Network Meta-analysis. JAMA Oncol. 2023, e227762. [Google Scholar] [CrossRef] [PubMed]
- ESMO 2022: Effect of Abiraterone-Prednisone in mCSPC with Neuroendocrine and Very High-Risk Features in the PEACE-1 Trial. Available online: https://www.urotoday.com/conference-highlights/esmo-2022/esmo-2022-prostate-cancer/139407-esmo-2022-effect-of-abiraterone-prednisone-in-mcspc-with-neuroendocrine-and-very-high-risk-features-in-the-peace-1-trial.html (accessed on 23 December 2022).
- Baciarello, G.; Özgüroğlu, M.; Mundle, S.; Leitz, G.; Richarz, U.; Hu, P.; Feyerabend, S.; Matsubara, N.; Chi, K.N.; Fizazi, K. Impact of abiraterone acetate plus prednisone in pa-tients with castration-sensitive prostate cancer and visceral metastases over four years of follow-up: A post-hoc exploratory analysis of the LATITUDE study. Eur. J. Cancer 2022, 162, 56–64. [Google Scholar] [CrossRef]
- Shiota, M.; Terada, N.; Saito, T.; Yokomizo, A.; Kohei, N.; Goto, T.; Kawamura, S.; Hashimoto, Y.; Takahashi, A.; Kimura, T.; et al. Differential prognostic factors in low- and high-burden de novo metastatic hormone-sensitive prostate cancer patients. Cancer Sci. 2020, 112, 1524–1533. [Google Scholar] [CrossRef]
- Swami, U.; Graf, R.P.; Nussenzveig, R.H.; Fisher, V.; Tukachinsky, H.; Schrock, A.B.; Li, G.; Ross, J.S.; Sayegh, N.; Tripathi, N.; et al. SPOP Mutations as a Predictive Biomarker for Androgen Receptor Axis–Targeted Therapy in De Novo Metastatic Castration-Sensitive Prostate Cancer. Clin. Cancer Res. 2022, 28, 4917–4925. [Google Scholar] [CrossRef]
- Gilson, C.; Ingleby, F.; Gilbert, D.C.; Parry, M.; Atako, N.B.; Mason, M.D.; Malik, Z.; Langley, R.E.; Simmons, A.; Loehr, A.; et al. Targeted next-generation sequencing (tNGS) of meta-static castrate-sensitive prostate cancer (M1 CSPC): A pilot molecular analysis in the STAMPEDE multi-center clinical trial. J. Clin. Oncol. 2019, 37, 5019. [Google Scholar] [CrossRef]
- Stopsack, K.H.; Nandakumar, S.; Wibmer, A.G.; Haywood, S.; Weg, E.S.; Barnett, E.S.; Kim, C.J.; Carbone, E.A.; Vasselman, S.E.; Nguyen, B.; et al. Oncogenic Genomic Alterations, Clinical Phenotypes, and Outcomes in Metastatic Castration-Sensitive Prostate Cancer. Clin. Cancer Res. 2020, 26, 3230–3238. [Google Scholar] [CrossRef]
- Abida, W.; Armenia, J.; Gopalan, A.; Brennan, R.; Walsh, M.; Barron, D.; Danila, D.; Rathkopf, D.; Morris, M.; Slovin, S.; et al. Prospective Genomic Profiling of Prostate Cancer Across Disease States Reveals Germline and Somatic Alterations That May Affect Clinical Decision Making. JCO Precis. Oncol. 2017, 1, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kappel, C.; Jiang, D.M.; Wong, B.; Zhang, T.; Selvarajah, S.; Warner, E.; Hansen, A.R.; Fallah-Rad, N.; Sacher, A.G.; Stockley, T.L.; et al. Comprehensive genomic profiling of treatment resistant metastatic castrate sensitive prostate cancer reveals high frequency of potential therapeutic targets. Clin. Genitourin. Cancer 2022, 20, 278–284. [Google Scholar] [CrossRef]
- Hamid, A.A.; Huang, H.C.; Wang, V.; Chen, Y.H.; Feng, F.; Den, R.; Attard, G.; Van Allen, E.M.; Tran, P.T.; Spratt, D.E.; et al. Transcriptional profiling of primary prostate tumor in meta-static hormone-sensitive prostate cancer and association with clinical outcomes: Correlative analysis of the E3805 CHAARTED trial. Ann. Oncol. 2021, 32, 1157–1166. [Google Scholar] [CrossRef]
- Diaz Mejia, N.M.; Stecca, C.; Jiang, D.M.; Fallah-Rad, N.; Bedard, P.L.; Kumar, V.; Abdelijalil, O.; Zahralliyali, A.; Alqaisi, H.; Al-Ezzi, E.M.; et al. A retrospective review of primary prophy-laxis with granulocyte-colony stimulating factor (G-CSF) for patients with genitourinary malignancies receiving chemo-therapy during the COVID-19 pandemic and implications for the future. J. Clin. Oncol. 2023, 41, 115. [Google Scholar] [CrossRef]
- Rauscher, I.; Düwel, C.; Haller, B.; Rischpler, C.; Heck, M.M.; Gschwend, J.E.; Schwaiger, M.; Maurer, T.; Eiber, M. Efficacy, Predictive Factors, and Prediction Nomograms for 68Ga-labeled Prostate-specific Membrane Antigen-ligand Positron-emission Tomography/Computed To-mography in Early Biochemical Recurrent Prostate Cancer after Radical Prostatectomy. Eur. Urol. 2018, 73, 656–661. [Google Scholar] [CrossRef]
- Fendler, W.P.; Calais, J.; Eiber, M.; Flavell, R.R.; Mishoe, A.; Feng, F.Y.; Nguyen, H.G.; Reiter, R.E.; Rettig, M.B.; Okamoto, S.; et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localiz-ing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol. 2019, 5, 856–863. [Google Scholar] [CrossRef]
- Phase 3 Randomized, Placebo-Controlled, Double-Blind Study of Niraparib in Combination with Abiraterone Acetate and Prednisone Versus Abiraterone Acetate and Prednisone for the Treatment of Participants with Deleterious Germline or Somatic Homologous Recombination Repair (HRR) Gene-Mutated Metastatic Castration-Sensitive Prostate Cancer (mCSPC). Available online: https://clinicaltrials.gov/ct2/show/NCT04497844 (accessed on 8 December 2022).
- Talapro-3: A Phase 3, Randomized, Double-Blind, Study of Talazoparib with Enzalutamide Versus Placebo with Enzalutamide in Men with Ddr Gene Mutated Metastatic Castration-Sensitive Prostate Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT04821622 (accessed on 8 December 2022).
- A Phase III Double-Blind, Randomised, Placebo-Controlled Study Assessing the Efficacy and Safety of Capi-Vasertib + Abiraterone Versus Placebo + Abiraterone as Treatment for Patients with DeNovo Metastatic Hormone-Sensitive Prostate Cancer Characterised by PTEN Deficiency. Available online: https://clinicaltrials.gov/ct2/show/NCT04493853 (accessed on 8 December 2022).
- CYCLONE 3: A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study of Abemaciclib in Combination with Abiraterone Plus Prednisone in Men with High-Risk Metastatic Hormone-Sensitive Prostate Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT05288166 (accessed on 15 December 2022).
- A Phase 3, Randomized, Double-Blind Trial of Pembrolizumab (MK-3475) Plus Enzalutamide Plus ADT Versus Placebo Plus Enzalutamide Plus ADT in Participants with Metastatic Hormone-Sensitive Prostate Cancer (mHSPC) (KEYNOTE-991). Available online: https://clinicaltrials.gov/ct2/show/NCT04191096 (accessed on 8 December 2022).
- An Open-Label, Randomized, Phase III Study Comparing 177Lu-PSMA-617 in Combination with Standard of Care, Versus Standard of Care Alone, in Adult Male Patients with Metastatic Hormone Sensitive Prostate Cancer (mHSPC). Available online: https://clinicaltrials.gov/ct2/show/NCT04720157 (accessed on 8 December 2022).
- ABX Advanced Biochemical Compounds GmbH. Phase III Study of [18F]PSMA-1007 Positron Emission Tomography for the Detection of Prostate Cancer Lesions in Patients with Biochemical Recurrence after Previous Definitive Treatment for Local-ized Prostate Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT04742361 (accessed on 1 February 2023).
- PSMA PET/CT Guided Intensification of Therapy in Patients at Risk of Advanced Prostate Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT04557501 (accessed on 1 February 2023).
- Phillips, R.; Shi, W.Y.; Deek, M.; Radwan, N.; Lim, S.J.; Antonarakis, E.S.; Rowe, S.P.; Ross, A.E.; Gorin, M.A.; Deville, C.; et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Ost, P.; Reynders, D.; Decaestecker, K.; Fonteyne, V.; Lumen, N.; De Bruycker, A.; Lambert, B.; Delrue, L.; Bultijnck, R.; Claeys, T.; et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J. Clin. Oncol. 2018, 36, 446–453. [Google Scholar] [CrossRef]
- A New Spark in Treating Oligorecurrent Prostate Cancer: Adding Systemic Treatment to Stereotactic Body Radiotherapy or Metastasectomy: Key to Long-Lasting Event-Free Survival? Available online: https://clinicaltrials.gov/ct2/show/NCT05352178 (accessed on 15 December 2022).
- Cheung, D.C.; Martin, L.J.; Alibhai, S.M.; Komisarenko, M.; Dharma, C.; Niu, Y.; Warde, P.; Sridhar, S.S.; Fleshner, N.E.; Kulkarni, G.S.; et al. Androgen deprivation therapy for prostate cancer: Prescribing behaviors and preferences among urologists. Can. Urol. Assoc. J. 2022, 16, 351–357. [Google Scholar] [CrossRef]
- Ryan, C.J.; Ke, X.; Lafeuille, M.H.; Romdhani, H.; Kinkead, F.; Lefebvre, P.; Petrilla, A.; Pulungan, Z.; Kim, S.; D’Andrea, D.M.; et al. Management of Patients with Metastatic Castration-Sensitive Prostate Cancer in the Real-World Setting in the United States. J. Urol. 2021, 206, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Mar, N.; Forsyth, M. Prescribing patterns in patients with metastatic castrate-sensitive prostate cancer (mCSPC). J. Clin. Oncol. 2021, 39, 21. [Google Scholar] [CrossRef]
| CHAARTED [4,11] | STAMPEDE (DOC) [10] | LATITUDE [15] | STAMPEDE (AAP) [14,16,17] | TITAN [18] | ARCHES [19,20] | |
|---|---|---|---|---|---|---|
| N | 790 | 1776 (61% of patients with mCSPC) | 1199 | 1917 | 1052 | 1150 |
| Treatment arms | ADT + docetaxel ADT | SOC (ADT) SOC + docetaxel | ADT + abiraterone + prednisone ADT + placebo | SOC (ADT) SOC + abiraterone + prednisolone | ADT + apalutamide ADT + placebo | ADT + enzalutamide ADT + placebo |
| Disease risk | 65% high volume | 56% high burden | 100% high risk | 52% high risk (among M1) | 63% high volume | 63% high volume |
| Synchronous | 73% | 58% | 100% | 49% | 81% | 67% |
| Primary end point | OS: HR 0.72 (0.59–0.89), p = 0.0017 | OS: HR 0.78 (0.66–0.93), p = 0.006 OS: HR 0.76 (0.62–0.92) for M1, p = NS | OS: HR 0.66 (0.51–0.76), p < 0.0001 rPFS: HR 0.47 (0.39–0.55), p < 0.001 | OS: HR 0.61 for M1, p = 0.005 | rPFS: HR 0.48 (0.39–0.60), p < 0.0001 OS: HR 0.65 (0.51–0.89), p < 0.0001 | rPFS: HR 0.39 (0.30–0.50), p < 0.0001 OS (final analysis): HR 0.66 (0.53–0.81), p < 0.0001 |
| High risk/High volume | OS: HR 0.63 (0.50–0.79), p < 0.001 | OS: HR 0.81 (0.64–1.02), p = 0.064 | OS HR: 0.58 (0.41–0.83) | OS: HR 0.54 (0.41–0.70), p < 0.05 | OS HR: 0.68 (0.50–0.92) rPFS HR: 0.53 (0.41–0.67) | OS: HR 66 (0.52–0.83) |
| Low risk/Low volume | OS: HR 1.04 (0.70–1.55), p = 0.68 | OS: HR 0.76 (0.54–1.07), p = 0.207 | OS HR: 0.69 (0.58–0.82) | OS: HR 0.66 (0.44–0.98), p < 0.05 | OS: HR0.67 (0.34–1.32) rPFS: HR 0.36 (0.22–0.57) | OS: HR 0.66 (0.43–1.02) |
| QOL | Worse for ADT + docetaxel at Month 3 (FACT-P = 116.3 vs. 118.3) but better by Month 12 (FACT-P = 119.2 vs. 116.4) | Improved for ADT + abiraterone + prednisone | No difference/ Maintained | No difference/ Maintained | ||
| Select adverse events (Gr ≥ 3) | Febrile neutropenia: 6% | Febrile neutropenia: 15% | Grade 3/4: 68% Most common: hypertension, hypokalemia | Grade 3–5: 47% Most common: endocrine, cardiovascular disorders | Grade 3–4: 42.2% Most common: hypertension, rash | Grade ≥ 3: 24.3% Most common: hypertension, fatigue |
| ARASENS [8,35] | PEACE-1 [7] | ENZAMET [21,22] | |
|---|---|---|---|
| N | 1306 | 1173 | 1125 |
| Treatment arms | ADT + Docetaxel + Darolutamide vs. ADT + Docetaxel | SOC vs. SOC + AAP (SOC included docetaxel in 710 patients) | ADT + enzalutamide ADT + NSAA |
| Disease volume | 77% high volume | 64.2% high volume | 52% high volume |
| Synchronous | 86.1% | 100% | 61% |
| Primary end point | OS | rPFS and OS | OS |
| Primary end point | OS: HR 0.68 (0.57–0.80), p < 0.001 | rPFS HR: 0.50 (0.40–0.62), p < 0.0001 OS: HR 0.75 (0.59–0.95), p = 0.017 | OS: HR 0.67 (0.52–0.86), p = 0.002 |
| Key Secondary end points | Time to CRPC: HR 0.36 (0.30–0.42), p < 0.0001 | CRPC free survival: HR 0·38, 95% CI 0·31–0·47; p < 0·0001 Prostate cancer specific survival: HR 0·69, 95% CI 0·53–0·90; p = 0·0062 | PFS; HR 0.40 (0.33–0.49), p < 0.001 |
| High volume | OS HR: 0.68 (0.57–0.82) | OS: HR 0.72 (0.55–0.95), p = 0.019 | NA |
| Low volume | OS HR: 0.68 (0.41–1.13) | OS: HR 0.83 (0.50–1.38), p = 0.66 | NA |
| QOL | Not reported yet | Not reported yet | No difference/ Maintained |
| Select adverse events (Gr ≥ 3) | Febrile Neutropenia rates: 7.8% vs. 7.4% Hypertension- 6.4% vs. 3.2% Increased liver enzymes- 5.4% vs. 2.8% | Febrile Neutropenia 5% with docetaxel Hypertension- 22% with AAP vs. 13% in SOC Hepatotoxicity 6% in AAP vs. 1% in SOC | Enzalutamide + docetaxel, 65% completed planned 6 cycles Among patients who received docetaxel, >grade2 neuropath 9% with docetaxel and 3% without |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mittal, A.; Sridhar, S.S.; Ong, M.; Jiang, D.M. Triplet Therapy in Metastatic Castrate Sensitive Prostate Cancer (mCSPC)—A Potential New Standard of Care. Curr. Oncol. 2023, 30, 4365-4378. https://doi.org/10.3390/curroncol30040332
Mittal A, Sridhar SS, Ong M, Jiang DM. Triplet Therapy in Metastatic Castrate Sensitive Prostate Cancer (mCSPC)—A Potential New Standard of Care. Current Oncology. 2023; 30(4):4365-4378. https://doi.org/10.3390/curroncol30040332
Chicago/Turabian StyleMittal, Abhenil, Srikala S. Sridhar, Michael Ong, and Di Maria Jiang. 2023. "Triplet Therapy in Metastatic Castrate Sensitive Prostate Cancer (mCSPC)—A Potential New Standard of Care" Current Oncology 30, no. 4: 4365-4378. https://doi.org/10.3390/curroncol30040332
APA StyleMittal, A., Sridhar, S. S., Ong, M., & Jiang, D. M. (2023). Triplet Therapy in Metastatic Castrate Sensitive Prostate Cancer (mCSPC)—A Potential New Standard of Care. Current Oncology, 30(4), 4365-4378. https://doi.org/10.3390/curroncol30040332





