Current Concepts in the Resection of Bone Tumors Using a Patient-Specific Three-Dimensional Printed Cutting Guide
Abstract
1. Introduction
2. Digital Imaging Applications for Surgical Interventions
2.1. Reproduction of 3D Images
2.2. Computer-Aided Surgical Simulation
3. 3D-printing Technique for Surgical Planning
3.1. Advantages of the 3D-Printing Technique
3.1.1. Resection with Safe Margins
3.1.2. Reconstruction of Bone Defects
3.1.3. Understanding of Anatomy and Surgical Planning
3.1.4. Reduction of Surgical Invasiveness and Operation Time
3.2. Limitations of the 3D-Printing Technique
3.2.1. Delay and Cost of Surgery
3.2.2. Learning Curves
3.2.3. Properties of Surgical Materials
3.3. Validation Studies
3.3.1. Cadaver Studies
3.3.2. Clinical Application
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gkagkalis, G.; Moerenhout, K.; Rüdiger, H.A.; Müller, D.A.; Letovanec, I.; Cherix, S. Pelvic chondrosarcoma treated by en bloc resection with patient-specific osteotomy guides and reimplantation of the extracorporeally irradiated bone as an osseocartilaginous structural orthotopic autograft: A report of two cases with description of the surgical technique. Case. Rep. Orthop. 2021, 2021, 5512143. [Google Scholar] [PubMed]
- Angelini, A.; Trovarelli, G.; Berizzi, A.; Pala, E.; Breda, A.; Ruggieri, P. Three-dimension-printed custom-made prosthetic reconstructions: From revision surgery to oncologic reconstructions. Int. Orthop. 2019, 43, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Vaishya, R.; Patralekh, M.K.; Vaish, A.; Agarwal, A.K.; Vijay, V. Publication trends and knowledge mapping in 3D printing in orthopaedics. J. Clin. Orthop. Trauma 2018, 9, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Wang, J.; Sun, T.; Zhang, W.; Zhang, J.; Shu, L.; Li, Z. Clinical applications and prospects of 3D printing guide templates in orthopaedics. J. Orthop. Translat. 2022, 34, 22–41. [Google Scholar] [CrossRef]
- Cartiaux, O.; Docquier, P.L.; Paul, L.; Francq, B.G.; Cornu, O.H.; Delloye, C.; Raucent, B.; Dehez, B.; Banse, X. Surgical inaccuracy of tumor resection and reconstruction within the pelvis: An experimental study. Acta Orthop. 2008, 79, 695–702. [Google Scholar] [CrossRef]
- Müller, D.A.; Stutz, Y.; Vlachopoulos, L.; Farshad, M.; Fürnstahl, P. The accuracy of three-dimensional planned bone tumor resection using patient-specific instrument. Cancer. Manag. Res. 2020, 12, 6533–6540. [Google Scholar] [CrossRef]
- Dong, C.; Beglinger, I.; Krieg, A.H. Personalized 3D-printed guide in malignant bone tumor resection and following reconstruction-17 cases in pelvic and extremities. J. Surg. Oncol. 2022, 42, 101733. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, Y.; Lin, Z.; Wang, Y.; Zhang, Y.; Xia, H.; Mao, C. 3D-printed guiding templates for improved osteosarcoma resection. Sci. Rep. 2016, 6, 23335. [Google Scholar] [CrossRef]
- Donati, D.; El Ghoneimy, A.; Bertoni, F.; Di Bella, C.; Mercuri, M. Surgical treatment and outcome of conventional pelvic chondrosarcoma. J. Bone Joint Surg. Br. 2005, 87, 1527–1530. [Google Scholar] [CrossRef]
- Gouin, F.; Paul, L.; Odri, G.A.; Cartiaux, O. Computer- assisted planning and patient-specific instruments for bone tumor resection within the pelvis: A series of 11 patients. Sarcoma 2014, 2014, 842709. [Google Scholar] [CrossRef]
- Biscaccianti, V.; Fragnaud, H.; Hascoët, J.Y.; Crenn, V.; Vidal, L. Digital chain for pelvic tumor resection with 3D-printed surgical cutting guides. Front. Bioeng. Biotechnol. 2022, 10, 991676. [Google Scholar] [CrossRef] [PubMed]
- Trace, A.P.; Ortiz, D.; Deal, A.; Retrouvey, M.; Elzie, C.; Goodmurphy, C.; Morey, J.; Hawkins, C.M. Radiology’s emerging role in 3-D printing applications in health care. J. Am. Coll. Radiol. 2016, 13, 856–862.e4. [Google Scholar] [CrossRef] [PubMed]
- Mensel, C.; Gundtoft, P.H.; Brink, O. Preoperative templating in orthopaedic fracture surgery: The past, present and future. Injury 2022, 53 (Suppl. S3), S42–S46. [Google Scholar] [CrossRef] [PubMed]
- Frizziero, L.; Santi, G.M.; Leon-Cardenas, C.; Donnici, G.; Liverani, A.; Papaleo, P.; Napolitano, F.; Pagliari, C.; Di Gennaro, G.L.; Stallone, S.; et al. In-house, fast FDM prototyping of a custom cutting guide for a lower-risk pediatric femoral osteotomy. Bioengineering 2021, 8, 71. [Google Scholar] [CrossRef]
- Han, Q.; Zhao, X.; Wang, C.; Chen, B.; Wang, X.; Zhang, Z.; Zhang, K.; Zheng, Y.; Wang, J. Individualized reconstruction for severe periprosthetic fractures around the tumor prosthesis of knee under assistance of 3D printing technology: A case report. Medicine (Baltimore) 2018, 97, e12726. [Google Scholar] [CrossRef]
- Hoang, D.; Perrault, D.; Stevanovic, M.; Ghiassi, A. Surgical applications of three-dimensional printing: A review of the current literature & how to get started. Ann. Transl. Med. 2016, 4, 456. [Google Scholar]
- Petzold, R.; Zeilhofer, H.F.; Kalender, W.A. Rapid protyping technology in medicine--basics and applications. Comput. Med. Imaging Graph. 1999, 23, 277–284. [Google Scholar] [CrossRef]
- García-Sevilla, M.; Mediavilla-Santos, L.; Ruiz-Alba, M.T.; Pérez-Mañanes, R.; Calvo-Haro, J.A.; Pascau, J. Patient-specific desktop 3D-printed guides for pelvic tumour resection surgery: A precision study on cadavers. Int. J. Comput. Assist. Radiol. Surg. 2021, 16, 397–406. [Google Scholar] [CrossRef]
- Park, J.W.; Kang, H.G. Application of 3-dimensional printing implants for bone tumors. Clin. Exp. Pediatr. 2022, 65, 476–482. [Google Scholar] [CrossRef]
- Khan, F.A.; Lipman, J.D.; Pearle, A.D.; Boland, P.J.; Healey, J.H. Surgical technique: Computer-generated custom jigs improve accuracy of wide resection of bone tumors. Clin. Orthop. Relat. Res. 2013, 471, 2007–2016. [Google Scholar] [CrossRef]
- Helguero, C.G.; Kao, I.; Komatsu, D.E.; Shaikh, S.; Hansen, D.; Franco, J.; Khan, F. Improving the accuracy of wide resection of bone tumors and enhancing implant fit: A cadaveric study. J. Orthop. 2015, 12 (Suppl. S2), S188–S194. [Google Scholar] [CrossRef]
- Bosma, S.E.; Wong, K.C.; Paul, L.; Gerbers, J.G.; Jutte, P.C. A cadaveric comparative study on the surgical accuracy of freehand, computer navigation, and patient-specific instruments in joint-preserving bone tumor resections. Sarcoma 2018, 2018, 4065846. [Google Scholar] [CrossRef] [PubMed]
- Sallent, A.; Vicente, M.; Reverté, M.M.; Lopez, A.; Rodríguez-Baeza, A.; Pérez-Domínguez, M.; Velez, R. How 3D patient-specific instruments improve accuracy of pelvic bone tumour resection in a cadaveric study. Bone Joint Res. 2017, 6, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Evrard, R.; Schubert, T.; Paul, L.; Docquier, P.L. Quality of resection margin with patient specific instrument for bone tumor resection. J. Bone Oncol. 2022, 34, 100434. [Google Scholar] [CrossRef] [PubMed]
- Bellanova, L.; Paul, L.; Docquier, P.L. Surgical guides (patient-specific instruments) for pediatric tibial bone sarcoma resection and allograft reconstruction. Sarcoma 2013, 2013, 787653. [Google Scholar] [CrossRef]
- Li, Z.; Chen, G.; Xiang, Y.; Muheremu, A.; Wu, X.; He, P.; Fan, H.; Liu, J.; Chen, C.; Yang, L.; et al. Treatment of massive iliac chondrosarcoma with personalized three-dimensional printed tantalum implant: A case report and literature review. J. Int. Med. Res. 2020, 48, 300060520959508. [Google Scholar] [CrossRef]
- Kieser, D.C.; Ailabouni, R.; Kieser, S.C.J.; Wyatt, M.C.; Armour, P.C.; Coates, M.H.; Hooper, G.J. The use of an Ossis custom 3D-printed tri-flanged acetabular implant for major bone loss: Minimum 2-year follow-up. Hip. Int. 2018, 28, 668–674. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Lu, W.; Liao, S.; Du, Q.; Deng, Z.; Lu, W. Combined application of modified three-dimensional printed anatomic templates and customized cutting blocks in pelvic reconstruction after pelvic tumor resection. J. Arthroplasty 2019, 34, 338–345.e1. [Google Scholar] [CrossRef]
- Riggs, K.W.; Dsouza, G.; Broderick, J.T.; Moore, R.A.; Morales, D.L.S. 3D-printed models optimize preoperative planning for pediatric cardiac tumor debulking. Transl. Pediatr. 2018, 7, 196–202. [Google Scholar] [CrossRef]
- Hu, H.; Liu, W.; Zeng, Q.; Wang, S.; Zhang, Z.; Liu, J.; Zhang, Y.; Shao, Z.; Wang, B. The personalized shoulder reconstruction assisted by 3d printing technology after resection of the proximal humerus tumours. Cancer. Manag. Res. 2019, 11, 10665–10673. [Google Scholar] [CrossRef]
- Kovler, I.; Joskowicz, L.; Weil, Y.A.; Khoury, A.; Kronman, A.; Mosheiff, R.; Liebergall, M.; Salavarrieta, J. Haptic computer-assisted patient-specific preoperative planning for orthopedic fractures surgery. Int. J. Comput. Assist. Radiol. Surg. 2015, 10, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Chua, C.; Chen, Y.S.; Murphy, D.; O ’Neill, G.K. Utility of 3D printed models as adjunct in acetabular fracture teaching for Orthopaedic trainees. BMC Med. Educ. 2022, 22, 595. [Google Scholar] [CrossRef] [PubMed]
- Ritacco, L.E.; Milano, F.E.; Farfalli, G.L.; Ayerza, M.A.; Muscolo, D.L.; Aponte-Tinao, L.A. Accuracy of 3-D planning and navigation in bone tumor resection. Orthopedics 2013, 36, e942–e950. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhu, J.; Peng, X.; Su, J. The application of 3D printed surgical guides in resection and reconstruction of malignant bone tumor. Oncol. Lett. 2017, 14, 4581–4584. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.C.; Sze, K.Y.; Wong, I.O.; Wong, C.M.; Kumta, S.M. Patient-specific instrument can achieve same accuracy with less resection time than navigation assistance in periacetabular pelvic tumor surgery: A cadaveric study. Int. J. Comput. Assist. Radiol. Surg. 2016, 11, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.C. 3D-printed patient-specific applications in orthopedics. Orthop. Res. Rev. 2016, 8, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Rustemeyer, J.; Melenberg, A.; Sari-Rieger, A. Costs incurred by applying computer-aided design/computer-aided manufacturing techniques for the reconstruction of maxillofacial defects. J. Craniomaxillofac. Surg. 2014, 42, 2049–2055. [Google Scholar] [CrossRef]
- Martelli, N.; Serrano, C.; van den Brink, H.; Pineau, J.; Prognon, P.; Borget, I.; El Batti, S. Advantages and disadvantages of 3-dimensional printing in surgery: A systematic review. Surgery 2016, 159, 1485–1500. [Google Scholar] [CrossRef]
- Kadakia, R.J.; Wixted, C.M.; Allen, N.B.; Hanselman, A.E.; Adams, S.B. Clinical applications of custom 3D printed implants in complex lower extremity reconstruction. 3D Print Med. 2020, 6, 29. [Google Scholar] [CrossRef]
- Calvo-Haro, J.A.; Pascau, J.; Mediavilla-Santos, L.; Sanz-Ruiz, P.; Sánchez-Pérez, C.; Vaquero-Martín, J.; Perez-Mañanes, R. Conceptual evolution of 3D printing in orthopedic surgery and traumatology: From “do it yourself” to “point of care manufacturing”. BMC Musculoskelet Disord. 2021, 22, 360. [Google Scholar] [CrossRef]
- Fidanza, A.; Perinetti, T.; Logroscino, G.; Saracco, M. 3D Printing Applications in Orthopaedic Surgery: Clinical Experience and Opportunities. Appl. Sci. 2022, 12, 3245. [Google Scholar] [CrossRef]
- Leeuwen, J.A.; Grøgaard, B.; Nordsletten, L.; Röhrl, S.M. Comparison of planned and achieved implant position in total knee arthroplasty with patient-specific positioning guides. Acta Orthop. 2015, 86, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.C.; Kumta, S.M.; Sze, K.Y.; Wong, C.M. Use of a patient-specific CAD/CAM surgical jig in extremity bone tumor resection and custom prosthetic reconstruction. Comput. Aided Surg. 2012, 17, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.C.; Sze, L.K.Y.; Kumta, S.M. Complex joint-preserving bone tumor resection and reconstruction using computer navigation and 3D-printed patient-specific guides: A technical note of three cases. J. Orthop. Translat. 2021, 29, 152–162. [Google Scholar] [CrossRef]
- Aponte-Tinao, L.A.; Ritacco, L.E.; Ayerza, M.A.; Muscolo, D.L.; Farfalli, G.L. Multiplanar osteotomies guided by navigation in chondrosarcoma of the knee. Orthopedics 2013, 36, e325–e330. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Zhong, L.; Qiu, L.; Xu, L.; Sun, Y.; Niu, X.; Zhang, L. New perspectives on surgical accuracy analysis of image-guided bone tumour resection surgery. Int. Orthop. 2020, 44, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Mustahsan, V.M.; He, G.; Helguero, C.G.; Blum, C.L.; Komatsu, D.E.; Pentyala, S.; Kao, I.; Khan, F. Novel positioning feedback system as a guidance in bone tumor resection. Surg. Innov. 2022, 30, 15533506221106070. [Google Scholar] [CrossRef] [PubMed]
- Van den Broeck, J.; Wirix-Speetjens, R.; Vander Sloten, J. Preoperative analysis of the stability of fit of a patient-specific surgical guide. Comput. Methods Biomech. Biomed. Eng. 2015, 18, 38–47. [Google Scholar] [CrossRef]
- Cho, H.S.; Park, Y.K.; Gupta, S.; Yoon, C.; Han, I.; Kim, H.S.; Choi, H.; Hong, J. Augmented reality in bone tumour resection: An experimental study. Bone Joint Res. 2017, 6, 137–143. [Google Scholar] [CrossRef]
- Bruns, N.; Krettek, C. 3D-printing in trauma surgery: Planning, printing and processing. Unfallchirurg 2019, 122, 270–277. [Google Scholar] [CrossRef]
- Gokuldoss, P.K.; Kolla, S.; Eckert, J. Additive manufacturing processes: Selective laser melting, electron beam melting and binder jetting-selection guidelines. Materials 2017, 10, 672. [Google Scholar] [CrossRef] [PubMed]
- Peniston, S.J.; Choi, S.J. Effect of sterilization on the physicochemical properties of molded poly(L-lactic acid). J. Biomed. Mater. Res. B Appl. Biomater. 2007, 80, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Mañanes, R.; Burró, J.A.; Manaute, J.R.; Rodriguez, F.C.; Martín, J.V. 3D surgical printing cutting guides for open-wedge high tibial osteotomy: Do it yourself. J. Knee Surg. 2016, 29, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yuchan, K.Z.; Chen, L.F. Mechanical characterization of biocompatible PEEK by FDM. J. Manuf. Process. 2020, 56, 28–42. [Google Scholar] [CrossRef]
- Chen, J.V.; Tanaka, K.S.; Dang, A.B.C.; Dang, A. Identifying a commercially-available 3D printing process that minimizes model distortion after annealing and autoclaving and the effect of steam sterilization on mechanical strength. 3D Print Med. 2020, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Evrard, R.; Schubert, T.; Paul, L.; Docquier, P.L. Resection margins obtained with patient-specific instruments for resecting primary pelvic bone sarcomas: A case-control study. Orthop. Traumatol. Surg. Res. 2019, 105, 781–787. [Google Scholar] [CrossRef]
- Park, J.W.; Kang, H.G.; Lim, K.M.; Park, D.W.; Kim, J.H.; Kim, H.S. Bone tumor resection guide using three-dimensional printing for limb salvage surgery. J. Surg. Oncol. 2018, 118, 898–905. [Google Scholar] [CrossRef]
- Liu, W.; Shao, Z.; Rai, S.; Hu, B.; Wu, Q.; Hu, H.; Zhang, S.; Wang, B. Three-dimensional-printed intercalary prosthesis for the reconstruction of large bone defect after joint-preserving tumor resection. J. Surg. Oncol. 2020, 121, 570–577. [Google Scholar] [CrossRef]
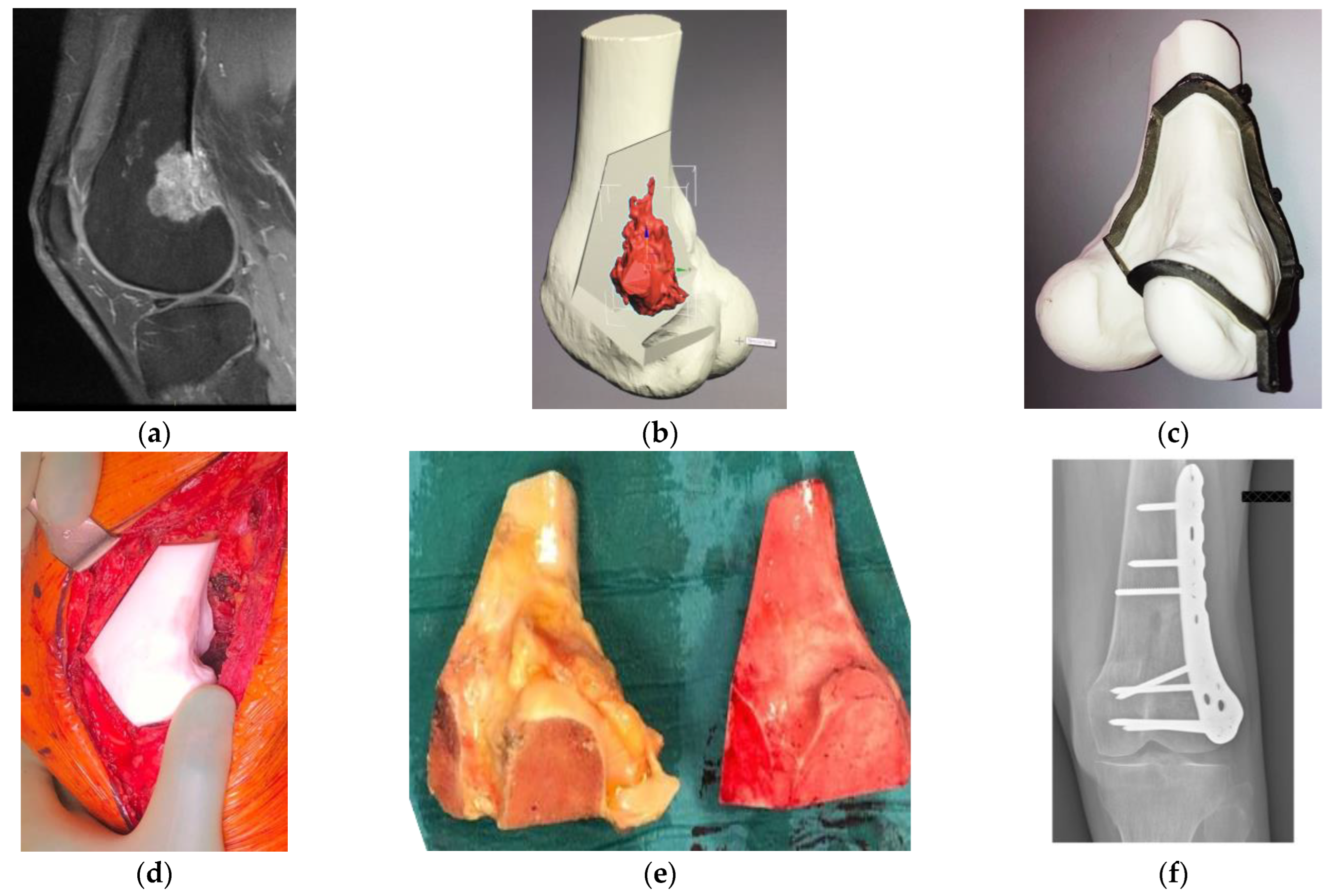
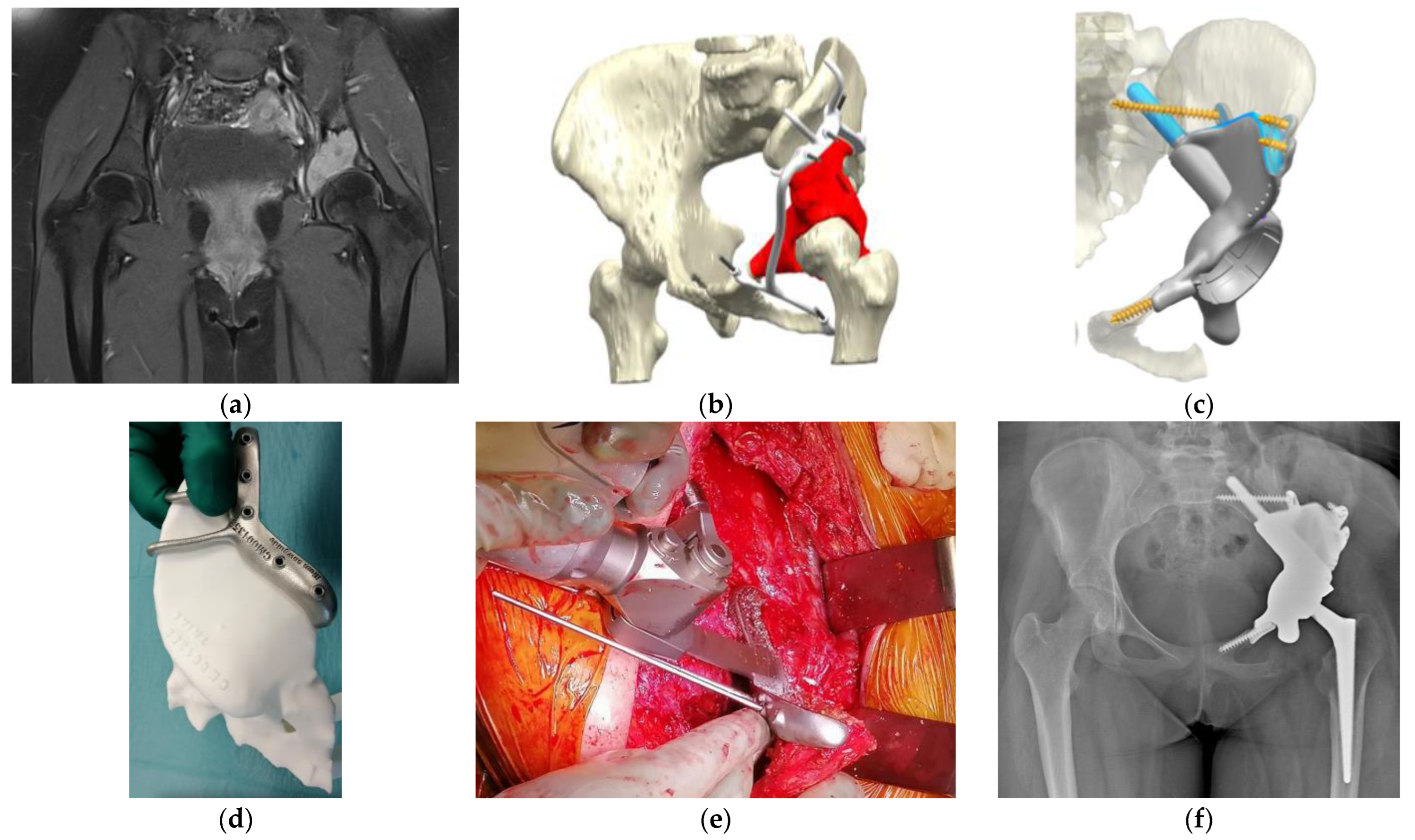
| Authors | Site | Technology | Evaluation | Results |
|---|---|---|---|---|
| Khan et al. [20] | Femur | MAN vs. PSG | Deviation from the planned osteotomy line | 9.0 mm (MAN) vs. 2.0 mm (PSG), p = 0.002 |
| Helguero et al. [21] | Femur | MAN vs. PSG | Deviation from the planned osteotomy angle | MAN: large gaps (>5 mm) between the implant and the bone PSG: no large gaps, no statistics |
| Bosma et al. [22] | Femur, tibia | MAN vs. NVI vs. PSG vs. NVI + PSG | Deviation from the planned osteotomy line | 9.2 mm (MAN), 3.6 mm (NVI), 1.9 mm (PSG), and 2.0 mm (NVI + PSG) *1 |
| Sallent et al. [23] | Pelvis | MAN vs. PSG | Deviation from the planned osteotomy line | Sacroiliac: 14.6 mm (MAN) vs. 5.0 mm (PSG), p = 0.008 Supra-acetabular: 10.2 mm (MAN) vs. 4.0 mm (PSG), p = 0.008) Ischial: 5.2 mm (MAN) vs. 2.2 mm (PSG), p = 0.016 Iliopubic: 3.0 mm (MAN) vs. 0.8 mm (PSG), p = 0.032 |
| García-Sevilla et al. [18] | Pelvis | PSG | Translations and rotations of the planned osteotomy plane | Iliac crest: mean translation, 5.3 mm; mean rotation, 6.7° Supra-acetabular: mean translation, 1.8 mm; mean rotation, 5.1° Ischial: mean translation, 1.5 mm; mean rotation, 3.4° Pubic: mean translation, 1.8 mm; mean rotation, 3.5° |
| Authors and Study Type | Tumor | Site | Patient Number | Surgical Technique | Negative Surgical Margin | Blood Loss (Mean) | Operation Time (Mean) | Local Recurrence | Accuracy of Osteotomy |
|---|---|---|---|---|---|---|---|---|---|
| Gouin et al. [10], case series | CS, EWS, SS | Pelvis | 11 | PSG | 100% | NA | NA | 9% | Mean cutting error * = 0.8 mm |
| Ma et al. [8], case series | OS | Femur | 8 | PSG + ALO | NA | 746 mL | 213 min | 0% | NA |
| Wang et al. [34], randomized control study | CS, GCT, OS | Femur, tibia | 33 | PSG + ALO | 90.9% (CTR) vs. 93.9% (PSG); NS | 689 mL (CTR) vs. 650 mL (PSG); p = 0.037 | 136 min (CTR) vs. 145 min (PSG); p = 0.685 | 15.2% (CTR) vs. 9.1% (PSG); p = 0.708 | NA |
| Evrard et al. [56], case-control study | CS, EWS, OS | Pelvis | 9 | PSG + ALO | 68.4% (CTR) vs. 89% (PSG); p = 0.479 | NA | 633 min (CTR) vs. 612 min (PSG); p = 0.877 | 37% (CTR) vs. 0% (PSG); p = 0.035 | NA |
| Park et al. [57], case series | CS, Meta, OS, SS | Various | 12 | PSG + PSI or ALO | 100% | NA | 118 min | 8.3% | Maximal cutting error = 3 mm |
| Hu et al. [30], case series | CS, GCT, OS | Shoulder | 7 | PSG + PSP + RSA | 100% | NA | NA | 0% | NA |
| Liu et al. [28], case-control study | CS, EWS, SS | Pelvis | 19 | PSG + PSI | 89.4% (CTR) vs. 100% (PSG), p = NA | 2,248 mL (CTR) vs. 1,390 mL (PSG); p = 0.002 | 272 min (CTR) vs. 209 min (PSG); p = 0.002 | 42% (CTR) vs. 5% (PSG); p = 0.008 | 5 mm deviation from the planned margin, 58% (CTR) vs. 0% (PSG), p = NA |
| Müller et al. [6], case series | CS, EWS, OS | Various | 12 | PSG + ALO | 92% | NA | NA | 0% | Range of cutting error = 0.7–3.6 mm |
| Liu et al. [58], case series | CS, EWS, SS | Femur, tibia (intercalary) | 19 | PSG + PSI | 100% | NA | 155 min | 8.3% | Mean cutting error = 1.9 mm |
| Wong et al. [44], case series | EWS, OS | Femur, tibia | 3 | PSG + NVI | 100% | NA | 276 min | 0% | Mean cutting error = 1.6 mm |
| Evrard et al. [24], case series | ADA, CS, EWS, FD GCT, OS, SS | Various | 31 | PSG | 100% | NA | NA | NA | Mean cutting error = 0.4 mm |
| Dong et al. [7], case series | EWS, Meta, OS, CS, GCT | Pelvis, femur, tibia | 17 | PSG + ALO or AUTO | 98% | Pelvis, 2,100 mL Limb, 957 mL | 618 min | 0% | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aiba, H.; Spazzoli, B.; Tsukamoto, S.; Mavrogenis, A.F.; Hermann, T.; Kimura, H.; Murakami, H.; Donati, D.M.; Errani, C. Current Concepts in the Resection of Bone Tumors Using a Patient-Specific Three-Dimensional Printed Cutting Guide. Curr. Oncol. 2023, 30, 3859-3870. https://doi.org/10.3390/curroncol30040292
Aiba H, Spazzoli B, Tsukamoto S, Mavrogenis AF, Hermann T, Kimura H, Murakami H, Donati DM, Errani C. Current Concepts in the Resection of Bone Tumors Using a Patient-Specific Three-Dimensional Printed Cutting Guide. Current Oncology. 2023; 30(4):3859-3870. https://doi.org/10.3390/curroncol30040292
Chicago/Turabian StyleAiba, Hisaki, Benedetta Spazzoli, Shinji Tsukamoto, Andreas F. Mavrogenis, Tomas Hermann, Hiroaki Kimura, Hideki Murakami, Davide Maria Donati, and Costantino Errani. 2023. "Current Concepts in the Resection of Bone Tumors Using a Patient-Specific Three-Dimensional Printed Cutting Guide" Current Oncology 30, no. 4: 3859-3870. https://doi.org/10.3390/curroncol30040292
APA StyleAiba, H., Spazzoli, B., Tsukamoto, S., Mavrogenis, A. F., Hermann, T., Kimura, H., Murakami, H., Donati, D. M., & Errani, C. (2023). Current Concepts in the Resection of Bone Tumors Using a Patient-Specific Three-Dimensional Printed Cutting Guide. Current Oncology, 30(4), 3859-3870. https://doi.org/10.3390/curroncol30040292







