Predictive Factors for Anastomotic Leakage Following Colorectal Cancer Surgery: Where Are We and Where Are We Going?
Abstract
1. Introduction
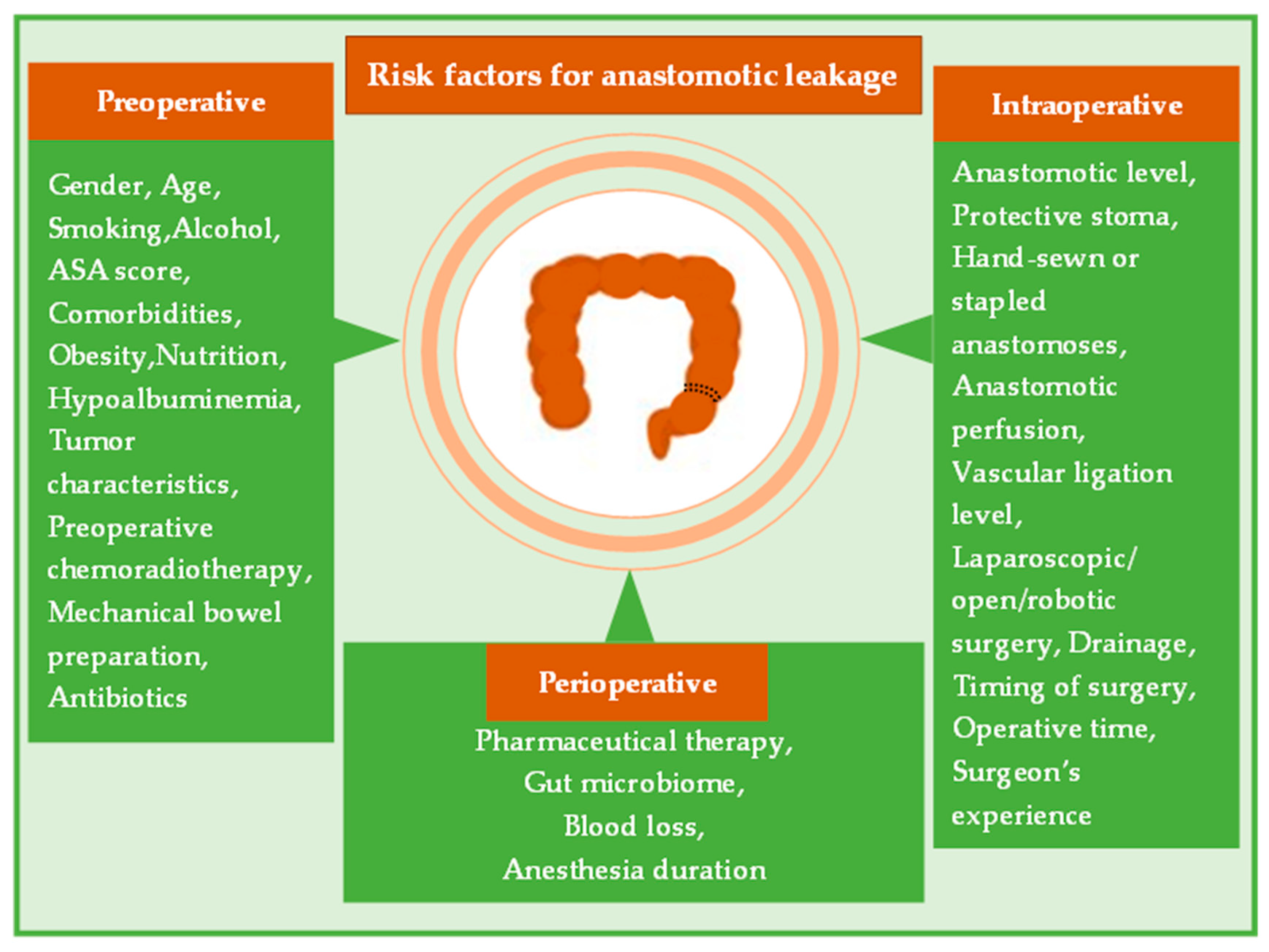
2. Risk Factors for Anastomotic Leakage
2.1. Preoperative Risk Factors
2.1.1. Gender and Age
2.1.2. Smoking and Alcohol
2.1.3. American Society of Anesthesiologists (ASA) Score and Comorbidities
2.1.4. Obesity, Nutrition and Hypoalbuminemia
2.1.5. Tumor Characteristics
2.1.6. Preoperative Chemoradiotherapy (PCRT)
2.1.7. Mechanical Bowel Preparation (MBP) and Antibiotics
2.2. Intraoperative Risk Factors
2.2.1. Anastomotic Level
2.2.2. Protective Stoma
2.2.3. Hand-Sewn vs. Stapled Anastomoses
2.2.4. Anastomotic Perfusion and Vascular Ligation Level
2.2.5. Laparoscopic vs. Open and Robotic Colorectal Surgery
2.2.6. Pelvic and Transanal Drain Placement
2.2.7. Timing of CRC Surgery (Elective vs. Emergency)
2.2.8. Operative Time
2.2.9. Surgeon’s Experience
2.3. Perioperative Risk Factors
2.3.1. Pharmaceutical Therapy
2.3.2. Gut Microbiome
2.3.3. Other Perioperative Complications and Events
3. Clinical Presentation
4. Diagnostic Workup
Scoring Systems and Other Predictive Biomarkers of Anastomotic Leakage
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Vilahur, N.; Bianchini, F.; Guha, N.; Straif, K.; International Agency for Research on Cancer Handbook Working Group. The IARC Perspective on Colorectal Cancer Screening. N. Engl. J. Med. 2018, 378, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Branagan, G.; Finnis, D.; Wessex Colorectal Cancer Audit Working Group. Prognosis after Anastomotic Leakage in Colorectal Surgery. Dis. Colon Rectum 2005, 48, 1021–1026. [Google Scholar] [CrossRef]
- Mirnezami, A.; Mirnezami, R.; Chandrakumaran, K.; Sasapu, K.; Sagar, P.; Finan, P. Increased Local Recurrence and Reduced Survival from Colorectal Cancer Following Anastomotic Leak: Systematic Review and Meta-Analysis: Systematic Review and Meta-Analysis. Ann. Surg. 2011, 253, 890–899. [Google Scholar] [CrossRef]
- Phillips, B. Reducing Gastrointestinal Anastomotic Leak Rates: Review of Challenges and Solutions. Open Access Surg. 2016, 5, 5–14. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Weitz, J.; Hohenberger, W.; Heald, R.J.; Moran, B.; Ulrich, A.; Holm, T.; Wong, W.D.; Tiret, E.; Moriya, Y.; et al. Definition and Grading of Anastomotic Leakage Following Anterior Resection of the Rectum: A Proposal by the International Study Group of Rectal Cancer. Surgery 2010, 147, 339–351. [Google Scholar] [CrossRef] [PubMed]
- van Helsdingen, C.P.; Jongen, A.C.; de Jonge, W.J.; Bouvy, N.D.; Derikx, J.P. Consensus on the Definition of Colorectal Anastomotic Leakage: A Modified Delphi Study. World J. Gastroenterol. 2020, 26, 3293–3303. [Google Scholar] [CrossRef] [PubMed]
- Hyman, N.; Manchester, T.L.; Osler, T.; Burns, B.; Cataldo, P.A. Anastomotic Leaks after Intestinal Anastomosis: It’s Later than You Think. Ann. Surg. 2007, 245, 254–258. [Google Scholar] [CrossRef]
- Kanellos, I.; Vasiliadis, K.; Angelopoulos, S.; Tsachalis, T.; Pramateftakis, M.G.; Mantzoros, I.; Betsis, D. Anastomotic Leakage Following Anterior Resection for Rectal Cancer. Tech. Coloproctol. 2004, 8 (Suppl. S1), s79–s81. [Google Scholar] [CrossRef]
- Zarnescu, E.C.; Zarnescu, N.O.; Costea, R. Updates of Risk Factors for Anastomotic Leakage after Colorectal Surgery. Diagnostics 2021, 11, 2382. [Google Scholar] [CrossRef]
- Ray, M.D. Management of Anastomotic Leak. In Multidisciplinary Approach to Surgical Oncology Patients; Springer: Singapore, 2021; pp. 233–237. [Google Scholar]
- Gray, M.; Marland, J.R.K.; Murray, A.F.; Argyle, D.J.; Potter, M.A. Predictive and Diagnostic Biomarkers of Anastomotic Leakage: A Precision Medicine Approach for Colorectal Cancer Patients. J. Pers. Med. 2021, 11, 471. [Google Scholar] [CrossRef] [PubMed]
- Brisinda, G.; Vanella, S.; Cadeddu, F.; Mazzeo, P. Colonic Anastomotic Leak: Risk Factors, Diagnosis, and Treatment. J. Am. Coll. Surg. 2009, 208, 1152–1153; author reply 1153–1154. [Google Scholar] [CrossRef]
- Alverdy, J.C.; Schardey, H.M. Anastomotic Leak: Toward an Understanding of Its Root Causes. J. Gastrointest. Surg. 2021, 25, 2966–2975. [Google Scholar] [CrossRef]
- Park, J.S.; Choi, G.-S.; Kim, S.H.; Kim, H.R.; Kim, N.K.; Lee, K.Y.; Kang, S.B.; Kim, J.Y.; Lee, K.Y.; Kim, B.C.; et al. Multicenter Analysis of Risk Factors for Anastomotic Leakage after Laparoscopic Rectal Cancer Excision: The Korean Laparoscopic Colorectal Surgery Study Group. Ann. Surg. 2013, 257, 665–671. [Google Scholar] [CrossRef]
- Law, W.I.; Chu, K.W.; Ho, J.W.; Chan, C.W. Risk Factors for Anastomotic Leakage after Low Anterior Resection with Total Mesorectal Excision. Am. J. Surg. 2000, 179, 92–96. [Google Scholar] [CrossRef]
- Ba, Z.F.; Yokoyama, Y.; Toth, B.; Rue, L.W., 3rd; Bland, K.I.; Chaudry, I.H. Gender Differences in Small Intestinal Endothelial Function: Inhibitory Role of Androgens. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G452–G457. [Google Scholar] [CrossRef]
- Yu, Z.-L.; Liu, X.-H.; Liu, H.-S.; Ke, J.; Zou, Y.-F.; Cao, W.-T.; Xiao, J.; Zhou, Z.-Y.; Lan, P.; Wu, X.-J.; et al. Impact of Pelvic Dimensions on Anastomotic Leak after Anterior Resection for Patients with Rectal Cancer. Surg. Endosc. 2021, 35, 2134–2143. [Google Scholar] [CrossRef] [PubMed]
- Verduin, W.M.; Warps, A.-L.K.; van den Helder, R.; Doodeman, H.J.; Houdijk, A.P.J.; INfluences of Fat And MUscle in colorectal Surgery Collaborative. Visceral Fat and Anastomotic Leakage after Colon Cancer Resection. Dis. Colon Rectum 2021, 64, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Rutegård, M.; Moshtaghi-Svensson, J.; Weibull, C.E.; Ottander, U.; Nordenvall, C.; Sund, M. Exposure to Oestrogen and Risk of Anastomotic Leakage after Colorectal Cancer Surgery—A Clue to the Different Leak Rates in Men and Women. Colorectal Dis. 2023, 25, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Alekseev, M.; Rybakov, E.; Khomyakov, E.; Zarodnyuk, I.; Shelygin, Y. Intraoperative Fluorescence Angiography as an Independent Factor of Anastomotic Leakage and a Nomogram for Predicting Leak for Colorectal Anastomoses. Ann. Coloproctol. 2022, 38, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Zaimi, I.; Sparreboom, C.L.; Lingsma, H.F.; Doornebosch, P.G.; Menon, A.G.; Kleinrensink, G.-J.; Jeekel, J.; Wouters, M.W.J.M.; Lange, J.F.; Dutch ColoRectal Audit Group. The Effect of Age on Anastomotic Leakage in Colorectal Cancer Surgery: A Population-Based Study. J. Surg. Oncol. 2018, 118, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Kim, H.; Park, H.; Lee, B.; Lee, K.Y. Risk Factors and Economic Burden of Postoperative Anastomotic Leakage Related Events in Patients Who Underwent Surgeries for Colorectal Cancer. PLoS ONE 2022, 17, e0267950. [Google Scholar]
- Boström, P.; Haapamäki, M.M.; Rutegård, J.; Matthiessen, P.; Rutegård, M. Population-Based Cohort Study of the Impact on Postoperative Mortality of Anastomotic Leakage after Anterior Resection for Rectal Cancer: Anastomotic Leakage and Mortality after Anterior Resection for Rectal Cancer. BJS Open 2019, 3, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-K.; Yueh, T.-C.; Chang, S.-C.; Lin, C.-C.; Lan, Y.-T.; Wang, H.-S.; Yang, S.-H.; Jiang, J.-K.; Chen, W.-S.; Lin, T.-C. The Influence of Fecal Diversion and Anastomotic Leakage on Survival after Resection of Rectal Cancer. J. Gastrointest. Surg. 2011, 15, 2251–2261. [Google Scholar] [CrossRef]
- Sripathi, S.; Khan, M.I.; Patel, N.; Meda, R.T.; Nuguru, S.P.; Rachakonda, S. Factors Contributing to Anastomotic Leakage Following Colorectal Surgery: Why, When, and Who Leaks? Cureus 2022, 14, e29964. [Google Scholar] [CrossRef]
- Pommergaard, H.C.; Gessler, B.; Burcharth, J.; Angenete, E.; Haglind, E.; Rosenberg, J. Preoperative Risk Factors for Anastomotic Leakage after Resection for Colorectal Cancer: A Systematic Review and Meta-Analysis. Colorectal Dis. 2014, 16, 662–671. [Google Scholar] [CrossRef]
- Konishi, T.; Watanabe, T.; Kishimoto, J.; Nagawa, H. Risk Factors for Anastomotic Leakage after Surgery for Colorectal Cancer: Results of Prospective Surveillance. J. Am. Coll. Surg. 2006, 202, 439–444. [Google Scholar] [CrossRef]
- Jannasch, O.; Klinge, T.; Otto, R.; Chiapponi, C.; Udelnow, A.; Lippert, H.; Bruns, C.J.; Mroczkowski, P. Risk Factors, Short and Long Term Outcome of Anastomotic Leaks in Rectal Cancer. Oncotarget 2015, 6, 36884–36893. [Google Scholar] [CrossRef]
- Bakker, I.S.; Grossmann, I.; Henneman, D.; Havenga, K.; Wiggers, T. Risk Factors for Anastomotic Leakage and Leak-Related Mortality after Colonic Cancer Surgery in a Nationwide Audit: Anastomotic Leakage after Colonic Cancer Surgery. Br. J. Surg. 2014, 101, 424–432; discussion 432. [Google Scholar] [CrossRef]
- Kwak, H.D.; Kim, S.-H.; Kang, D.W.; Baek, S.-J.; Kwak, J.M.; Kim, J. Risk Factors and Oncologic Outcomes of Anastomosis Leakage after Laparoscopic Right Colectomy. Surg. Laparosc. Endosc. Percutan. Tech. 2017, 27, 440–444. [Google Scholar] [CrossRef]
- Kim, M.J.; Shin, R.; Oh, H.-K.; Park, J.W.; Jeong, S.-Y.; Park, J.-G. The Impact of Heavy Smoking on Anastomotic Leakage and Stricture after Low Anterior Resection in Rectal Cancer Patients. World J. Surg. 2011, 35, 2806–2810. [Google Scholar] [CrossRef]
- Sørensen, L.T.; Jørgensen, T.; Kirkeby, L.T.; Skovdal, J.; Vennits, B.; Wille-Jørgensen, P. Smoking and Alcohol Abuse Are Major Risk Factors for Anastomotic Leakage in Colorectal Surgery: Anastomotic Leakage in Colorectal Surgery. Br. J. Surg. 1999, 86, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.P.; Talbott, V.A.; Leong, Q.Q.; Isenberg, G.A.; Goldstein, S.D. American Society of Anesthesiologists Class and Charlson’s Comorbidity Index as Predictors of Postoperative Colorectal Anastomotic Leak: A Single-Institution Experience. J. Surg. Res. 2013, 184, 115–119. [Google Scholar] [CrossRef]
- Cong, Z.-J.; Fu, C.-G.; Wang, H.-T.; Liu, L.-J.; Zhang, W.; Wang, H. Influencing Factors of Symptomatic Anastomotic Leakage after Anterior Resection of the Rectum for Cancer. World J. Surg. 2009, 33, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Slieker, J.C.; Komen, N.; Mannaerts, G.H.; Karsten, T.M.; Willemsen, P.; Murawska, M.; Jeekel, J.; Lange, J.F. Long-Term and Perioperative Corticosteroids in Anastomotic Leakage: A Prospective Study of 259 Left-Sided Colorectal Anastomoses: A Prospective Study of 259 Left-Sided Colorectal Anastomoses. Arch. Surg. 2012, 147, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Warschkow, R.; Steffen, T.; Thierbach, J.; Bruckner, T.; Lange, J.; Tarantino, I. Risk Factors for Anastomotic Leakage after Rectal Cancer Resection and Reconstruction with Colorectostomy. A Retrospective Study with Bootstrap Analysis. Ann. Surg. Oncol. 2011, 18, 2772–2782. [Google Scholar] [CrossRef]
- Krysa, J.; Patel, V.; Taylor, J.; Williams, A.B.; Carapeti, E.; George, M.L. Outcome of Patients on Renal Replacement Therapy after Colorectal Surgery. Dis. Colon Rectum 2008, 51, 961–965. [Google Scholar] [CrossRef]
- Frasson, M.; Flor-Lorente, B.; Ramos Rodríguez, J.L.; Granero-Castro, P.; Hervás, D.; Alvarez Rico, M.A.; Brao, M.J.G.; Sánchez González, J.M.; Garcia-Granero, E. Risk Factors for Anastomotic Leak after Colon Resection for Cancer: Multivariate Analysis and Nomogram from a Multicentric, Prospective, National Study with 3193 Patients. Ann. Surg. 2015, 262, 321–330. [Google Scholar] [CrossRef]
- Nikolian, V.C.; Kamdar, N.S.; Regenbogen, S.E.; Morris, A.M.; Byrn, J.C.; Suwanabol, P.A.; Campbell, D.A., Jr.; Hendren, S. Anastomotic Leak after Colorectal Resection: A Population-Based Study of Risk Factors and Hospital Variation. Surgery 2017, 161, 1619–1627. [Google Scholar] [CrossRef]
- Akiyoshi, T.; Ueno, M.; Fukunaga, Y.; Nagayama, S.; Fujimoto, Y.; Konishi, T.; Kuroyanagi, H.; Yamaguchi, T. Effect of Body Mass Index on Short-Term Outcomes of Patients Undergoing Laparoscopic Resection for Colorectal Cancer: A Single Institution Experience in Japan: A Single Institution Experience in Japan. Surg. Laparosc. Endosc. Percutan. Tech. 2011, 21, 409–414. [Google Scholar] [CrossRef]
- Watanabe, J.; Tatsumi, K.; Ota, M.; Suwa, Y.; Suzuki, S.; Watanabe, A.; Ishibe, A.; Watanabe, K.; Akiyama, H.; Ichikawa, Y.; et al. The Impact of Visceral Obesity on Surgical Outcomes of Laparoscopic Surgery for Colon Cancer. Int. J. Colorectal Dis. 2014, 29, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Cakir, H.; Heus, C.; Verduin, W.M.; Lak, A.; Doodeman, H.J.; Bemelman, W.A.; Houdijk, A.P. Visceral Obesity, Body Mass Index and Risk of Complications after Colon Cancer Resection: A Retrospective Cohort Study. Surgery 2015, 157, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wei, M.; He, Y.; Deng, X.; Wang, Z. Impact of Visceral Obesity on Outcomes of Laparoscopic Colorectal Surgery: A Meta-Analysis: Visceral Obesity on Laparoscopic Surgery. ANZ J. Surg. 2015, 85, 507–513. [Google Scholar] [CrossRef]
- Mäkelä, J.T.; Kiviniemi, H.; Laitinen, S. Risk Factors for Anastomotic Leakage after Left-Sided Colorectal Resection with Rectal Anastomosis. Dis. Colon Rectum 2003, 46, 653–660. [Google Scholar] [CrossRef]
- Kwag, S.-J.; Kim, J.-G.; Kang, W.-K.; Lee, J.-K.; Oh, S.-T. The Nutritional Risk Is a Independent Factor for Postoperative Morbidity in Surgery for Colorectal Cancer. Ann. Surg. Treat. Res. 2014, 86, 206–211. [Google Scholar] [CrossRef]
- Kang, C.Y.; Halabi, W.J.; Chaudhry, O.O.; Nguyen, V.; Pigazzi, A.; Carmichael, J.C.; Mills, S.; Stamos, M.J. Risk Factors for Anastomotic Leakage after Anterior Resection for Rectal Cancer. JAMA Surg. 2013, 148, 65–71. [Google Scholar] [CrossRef]
- Telem, D.A.; Chin, E.H.; Nguyen, S.Q.; Divino, C.M. Risk Factors for Anastomotic Leak Following Colorectal Surgery: A Case-Control Study: A Case-Control Study. Arch. Surg. 2010, 145, 371–376; discussion 376. [Google Scholar] [CrossRef] [PubMed]
- Sciuto, A.; Merola, G.; Palma, G.D.D.; Sodo, M.; Pirozzi, F.; Bracale, U.M.; Bracale, U. Predictive Factors for Anastomotic Leakage after Laparoscopic Colorectal Surgery. World J. Gastroenterol. 2018, 24, 2247–2260. [Google Scholar] [CrossRef]
- Zhu, Q.-L.; Feng, B.; Lu, A.-G.; Wang, M.-L.; Hu, W.-G.; Li, J.-W.; Mao, Z.-H.; Zheng, M.-H. Laparoscopic Low Anterior Resection for Rectal Carcinoma: Complications and Management in 132 Consecutive Patients. World J. Gastroenterol. 2010, 16, 4605–4610. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Elbers, H.; Askoxylakis, V.; Motschall, E.; Bork, U.; Büchler, M.W.; Weitz, J.; Koch, M. Neoadjuvant Radiotherapy for Rectal Cancer: Meta-Analysis of Randomized Controlled Trials. Ann. Surg. Oncol. 2013, 20, 4169–4182. [Google Scholar] [CrossRef]
- Martin, S.T.; Heneghan, H.M.; Winter, D.C. Systematic Review and Meta-Analysis of Outcomes Following Pathological Complete Response to Neoadjuvant Chemoradiotherapy for Rectal Cancer. Br. J. Surg. 2012, 99, 918–928. [Google Scholar] [CrossRef]
- Park, E.J.; Kang, J.; Hur, H.; Min, B.S.; Baik, S.H.; Lee, K.Y.; Kim, N.K. Different Clinical Features According to the Anastomotic Leakage Subtypes after Rectal Cancer Surgeries: Contained vs. Free Leakages. PLoS ONE 2018, 13, e0208572. [Google Scholar] [CrossRef] [PubMed]
- Hamabe, A.; Ito, M.; Nishigori, H.; Nishizawa, Y.; Sasaki, T. Preventive Effect of Diverting Stoma on Anastomotic Leakage after Laparoscopic Low Anterior Resection with Double Stapling Technique Reconstruction Applied Based on Risk Stratification. Asian J. Endosc. Surg. 2018, 11, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Ma, T.; Deng, Y.; Zheng, J.; Zhou, Z.; Wang, H.; Wang, L.; Wang, J. Impact of Preoperative Radiotherapy on Anastomotic Leakage and Stenosis after Rectal Cancer Resection: Post Hoc Analysis of a Randomized Controlled Trial. Dis. Colon Rectum 2016, 59, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Borstlap, W.A.A.; Westerduin, E.; Aukema, T.S.; Bemelman, W.A.; Tanis, P.J.; Dutch Snapshot Research Group. Anastomotic Leakage and Chronic Presacral Sinus Formation after Low Anterior Resection: Results from a Large Cross-Sectional Study. Ann. Surg. 2017, 266, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Keum, K.C.; Kim, N.K.; Baik, S.H.; Min, B.S.; Huh, H.; Lee, C.G.; Koom, W.S. Preoperative Chemoradiotherapy Effects on Anastomotic Leakage after Rectal Cancer Resection: A Propensity Score Matching Analysis. Ann. Surg. 2014, 259, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Marijnen, C.A.M.; Kapiteijn, E.; van de Velde, C.J.H.; Martijn, H.; Steup, W.H.; Wiggers, T.; Kranenbarg, E.K.; Leer, J.W.H.; Cooperative Investigators of the Dutch Colorectal Cancer Group. Acute Side Effects and Complications after Short-Term Preoperative Radiotherapy Combined with Total Mesorectal Excision in Primary Rectal Cancer: Report of a Multicenter Randomized Trial. J. Clin. Oncol. 2002, 20, 817–825. [Google Scholar] [CrossRef]
- Qin, C.; Ren, X.; Xu, K.; Chen, Z.; He, Y.; Song, X. Does Preoperative Radio(Chemo)Therapy Increase Anastomotic Leakage in Rectal Cancer Surgery? A Meta-Analysis of Randomized Controlled Trials. Gastroenterol. Res. Pract. 2014, 2014, 910956. [Google Scholar] [CrossRef]
- Yang, J.; Luo, Y.; Tian, T.; Dong, P.; Fu, Z. Effects of Neoadjuvant Radiotherapy on Postoperative Complications in Rectal Cancer: A Meta-Analysis. J. Oncol. 2022, 2022, 8197701. [Google Scholar] [CrossRef]
- Hu, M.-H.; Huang, R.-K.; Zhao, R.-S.; Yang, K.-L.; Wang, H. Does Neoadjuvant Therapy Increase the Incidence of Anastomotic Leakage after Anterior Resection for Mid and Low Rectal Cancer? A Systematic Review and Meta-Analysis. Colorectal Dis. 2017, 19, 16–26. [Google Scholar] [CrossRef]
- Ma, B.; Gao, P.; Wang, H.; Xu, Q.; Song, Y.; Huang, X.; Sun, J.; Zhao, J.; Luo, J.; Sun, Y.; et al. What Has Preoperative Radio(Chemo)Therapy Brought to Localized Rectal Cancer Patients in Terms of Perioperative and Long-Term Outcomes over the Past Decades? A Systematic Review and Meta-Analysis Based on 41,121 Patients: PRT/PCRT-Perioperative and Long-Term Outcomes over the Past Decades. Int. J. Cancer 2017, 141, 1052–1065. [Google Scholar] [PubMed]
- Bretagnol, F.; Panis, Y.; Rullier, E.; Rouanet, P.; Berdah, S.; Dousset, B.; Portier, G.; Benoist, S.; Chipponi, J.; Vicaut, E.; et al. Rectal Cancer Surgery with or without Bowel Preparation: The French GRECCAR III Multicenter Single-Blinded Randomized Trial. Ann. Surg. 2010, 252, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Kiran, R.P.; Murray, A.C.A.; Chiuzan, C.; Estrada, D.; Forde, K. Combined Preoperative Mechanical Bowel Preparation with Oral Antibiotics Significantly Reduces Surgical Site Infection, Anastomotic Leak, and Ileus after Colorectal Surgery. Ann. Surg. 2015, 262, 416–425; discussion 423–425. [Google Scholar] [CrossRef] [PubMed]
- Scarborough, J.E.; Mantyh, C.R.; Sun, Z.; Migaly, J. Combined Mechanical and Oral Antibiotic Bowel Preparation Reduces Incisional Surgical Site Infection and Anastomotic Leak Rates after Elective Colorectal Resection: An Analysis of Colectomy-Targeted ACS NSQIP: An Analysis of Colectomy-Targeted ACS NSQIP. Ann. Surg. 2015, 262, 331–337. [Google Scholar] [CrossRef]
- van’t Sant, H.P.; Weidema, W.F.; Hop, W.C.J.; Lange, J.F.; Contant, C.M.E. The Influence of Mechanical Bowel Preparation in Elective Lower Colorectal Surgery. Ann. Surg. 2010, 252, 575–576. [Google Scholar] [CrossRef]
- Zmora, O.; Mahajna, A.; Bar-Zakai, B.; Hershko, D.; Shabtai, M.; Krausz, M.M.; Ayalon, A. Is Mechanical Bowel Preparation Mandatory for Left-Sided Colonic Anastomosis? Results of a Prospective Randomized Trial. Tech. Coloproctol. 2006, 10, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Vicaut, E.; Launay-Savary, M.-V.; Contant, C.; Chipponi, J. Updated Systematic Review and Meta-Analysis of Randomized Clinical Trials on the Role of Mechanical Bowel Preparation before Colorectal Surgery. Ann. Surg. 2009, 249, 203–209. [Google Scholar] [CrossRef]
- Güenaga, K.F.; Matos, D.; Wille-Jørgensen, P. Mechanical Bowel Preparation for Elective Colorectal Surgery. Cochrane Database Syst. Rev. 2011, 9, CD001544. [Google Scholar] [CrossRef]
- Rollins, K.E.; Javanmard-Emamghissi, H.; Lobo, D.N. Impact of Mechanical Bowel Preparation in Elective Colorectal Surgery: A Meta-Analysis. World J. Gastroenterol. 2018, 24, 519–536. [Google Scholar] [CrossRef]
- Moghadamyeghaneh, Z.; Hanna, M.H.; Carmichael, J.C.; Mills, S.D.; Pigazzi, A.; Nguyen, N.T.; Stamos, M.J. Nationwide Analysis of Outcomes of Bowel Preparation in Colon Surgery. J. Am. Coll. Surg. 2015, 220, 912–920. [Google Scholar] [CrossRef]
- Morris, M.S.; Graham, L.A.; Chu, D.I.; Cannon, J.A.; Hawn, M.T. Oral Antibiotic Bowel Preparation Significantly Reduces Surgical Site Infection Rates and Readmission Rates in Elective Colorectal Surgery. Ann. Surg. 2015, 261, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Garfinkle, R.; Abou-Khalil, J.; Morin, N.; Ghitulescu, G.; Vasilevsky, C.-A.; Gordon, P.; Demian, M.; Boutros, M. Is There a Role for Oral Antibiotic Preparation Alone before Colorectal Surgery? ACS-NSQIP Analysis by Coarsened Exact Matching. Dis. Colon Rectum 2017, 60, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Roos, D.; Dijksman, L.M.; Tijssen, J.G.; Gouma, D.J.; Gerhards, M.F.; Oudemans-van Straaten, H.M. Systematic Review of Perioperative Selective Decontamination of the Digestive Tract in Elective Gastrointestinal Surgery: Perioperative Selective Decontamination of the Digestive Tract. Br. J. Surg. 2013, 100, 1579–1588. [Google Scholar] [CrossRef]
- Grewal, S.; Reuvers, J.R.D.; Abis, G.S.A.; Otten, R.H.J.; Kazemier, G.; Stockmann, H.B.A.C.; van Egmond, M.; Oosterling, S.J. Oral Antibiotic Prophylaxis Reduces Surgical Site Infection and Anastomotic Leakage in Patients Undergoing Colorectal Cancer Surgery. Biomedicines 2021, 9, 1184. [Google Scholar] [CrossRef] [PubMed]
- Koskenvuo, L.; Lehtonen, T.; Koskensalo, S.; Rasilainen, S.; Klintrup, K.; Ehrlich, A.; Pinta, T.; Scheinin, T.; Sallinen, V. Mechanical and Oral Antibiotic Bowel Preparation versus No Bowel Preparation in Right and Left Colectomy: Subgroup Analysis of MOBILE Trial. BJS Open 2021, 5, zrab011. [Google Scholar] [CrossRef]
- Rudnicki, Y.; White, I.; Tiomkin, V.; Lahav, L.; Raguan, B.; Avital, S. Intraoperative Evaluation of Colorectal Anastomotic Integrity: A Comparison of Air Leak and Dye Leak Tests. Tech. Coloproctol. 2021, 25, 841–847. [Google Scholar] [CrossRef]
- Choi, D.H.; Hwang, J.K.; Ko, Y.T.; Jang, H.J.; Shin, H.K.; Lee, Y.C.; Lim, C.H.; Jeong, S.K.; Yang, H.K. Risk Factors for Anastomotic Leakage after Laparoscopic Rectal Resection. J. Korean Soc. Coloproctol. 2010, 26, 265–273. [Google Scholar] [CrossRef]
- Huh, J.W.; Kim, H.R.; Kim, Y.J. Anastomotic Leakage after Laparoscopic Resection of Rectal Cancer: The Impact of Fibrin Glue. Am. J. Surg. 2010, 199, 435–441. [Google Scholar] [CrossRef]
- Zhang, W.; Lou, Z.; Liu, Q.; Meng, R.; Gong, H.; Hao, L.; Liu, P.; Sun, G.; Ma, J.; Zhang, W. Multicenter Analysis of Risk Factors for Anastomotic Leakage after Middle and Low Rectal Cancer Resection without Diverting Stoma: A Retrospective Study of 319 Consecutive Patients. Int. J. Colorectal Dis. 2017, 32, 1431–1437. [Google Scholar] [CrossRef]
- Kim, J.S.; Cho, S.Y.; Min, B.S.; Kim, N.K. Risk Factors for Anastomotic Leakage after Laparoscopic Intracorporeal Colorectal Anastomosis with a Double Stapling Technique. J. Am. Coll. Surg. 2009, 209, 694–701. [Google Scholar] [CrossRef]
- Killingback, M.; Barron, P.; Dent, O. Elective Resection and Anastomosis for Colorectal Cancer: A Prospective Audit of Mortality and Morbidity 1976–1998: Colorectal Cancer: Resection and Anastomosis. ANZ J. Surg. 2002, 72, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Rullier, E.; Laurent, C.; Garrelon, J.L.; Michel, P.; Saric, J.; Parneix, M. Risk Factors for Anastomotic Leakage after Resection of Rectal Cancer. Br. J. Surg. 1998, 85, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Kayano, H.; Okuda, J.; Tanaka, K.; Kondo, K.; Tanigawa, N. Evaluation of the Learning Curve in Laparoscopic Low Anterior Resection for Rectal Cancer. Surg. Endosc. 2011, 25, 2972–2979. [Google Scholar] [CrossRef]
- Ito, T.; Obama, K.; Sato, T.; Matsuo, K.; Inoue, H.; Kubota, K.; Tamaki, N.; Kami, K.; Yoshimura, N.; Shono, T.; et al. Usefulness of Transanal Tube Placement for Prevention of Anastomotic Leakage Following Laparoscopic Low Anterior Resection: Transanal Tube Prevents AL Following LLAR. Asian J. Endosc. Surg. 2017, 10, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Akiyoshi, T.; Ueno, M.; Fukunaga, Y.; Nagayama, S.; Fujimoto, Y.; Konishi, T.; Kuroyanagi, H.; Yamaguchi, T. Incidence of and Risk Factors for Anastomotic Leakage after Laparoscopic Anterior Resection with Intracorporeal Rectal Transection and Double-Stapling Technique Anastomosis for Rectal Cancer. Am. J. Surg. 2011, 202, 259–264. [Google Scholar] [CrossRef]
- Peeters, K.C.M.J.; Tollenaar, R.A.E.M.; Marijnen, C.A.M.; Klein Kranenbarg, E.; Steup, W.H.; Wiggers, T.; Rutten, H.J.; van de Velde, C.J.H.; Dutch Colorectal Cancer Group. Risk Factors for Anastomotic Failure after Total Mesorectal Excision of Rectal Cancer. Br. J. Surg. 2005, 92, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.-L.; Wu, S.-W. Meta-Analysis of Defunctioning Stoma in Low Anterior Resection with Total Mesorectal Excision for Rectal Cancer: Evidence Based on Thirteen Studies. World J. Surg. Oncol. 2015, 13, 9. [Google Scholar] [CrossRef]
- Chen, J.; Wang, D.-R.; Yu, H.-F.; Zhao, Z.-K.; Wang, L.-H.; Li, Y.-K. Defunctioning Stoma in Low Anterior Resection for Rectal Cancer: A Meta- Analysis of Five Recent Studies. Hepatogastroenterology 2012, 59, 1828–1831. [Google Scholar] [CrossRef]
- Mrak, K.; Uranitsch, S.; Pedross, F.; Heuberger, A.; Klingler, A.; Jagoditsch, M.; Weihs, D.; Eberl, T.; Tschmelitsch, J. Diverting Ileostomy versus No Diversion after Low Anterior Resection for Rectal Cancer: A Prospective, Randomized, Multicenter Trial. Surgery 2016, 159, 1129–1139. [Google Scholar] [CrossRef]
- Gastinger, I.; Marusch, F.; Steinert, R.; Wolff, S.; Koeckerling, F.; Lippert, H.; Working Group “Colon/Rectum Carcinoma”. Protective Defunctioning Stoma in Low Anterior Resection for Rectal Carcinoma. Br. J. Surg. 2005, 92, 1137–1142. [Google Scholar] [CrossRef]
- Hüser, N.; Michalski, C.W.; Erkan, M.; Schuster, T.; Rosenberg, R.; Kleeff, J.; Friess, H. Systematic Review and Meta-Analysis of the Role of Defunctioning Stoma in Low Rectal Cancer Surgery. Ann. Surg. 2008, 248, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Tortorelli, A.P.; Alfieri, S.; Sanchez, A.M.; Rosa, F.; Papa, V.; Di Miceli, D.; Bellantone, C.; Doglietto, G.B. Anastomotic Leakage after Anterior Resection for Rectal Cancer with Mesorectal Excision: Incidence, Risk Factors, and Management. Am. Surg. 2015, 81, 41–47. [Google Scholar] [CrossRef]
- Abudeeb, H.; Hammad, A.; Ugwu, A.; Darabnia, J.; Malcomson, L.; Maung, M.; Khan, K.; Mclaughlin, C.; Mukherjee, A. Defunctioning Stoma-a Prognosticator for Leaks in Low Rectal Restorative Cancer Resection: A Retrospective Analysis of Stoma Database. Ann. Med. Surg. 2017, 21, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, K.; Häggström, J.; Haapamäki, M.M.; Matthiessen, P.; Rutegård, J.; Rutegård, M. Defunctioning Stomas May Reduce Chances of a Stoma-Free Outcome after Anterior Resection for Rectal Cancer. Colorectal Dis. 2021, 23, 2859–2869. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Y.; Jiang, C.; Yu, H.; Zhang, K.; Zhang, M.; Zhang, G.-Q.; Zhou, S.-J. Temporary Ileostomy versus Colostomy for Colorectal Anastomosis: Evidence from 12 Studies. Scand. J. Gastroenterol. 2013, 48, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Rondelli, F.; Reboldi, P.; Rulli, A.; Barberini, F.; Guerrisi, A.; Izzo, L.; Bolognese, A.; Covarelli, P.; Boselli, C.; Becattini, C.; et al. Loop Ileostomy versus Loop Colostomy for Fecal Diversion after Colorectal or Coloanal Anastomosis: A Meta-Analysis. Int. J. Colorectal Dis. 2009, 24, 479–488. [Google Scholar] [CrossRef]
- Åkesson, O.; Syk, I.; Lindmark, G.; Buchwald, P. Morbidity Related to Defunctioning Loop Ileostomy in Low Anterior Resection. Int. J. Colorectal Dis. 2012, 27, 1619–1623. [Google Scholar] [CrossRef] [PubMed]
- Gavriilidis, P.; Azoulay, D.; Taflampas, P. Loop Transverse Colostomy versus Loop Ileostomy for Defunctioning of Colorectal Anastomosis: A Systematic Review, Updated Conventional Meta-Analysis, and Cumulative Meta-Analysis. Surg. Today 2019, 49, 108–117. [Google Scholar] [CrossRef]
- Smith, S.A.; Ronksley, P.E.; Tan, Z.; Dixon, E.; Hemmelgarn, B.R.; Buie, W.D.; Pannu, N.; James, M.T. New Ileostomy Formation and Subsequent Community-Onset Acute and Chronic Kidney Disease: A Population-Based Cohort Study. Ann. Surg. 2021, 274, 352–358. [Google Scholar] [CrossRef]
- Neutzling, C.B.; Lustosa, S.A.S.; Proenca, I.M.; da Silva, E.M.K.; Matos, D. Stapled versus Handsewn Methods for Colorectal Anastomosis Surgery. Cochrane Database Syst. Rev. 2012, 2, CD003144. [Google Scholar] [CrossRef]
- Choy, P.Y.G.; Bissett, I.P.; Docherty, J.G.; Parry, B.R.; Merrie, A.; Fitzgerald, A. Stapled versus Handsewn Methods for Ileocolic Anastomoses. Cochrane Database Syst. Rev. 2011, 9, CD004320. [Google Scholar] [CrossRef] [PubMed]
- Puleo, S.; Sofia, M.; Trovato, M.A.; Pesce, A.; Portale, T.R.; Russello, D.; La Greca, G. Ileocolonic Anastomosis: Preferred Techniques in 999 Patients. A Multicentric Study. Surg. Today 2013, 43, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- 2015 European Society of Coloproctology collaborating group. The Relationship between Method of Anastomosis and Anastomotic Failure after Right Hemicolectomy and Ileo-Caecal Resection: An International Snapshot Audit. Colorectal Dis. 2017, 19, e296–e311. [Google Scholar]
- Frasson, M.; Granero-Castro, P.; Ramos Rodríguez, J.L.; Flor-Lorente, B.; Braithwaite, M.; Martí Martínez, E.; Álvarez Pérez, J.A.; Codina Cazador, A.; Espí, A.; Garcia-Granero, E.; et al. Risk Factors for Anastomotic Leak and Postoperative Morbidity and Mortality after Elective Right Colectomy for Cancer: Results from a Prospective, Multicentric Study of 1102 Patients. Int. J. Colorectal Dis. 2016, 31, 105–114. [Google Scholar] [CrossRef]
- Nordholm-Carstensen, A.; Schnack Rasmussen, M.; Krarup, P.-M. Increased Leak Rates Following Stapled versus Handsewn Ileocolic Anastomosis in Patients with Right-Sided Colon Cancer: A Nationwide Cohort Study: A Nationwide Cohort Study. Dis. Colon Rectum 2019, 62, 542–548. [Google Scholar] [CrossRef]
- Nakayama, S.; Hasegawa, S.; Nagayama, S.; Kato, S.; Hida, K.; Tanaka, E.; Itami, A.; Kubo, H.; Sakai, Y. The Importance of Precompression Time for Secure Stapling with a Linear Stapler. Surg. Endosc. 2011, 25, 2382–2386. [Google Scholar] [CrossRef]
- Kim, J.-S.; Park, S.-H.; Kim, N.-S.; Lee, I.Y.; Jung, H.S.; Ahn, H.-M.; Son, G.M.; Baek, K.-R. Compression Automation of Circular Stapler for Preventing Compression Injury on Gastrointestinal Anastomosis. Int. J. Med. Robot. 2022, 18, e2374. [Google Scholar] [CrossRef]
- Braunschmid, T.; Hartig, N.; Baumann, L.; Dauser, B.; Herbst, F. Influence of Multiple Stapler Firings Used for Rectal Division on Colorectal Anastomotic Leak Rate. Surg. Endosc. 2017, 31, 5318–5326. [Google Scholar] [CrossRef]
- Balciscueta, Z.; Uribe, N.; Caubet, L.; López, M.; Torrijo, I.; Tabet, J.; Martín, M.C. Impact of the Number of Stapler Firings on Anastomotic Leakage in Laparoscopic Rectal Surgery: A Systematic Review and Meta-Analysis. Tech. Coloproctol. 2020, 24, 919–925. [Google Scholar] [CrossRef]
- Ito, M.; Sugito, M.; Kobayashi, A.; Nishizawa, Y.; Tsunoda, Y.; Saito, N. Relationship between Multiple Numbers of Stapler Firings during Rectal Division and Anastomotic Leakage after Laparoscopic Rectal Resection. Int. J. Colorectal Dis. 2008, 23, 703–707. [Google Scholar] [CrossRef]
- Kawada, K.; Hasegawa, S.; Hida, K.; Hirai, K.; Okoshi, K.; Nomura, A.; Kawamura, J.; Nagayama, S.; Sakai, Y. Risk Factors for Anastomotic Leakage after Laparoscopic Low Anterior Resection with DST Anastomosis. Surg. Endosc. 2014, 28, 2988–2995. [Google Scholar] [CrossRef] [PubMed]
- Huang, E. Constructing a Sound Anastomosis. Semin. Colon Rectal Surg. 2022, 33, 100878. [Google Scholar] [CrossRef]
- Vignali, A.; Gianotti, L.; Braga, M.; Radaelli, G.; Malvezzi, L.; Di Carlo, V. Altered Microperfusion at the Rectal Stump Is Predictive for Rectal Anastomotic Leak. Dis. Colon Rectum 2000, 43, 76–82. [Google Scholar] [CrossRef] [PubMed]
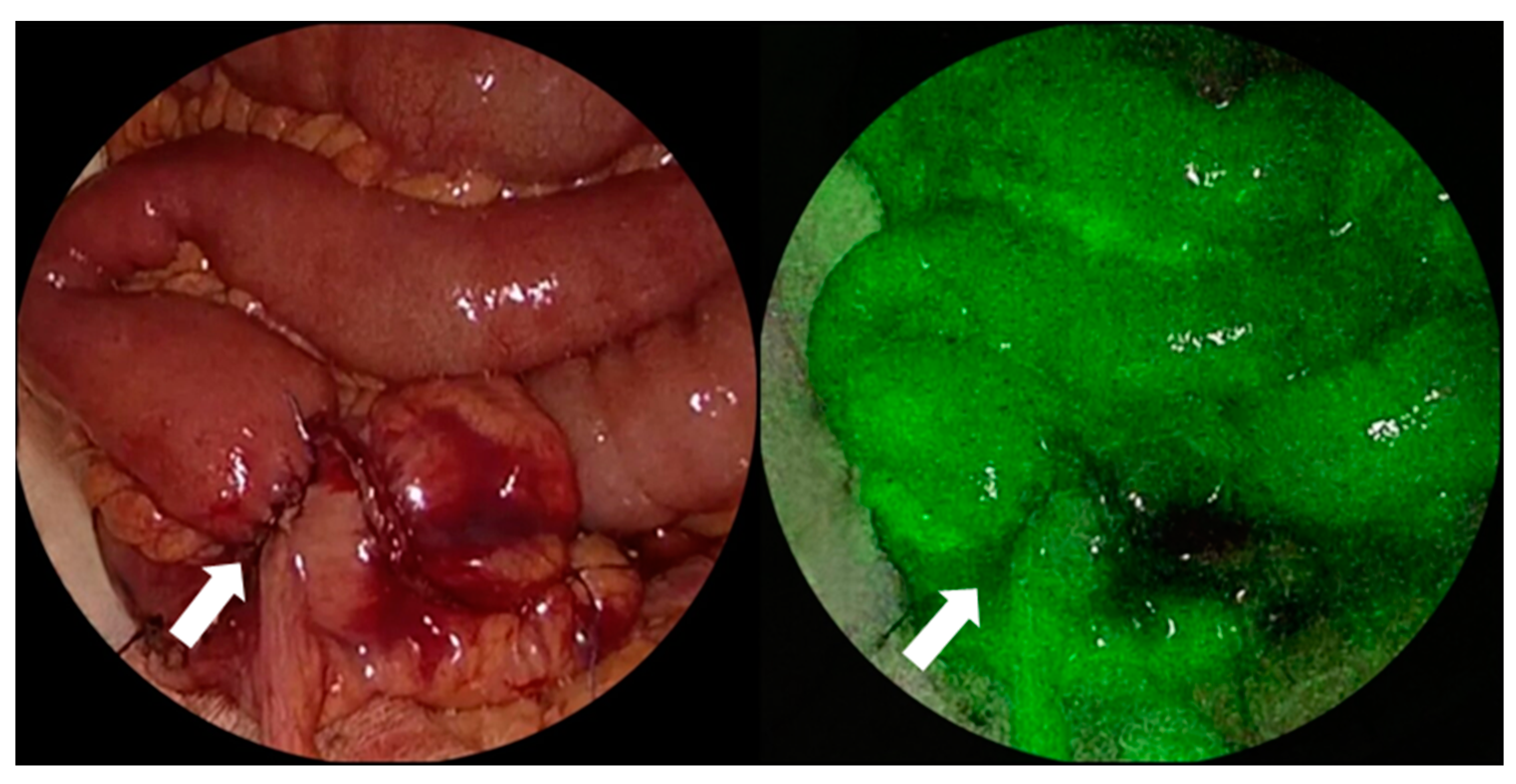
- Watanabe, J.; Ishibe, A.; Suwa, Y.; Suwa, H.; Ota, M.; Kunisaki, C.; Endo, I. Indocyanine Green Fluorescence Imaging to Reduce the Risk of Anastomotic Leakage in Laparoscopic Low Anterior Resection for Rectal Cancer: A Propensity Score-Matched Cohort Study. Surg. Endosc. 2020, 34, 202–208. [Google Scholar] [CrossRef]
- Wada, T.; Kawada, K.; Hoshino, N.; Inamoto, S.; Yoshitomi, M.; Hida, K.; Sakai, Y. The Effects of Intraoperative ICG Fluorescence Angiography in Laparoscopic Low Anterior Resection: A Propensity Score-Matched Study. Int. J. Clin. Oncol. 2019, 24, 394–402. [Google Scholar] [CrossRef]
- Ohya, H.; Watanabe, J.; Suwa, H.; Suwa, Y.; Ishibe, A.; Masui, H.; Nagahori, K.; Kunisaki, C.; Endo, I. Incidence and Risk Factors for Fluorescence Abnormalities on Near-Infrared Imaging Using Indocyanine Green in Stapled Functional End-to-End Anastomosis in Laparoscopic Colectomy. Int. J. Colorectal Dis. 2020, 35, 2011–2018. [Google Scholar] [CrossRef]
- Hinoi, T.; Okajima, M.; Shimomura, M.; Egi, H.; Ohdan, H.; Konishi, F.; Sugihara, K.; Watanabe, M. Effect of Left Colonic Artery Preservation on Anastomotic Leakage in Laparoscopic Anterior Resection for Middle and Low Rectal Cancer. World J. Surg. 2013, 37, 2935–2943. [Google Scholar] [CrossRef]
- Kachlik, D.; Baca, V. Macroscopic and Microscopic Intermesenteric Communications. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 2006, 150, 121–124. [Google Scholar] [CrossRef]
- Chen, J.-N.; Liu, Z.; Wang, Z.-J.; Zhao, F.-Q.; Wei, F.-Z.; Mei, S.-W.; Shen, H.-Y.; Li, J.; Pei, W.; Wang, Z.; et al. Low Ligation Has a Lower Anastomotic Leakage Rate after Rectal Cancer Surgery. World J. Gastrointest. Oncol. 2020, 12, 632–641. [Google Scholar] [CrossRef]
- Si, M.-B.; Yan, P.-J.; Du, Z.-Y.; Li, L.-Y.; Tian, H.-W.; Jiang, W.-J.; Jing, W.-T.; Yang, J.; Han, C.-W.; Shi, X.-E.; et al. Lymph Node Yield, Survival Benefit, and Safety of High and Low Ligation of the Inferior Mesenteric Artery in Colorectal Cancer Surgery: A Systematic Review and Meta-Analysis. Int. J. Colorectal Dis. 2019, 34, 947–962. [Google Scholar] [CrossRef]
- Draginov, A.; Chesney, T.R.; Quereshy, H.A.; Chadi, S.A.; Quereshy, F.A. Association of High Ligation versus Low Ligation of the Inferior Mesenteric Artery on Anastomotic Leak, Postoperative Complications, and Mortality after Minimally Invasive Surgery for Distal Sigmoid and Rectal Cancer. Surg. Endosc. 2020, 34, 4593–4600. [Google Scholar] [CrossRef] [PubMed]
- De Nardi, P.; Elmore, U.; Maggi, G.; Maggiore, R.; Boni, L.; Cassinotti, E.; Fumagalli, U.; Gardani, M.; De Pascale, S.; Parise, P.; et al. Intraoperative Angiography with Indocyanine Green to Assess Anastomosis Perfusion in Patients Undergoing Laparoscopic Colorectal Resection: Results of a Multicenter Randomized Controlled Trial. Surg. Endosc. 2020, 34, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.D.; Pigazzi, A.; McLemore, E.C.; Mutch, M.G.; Haas, E.; Rasheid, S.H.; Wait, A.D.; Paquette, I.M.; Bardakcioglu, O.; Safar, B.; et al. Perfusion Assessment in Left-Sided/Low Anterior Resection (PILLAR III): A Randomized, Controlled, Parallel, Multicenter Study Assessing Perfusion Outcomes with PINPOINT near-Infrared Fluorescence Imaging in Low Anterior Resection: A Randomized, Controlled, Parallel, Multicenter Study Assessing Perfusion Outcomes with PINPOINT near-Infrared Fluorescence Imaging in Low Anterior Resection. Dis. Colon Rectum 2021, 64, 995–1002. [Google Scholar] [PubMed]
- Rutegård, M.; Hemmingsson, O.; Matthiessen, P.; Rutegård, J. High Tie in Anterior Resection for Rectal Cancer Confers No Increased Risk of Anastomotic Leakage. Br. J. Surg. 2012, 99, 127–132. [Google Scholar] [CrossRef]
- Kong, M.; Chen, H.; Xin, Y.; Jiang, Y.; Han, Y.; Sheng, H. High Ligation of the Inferior Mesenteric Artery and Anastomotic Leakage in Anterior Resection for Rectal Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trial Studies. Colorectal Dis. 2021, 23, 614–624. [Google Scholar] [CrossRef]
- Rutegård, M.; Hassmén, N.; Hemmingsson, O.; Haapamäki, M.M.; Matthiessen, P.; Rutegård, J. Anterior Resection for Rectal Cancer and Visceral Blood Flow: An Explorative Study. Scand. J. Surg. 2016, 105, 78–83. [Google Scholar] [CrossRef]
- Back, E.; Brännström, F.; Svensson, J.; Rutegård, J.; Matthiessen, P.; Haapamäki, M.M.; Rutegård, M. Mucosal Blood Flow in the Remaining Rectal Stump Is More Affected by Total than Partial Mesorectal Excision in Patients Undergoing Anterior Resection: A Key to Understanding Differing Rates of Anastomotic Leakage? Langenbecks Arch. Surg. 2021, 406, 1971–1977. [Google Scholar] [CrossRef]
- Kim, C.W.; Baek, S.J.; Hur, H.; Min, B.S.; Baik, S.H.; Kim, N.K. Anastomotic Leakage after Low Anterior Resection for Rectal Cancer Is Different between Minimally Invasive Surgery and Open Surgery. Ann. Surg. 2016, 263, 130–137. [Google Scholar] [CrossRef]
- van der Pas, M.H.; Haglind, E.; Cuesta, M.A.; Fürst, A.; Lacy, A.M.; Hop, W.C.; Bonjer, H.J.; COlorectal cancer Laparoscopic or Open Resection II (COLOR II) Study Group. Laparoscopic versus Open Surgery for Rectal Cancer (COLOR II): Short-Term Outcomes of a Randomised, Phase 3 Trial. Lancet Oncol. 2013, 14, 210–218. [Google Scholar] [CrossRef]
- Fleshman, J.; Branda, M.; Sargent, D.J.; Boller, A.M.; George, V.; Abbas, M.; Peters, W.R., Jr.; Maun, D.; Chang, G.; Herline, A.; et al. Effect of Laparoscopic-Assisted Resection vs Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. JAMA 2015, 314, 1346. [Google Scholar] [CrossRef]
- Kang, S.-B.; Park, J.W.; Jeong, S.-Y.; Nam, B.H.; Choi, H.S.; Kim, D.-W.; Lim, S.-B.; Lee, T.-G.; Kim, D.Y.; Kim, J.-S.; et al. Open versus Laparoscopic Surgery for Mid or Low Rectal Cancer after Neoadjuvant Chemoradiotherapy (COREAN Trial): Short-Term Outcomes of an Open-Label Randomised Controlled Trial. Lancet Oncol. 2010, 11, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ma, B.; Gao, P.; Wang, H.; Song, Y.; Tong, L.; Li, P.; Wang, Z. Laparoscopic Intersphincteric Resection versus an Open Approach for Low Rectal Cancer: A Meta-Analysis. World J. Surg. Oncol. 2017, 15, 229. [Google Scholar] [CrossRef] [PubMed]
- Allaix, M.E.; Degiuli, M.; Arezzo, A.; Arolfo, S.; Morino, M. Does Conversion Affect Short-Term and Oncologic Outcomes after Laparoscopy for Colorectal Cancer? Surg. Endosc. 2013, 27, 4596–4607. [Google Scholar] [CrossRef]
- Majbar, A.M.; Abid, M.; Alaoui, M.; Sabbah, F.; Raiss, M.; Ahallat, M.; Hrora, A. Impact of Conversion to Open Surgery on Early Postoperative Morbidity after Laparoscopic Resection for Rectal Adenocarcinoma: A Retrospective Study. J. Laparoendosc. Adv. Surg. Tech. A 2016, 26, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, R.; Di Lernia, S.; Sansonna, F.; Scandroglio, I.; Maggioni, D.; Ferrari, G.C.; Costanzi, A.; Magistro, C.; De Carli, S. Results of Laparoscopic Anterior Resection for Rectal Adenocarcinoma: Retrospective Analysis of 157 Cases. Am. J. Surg. 2008, 195, 233–238. [Google Scholar] [CrossRef]
- de’Angelis, N.; Portigliotti, L.; Brunetti, F. Robot-Assisted Rectal Cancer Surgery Deserves a Fair Trial. Colorectal Dis. 2015, 17, 824–825. [Google Scholar] [CrossRef]
- Aly, E.H. Robotic Colorectal Surgery: Summary of the Current Evidence. Int. J. Colorectal Dis. 2014, 29, 1–8. [Google Scholar] [CrossRef]
- Petrucciani, N.; Sirimarco, D.; Nigri, G.R.; Magistri, P.; La Torre, M.; Aurello, P.; D’Angelo, F.; Ramacciato, G. Robotic Right Colectomy: A Worthwhile Procedure? Results of a Meta-Analysis of Trials Comparing Robotic versus Laparoscopic Right Colectomy. J. Minim. Access Surg. 2015, 11, 22–28. [Google Scholar] [CrossRef]
- Ahmed, J.; Cao, H.; Panteleimonitis, S.; Khan, J.; Parvaiz, A. Robotic vs Laparoscopic Rectal Surgery in High-Risk Patients. Colorectal Dis. 2017, 19, 1092–1099. [Google Scholar] [CrossRef]
- Al-Mazrou, A.M.; Chiuzan, C.; Kiran, R.P. The Robotic Approach Significantly Reduces Length of Stay after Colectomy: A Propensity Score-Matched Analysis. Int. J. Colorectal Dis. 2017, 32, 1415–1421. [Google Scholar] [CrossRef]
- Emile, S.H.; Abd El-Hamed, T.M. Routine Drainage of Colorectal Anastomoses: An Evidence-Based Review of the Current Literature. Gastroenterol. Res. Pract. 2017, 2017, 6253898. [Google Scholar] [CrossRef] [PubMed]
- Denost, Q.; Rouanet, P.; Faucheron, J.-L.; Panis, Y.; Meunier, B.; Cotte, E.; Meurette, G.; Kirzin, S.; Sabbagh, C.; Loriau, J.; et al. French Research Group of Rectal Cancer Surgery (GRECCAR). To Drain or Not to Drain Infraperitoneal Anastomosis after Rectal Excision for Cancer: The GRECCAR 5 Randomized Trial. Ann. Surg. 2017, 265, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Liu, Y.; Bi, D.-S. Clinical Risk Factors for Anastomotic Leakage after Laparoscopic Anterior Resection for Rectal Cancer: A Systematic Review and Meta-Analysis. Surg. Endosc. 2015, 29, 3608–3617. [Google Scholar] [CrossRef] [PubMed]
- Rondelli, F.; Bugiantella, W.; Vedovati, M.C.; Balzarotti, R.; Avenia, N.; Mariani, E.; Agnelli, G.; Becattini, C. To Drain or Not to Drain Extraperitoneal Colorectal Anastomosis? A Systematic Review and Meta-Analysis. Colorectal Dis. 2014, 16, O35–O42. [Google Scholar] [CrossRef]
- Menahem, B.; Vallois, A.; Alves, A.; Lubrano, J. Prophylactic Pelvic Drainage after Rectal Resection with Extraperitoneal Anastomosis: Is It Worthwhile? A Meta-Analysis of Randomized Controlled Trials. Int. J. Colorectal Dis. 2017, 32, 1531–1538. [Google Scholar] [CrossRef]
- Guerra, F.; Giuliani, G.; Coletta, D.; Boni, M.; Rondelli, F.; Bianchi, P.P.; Coratti, A. A Meta-Analysis of Randomized Controlled Trials on the Use of Suction Drains Following Rectal Surgery. Dig. Surg. 2018, 35, 482–490. [Google Scholar] [CrossRef]
- Tanaka, K.; Okuda, J.; Yamamoto, S.; Ito, M.; Sakamoto, K.; Kokuba, Y.; Yoshimura, K.; Watanabe, M. Risk Factors for Anastomotic Leakage after Laparoscopic Surgery with the Double Stapling Technique for Stage 0/I Rectal Carcinoma: A Subgroup Analysis of a Multicenter, Single-Arm Phase II Trial. Surg. Today 2017, 47, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-K.; Law, W.-L.; Ho, J.W.C. Leakage after Resection and Intraperitoneal Anastomosis for Colorectal Malignancy: Analysis of Risk Factors. Dis. Colon Rectum 2006, 49, 1719–1725. [Google Scholar] [CrossRef]
- Midura, E.F.; Hanseman, D.; Davis, B.R.; Atkinson, S.J.; Abbott, D.E.; Shah, S.A.; Paquette, I.M. Risk Factors and Consequences of Anastomotic Leak after Colectomy: A National Analysis: A National Analysis. Dis. Colon Rectum 2015, 58, 333–338. [Google Scholar] [CrossRef]
- García-Granero, E.; Navarro, F.; Cerdán Santacruz, C.; Frasson, M.; García-Granero, A.; Marinello, F.; Flor-Lorente, B.; Espí, A. Individual Surgeon Is an Independent Risk Factor for Leak after Double-Stapled Colorectal Anastomosis: An Institutional Analysis of 800 Patients. Surgery 2017, 162, 1006–1016. [Google Scholar] [CrossRef]
- Marinello, F.G.; Baguena, G.; Lucas, E.; Frasson, M.; Hervás, D.; Flor-Lorente, B.; Esclapez, P.; Espí, A.; García-Granero, E. Anastomotic Leakage after Colon Cancer Resection: Does the Individual Surgeon Matter? Colorectal Dis. 2016, 18, 562–569. [Google Scholar] [CrossRef]
- Biondo, S.; Kreisler, E.; Millan, M.; Fraccalvieri, D.; Golda, T.; Frago, R.; Miguel, B. Impact of Surgical Specialization on Emergency Colorectal Surgery Outcomes. Arch. Surg. 2010, 145, 79–86. [Google Scholar] [CrossRef]
- Kelly, M.; Bhangu, A.; Singh, P.; Fitzgerald, J.E.F.; Tekkis, P.P. Systematic Review and Meta-Analysis of Trainee- versus Expert Surgeon-Performed Colorectal Resection: Trainee-Versus expert Surgeon-Performed Colorectal Resection. Br. J. Surg. 2014, 101, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, T.F.; Lassen, C.B.; Gögenur, I. Treatment with Corticosteroids and the Risk of Anastomotic Leakage Following Lower Gastrointestinal Surgery: A Literature Survey. Colorectal Dis. 2014, 16, O154–O160. [Google Scholar] [CrossRef] [PubMed]
- Kverneng Hultberg, D.; Angenete, E.; Lydrup, M.-L.; Rutegård, J.; Matthiessen, P.; Rutegård, M. Nonsteroidal Anti-Inflammatory Drugs and the Risk of Anastomotic Leakage after Anterior Resection for Rectal Cancer. Eur. J. Surg. Oncol. 2017, 43, 1908–1914. [Google Scholar] [CrossRef]
- Rutegård, M.; Westermark, S.; Kverneng Hultberg, D.; Haapamäki, M.; Matthiessen, P.; Rutegård, J. Non-Steroidal Anti-Inflammatory Drug Use and Risk of Anastomotic Leakage after Anterior Resection: A Protocol-Based Study. Dig. Surg. 2016, 33, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, K.J.; Benning, D.; Berghmans, T.; Snoeijs, M.G.; Sosef, M.N.; Hulsewe, K.W.E.; Luyer, M.D.P. Risk of Anastomotic Leakage with Non-Steroidal Anti-Inflammatory Drugs in Colorectal Surgery. Br. J. Surg. 2012, 99, 721–727. [Google Scholar] [CrossRef]
- Zeeh, J.; Inglin, R.; Baumann, G.; Dirsch, O.; Riley, N.E.; Gerken, G.; Buchler, M.W.; Egger, B. Mycophenolate Mofetil Impairs Healing of Left-Sided Colon Anastomoses 1. Transplantation 2001, 71, 1429–1435. [Google Scholar] [CrossRef]
- Petri, J.B.; Schurk, S.; Gebauer, S.; Haustein, U.F. Cyclosporine A Delays Wound Healing and Apoptosis and Suppresses Activin Beta-A Expression in Rats. Eur. J. Dermatol. 1998, 8, 104–113. [Google Scholar]
- Schäffer, M.R.; Fuchs, N.; Proksch, B.; Bongartz, M.; Beiter, T.; Becker, H.-D. Tacrolimus Impairs Wound Healing: A Possible Role of Decreased Nitric Oxide Synthesis1. Transplantation 1998, 65, 813–818. [Google Scholar] [CrossRef]
- van der Vliet, J.A.; Willems, M.C.M.; de Man, B.M.; Lomme, R.M.L.M.; Hendriks, T. Everolimus Interferes with Healing of Experimental Intestinal Anastomoses. Transplantation 2006, 82, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Machida, E.; Miyakura, Y.; Takahashi, J.; Tamaki, S.; Ishikawa, H.; Hasegawa, F.; Kikugawa, R.; Tsujinaka, S.; Lefor, A.K.; Rikiyama, T. Bevacizumab Is Associated with Delayed Anastomotic Leak after Low Anterior Resection with Preoperative Radiotherapy for Rectal Cancer: A Case Report. Surg. Case Rep. 2019, 5, 14. [Google Scholar] [CrossRef]
- Krane, M.K.; Allaix, M.E.; Zoccali, M.; Umanskiy, K.; Rubin, M.A.; Villa, A.; Hurst, R.D.; Fichera, A. Preoperative Infliximab Therapy Does Not Increase Morbidity and Mortality after Laparoscopic Resection for Inflammatory Bowel Disease. Dis. Colon Rectum 2013, 56, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Shogan, B.D.; Carlisle, E.M.; Alverdy, J.C.; Umanskiy, K. Do We Really Know Why Colorectal Anastomoses Leak? J. Gastrointest. Surg. 2013, 17, 1698–1707. [Google Scholar] [CrossRef] [PubMed]
- Shogan, B.D.; Belogortseva, N.; Luong, P.M.; Zaborin, A.; Lax, S.; Bethel, C.; Ward, M.; Muldoon, J.P.; Singer, M.; An, G.; et al. Collagen Degradation and MMP9 Activation by Enterococcus Faecalis Contribute to Intestinal Anastomotic Leak. Sci. Transl. Med. 2015, 7, 286ra68. [Google Scholar] [CrossRef] [PubMed]
- Russ, A.J.; Casillas, M.A. Gut Microbiota and Colorectal Surgery: Impact on Postoperative Complications. Clin. Colon Rectal Surg. 2016, 29, 253–257. [Google Scholar]
- van Praagh, J.B.; de Goffau, M.C.; Bakker, I.S.; Harmsen, H.J.M.; Olinga, P.; Havenga, K. Intestinal Microbiota and Anastomotic Leakage of Stapled Colorectal Anastomoses: A Pilot Study. Surg. Endosc. 2016, 30, 2259–2265. [Google Scholar] [CrossRef]
- van Praagh, J.B.; de Goffau, M.C.; Bakker, I.S.; van Goor, H.; Harmsen, H.J.M.; Olinga, P.; Havenga, K. Mucus Microbiome of Anastomotic Tissue during Surgery Has Predictive Value for Colorectal Anastomotic Leakage. Ann. Surg. 2019, 269, 911–916. [Google Scholar] [CrossRef]
- Iancu, C.; Mocan, L.C.; Todea-Iancu, D.; Mocan, T.; Acalovschi, I.; Ionescu, D.; Zaharie, F.V.; Osian, G.; Puia, C.I.; Muntean, V. Host-Related Predictive Factors for Anastomotic Leakage Following Large Bowel Resections for Colorectal Cancer. J. Gastrointestin. Liver Dis. 2008, 17, 299–303. [Google Scholar]
- Hayden, D.M.; Mora Pinzon, M.C.; Francescatti, A.B.; Saclarides, T.J. Patient Factors May Predict Anastomotic Complications after Rectal Cancer Surgery: Anastomotic Complications in Rectal Cancer. Ann. Med. Surg. 2015, 4, 11–16. [Google Scholar] [CrossRef]
- Bertelsen, C.A.; Andreasen, A.H.; Jørgensen, T.; Harling, H.; Danish Colorectal Cancer Group. Anastomotic Leakage after Anterior Resection for Rectal Cancer: Risk Factors: Risk Factors of Anastomotic Leakage in Rectal Cancer. Colorectal Dis. 2010, 12, 37–43. [Google Scholar] [CrossRef]
- Krarup, P.-M.; Jorgensen, L.N.; Andreasen, A.H.; Harling, H.; Danish Colorectal Cancer Group. A Nationwide Study on Anastomotic Leakage after Colonic Cancer Surgery: A Nationwide Study on Anastomotic Leakage. Colorectal Dis. 2012, 14, e661–e667. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.; Lu, Y.; Li, Q.; Guo, J.; Chen, G.; Zeng, W. Risk Factors for Anastomotic Leakage Following Anterior Resection for Colorectal Cancer: The Effect of Epidural Analgesia on Occurrence. Int. J. Colorectal Dis. 2013, 28, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Saikaly, E.; Saad, M.K. Anastomotic Leak in Colorectal Surgery: A Comprehensive Review. Surg. Clin. J. 2020, 2, 4. [Google Scholar]
- Boström, P.; Svensson, J.; Brorsson, C.; Rutegård, M. Early Postoperative Pain as a Marker of Anastomotic Leakage in Colorectal Cancer Surgery. Int. J. Colorectal Dis. 2021, 36, 1955–1963. [Google Scholar] [CrossRef]
- Morks, A.N.; Ploeg, R.J.; Sijbrand Hofker, H.; Wiggers, T.; Havenga, K. Late Anastomotic Leakage in Colorectal Surgery: A Significant Problem. Colorectal Dis. 2013, 15, e271–e275. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.-B.; Yu, C.S.; Kim, C.W.; Yoon, Y.S.; Park, I.J.; Kim, J.C. Late Anastomotic Leakage after Low Anterior Resection in Rectal Cancer Patients: Clinical Characteristics and Predisposing Factors. Colorectal Dis. 2016, 18, O135–O140. [Google Scholar] [CrossRef] [PubMed]
- Matthiessen, P.; Lindgren, R.; Hallböök, O.; Rutegård, J.; Sjödahl, R.; Rectal Cancer Trial on Defunctioning Stoma Study Group. Symptomatic Anastomotic Leakage Diagnosed after Hospital Discharge Following Low Anterior Resection for Rectal Cancer. Colorectal Dis. 2010, 12, e82–e87. [Google Scholar]
- Li, Y.-W.; Lian, P.; Huang, B.; Zheng, H.-T.; Wang, M.-H.; Gu, W.-L.; Li, X.-X.; Xu, Y.; Cai, S.-J. Very Early Colorectal Anastomotic Leakage within 5 Post-Operative Days: A More Severe Subtype Needs Relaparatomy. Sci. Rep. 2017, 7. [Google Scholar]
- Sparreboom, C.L.; van Groningen, J.T.; Lingsma, H.F.; Wouters, M.W.J.M.; Menon, A.G.; Kleinrensink, G.-J.; Jeekel, J.; Lange, J.F.; Dutch ColoRectal Audit group. Different Risk Factors for Early and Late Colorectal Anastomotic Leakage in a Nationwide Audit. Dis. Colon Rectum 2018, 61, 1258–1266. [Google Scholar]
- Jutesten, H.; Buchwald, P.L.; Angenete, E.; Rutegård, M.; Lydrup, M.-L. High Risk of Low Anterior Resection Syndrome in Long-Term Follow-up after Anastomotic Leakage in Anterior Resection for Rectal Cancer. Dis. Colon Rectum 2022, 65, 1264–1273. [Google Scholar] [CrossRef]
- Holmgren, K.; Kverneng Hultberg, D.; Haapamäki, M.M.; Matthiessen, P.; Rutegård, J.; Rutegård, M. High Stoma Prevalence and Stoma Reversal Complications Following Anterior Resection for Rectal Cancer: A Population-Based Multicentre Study. Colorectal Dis. 2017, 19, 1067–1075. [Google Scholar] [CrossRef]
- McDermott, F.D.; Heeney, A.; Kelly, M.E.; Steele, R.J.; Carlson, G.L.; Winter, D.C. Systematic Review of Preoperative, Intraoperative and Postoperative Risk Factors for Colorectal Anastomotic Leaks. Br. J. Surg. 2015, 102, 462–479. [Google Scholar] [CrossRef]
- Bertoni, C.B.; Mendible, M.; Fleury, A.R.; VanderMeer, T.J.; Skeist, B.P.; Cagir, B. M1545 Utility of Pelvic CT with Rectal Contrast to Identify Pelvic Abscess and Anastomotic Leaks. Gastroenterology 2009, 136, A-893. [Google Scholar] [CrossRef]
- Marres, C.C.M.; Engelmann, E.W.M.; Buskens, C.J.; Haak, H.E.; Bemelman, W.A.; van de Ven, A.W.H. The Importance of Rectal Contrast in CT Assessment to Detect Anastomotic Leakage after Colorectal Surgery. Colorectal Dis. 2021, 23, 2466–2471. [Google Scholar] [CrossRef] [PubMed]
- Daams, F.; Wu, Z.; Lahaye, M.J.; Jeekel, J.; Lange, J.F. Prediction and Diagnosis of Colorectal Anastomotic Leakage: A Systematic Review of Literature. World J. Gastrointest. Surg. 2014, 6, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Dekker, J.W.T.; Liefers, G.J.; de Mol van Otterloo, J.C.A.; Putter, H.; Tollenaar, R.A.E.M. Predicting the Risk of Anastomotic Leakage in Left-Sided Colorectal Surgery Using a Colon Leakage Score. J. Surg. Res. 2011, 166, e27–e34. [Google Scholar] [CrossRef]
- Yang, S.U.; Park, E.J.; Baik, S.H.; Lee, K.Y.; Kang, J. Modified Colon Leakage Score to Predict Anastomotic Leakage in Patients Who Underwent Left-Sided Colorectal Surgery. J. Clin. Med. 2019, 8, 1450. [Google Scholar] [CrossRef]
- den Dulk, M.; Witvliet, M.J.; Kortram, K.; Neijenhuis, P.A.; de Hingh, I.H.; Engel, A.F.; van de Velde, C.J.H.; de Brauw, L.M.; Putter, H.; Brouwers, M.A.M.; et al. The DULK (Dutch Leakage) and Modified DULK Score Compared: Actively Seek the Leak. Colorectal Dis. 2013, 15, e528–e533. [Google Scholar] [CrossRef]
- Hu, X.; Cheng, Y. A Clinical Parameters-Based Model Predicts Anastomotic Leakage after a Laparoscopic Total Mesorectal Excision: A Large Study with Data from China: A Large Study with Data from China. Medicine 2015, 94, e1003. [Google Scholar] [CrossRef]
- Han, Z.; Chen, D.; Li, Y.; Zhou, G.; Wang, M.; Zhang, C. Development of a Risk Scoring System for Predicting Anastomotic Leakage Following Laparoscopic Rectal Cancer Surgery. Ther. Clin. Risk Manag. 2021, 17, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Shiwakoti, E.; Song, J.; Li, J.; Wu, S.; Zhang, Z. Prediction Model for Anastomotic Leakage after Laparoscopic Rectal Cancer Resection. J. Int. Med. Res. 2020, 48, 300060520957547. [Google Scholar] [CrossRef]
- Hoek, V.T.; Buettner, S.; Sparreboom, C.L.; Detering, R.; Menon, A.G.; Kleinrensink, G.J.; Wouters, M.W.J.M.; Lange, J.F.; Wiggers, J.K.; Dutch ColoRectal Audit group. A Preoperative Prediction Model for Anastomotic Leakage after Rectal Cancer Resection Based on 13.175 Patients. Eur. J. Surg. Oncol. 2022, 48, 2495–2501. [Google Scholar] [CrossRef] [PubMed]
- Messias, B.A.; Botelho, R.V.; Saad, S.S.; Mocchetti, E.R.; Turke, K.C.; Waisberg, J. Serum C-Reactive Protein Is a Useful Marker to Exclude Anastomotic Leakage after Colorectal Surgery. Sci. Rep. 2020, 10, 1687. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, T.; Roikjær, O.; Jess, P. Increased Levels of C-Reactive Protein and Leukocyte Count Are Poor Predictors of Anastomotic Leakage Following Laparoscopic Colorectal Resection. Dan. Med. J. 2012, 59, A4552. [Google Scholar]
- Singh, P.P.; Zeng, I.S.L.; Srinivasa, S.; Lemanu, D.P.; Connolly, A.B.; Hill, A.G. Systematic Review and Meta-Analysis of Use of Serum C-Reactive Protein Levels to Predict Anastomotic Leak after Colorectal Surgery: Use of C-Reactive Protein Levels to Predict Anastomotic Leak after Colorectal Surgery. Br. J. Surg. 2014, 101, 339–346. [Google Scholar] [CrossRef]
- Garcia-Granero, A.; Frasson, M.; Flor-Lorente, B.; Blanco, F.; Puga, R.; Carratalá, A.; Garcia-Granero, E. Procalcitonin and C-Reactive Protein as Early Predictors of Anastomotic Leak in Colorectal Surgery: A Prospective Observational Study: A Prospective Observational Study. Dis. Colon Rectum 2013, 56, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Chen, L. Early Prediction of Anastomotic Leakage after Laparoscopic Rectal Surgery Using Creactive Protein. Medicine 2021, 100, e26196. [Google Scholar] [CrossRef]
- Ahmadi Amoli, H.; Mahmoudabadi, H.; Ghorbani, S.; Hajebi, R.; Rahimpour, E.; Vaghef Davari, F. Exclusion of Anastomosis Leakage after Colorectal Surgery Using C-Reactive Protein: A Retrospective Study. Iran. Red Crescent Med. J. 2022, 24, e1467. [Google Scholar]
- Reynolds, I.S.; Boland, M.R.; Reilly, F.; Deasy, A.; Majeed, M.H.; Deasy, J.; Burke, J.P.; McNamara, D.A. C-Reactive Protein as a Predictor of Anastomotic Leak in the First Week after Anterior Resection for Rectal Cancer. Colorectal Dis. 2017, 19, 812–818. [Google Scholar] [CrossRef]
- Italian ColoRectal Anastomotic Leakage (iCral) Study Group. Anastomotic Leakage after Elective Colorectal Surgery: A Prospective Multicentre Observational Study on Use of the Dutch Leakage Score, Serum Procalcitonin and Serum C-Reactive Protein for Diagnosis. BJS Open 2020, 4, 499–507. [Google Scholar] [CrossRef]
- El Zaher, H.A.; Ghareeb, W.M.; Fouad, A.M.; Madbouly, K.; Fathy, H.; Vedin, T.; Edelhamre, M.; Emile, S.H.; Faisal, M. Role of the Triad of Procalcitonin, C-Reactive Protein, and White Blood Cell Count in the Prediction of Anastomotic Leak Following Colorectal Resections. World J. Surg. Oncol. 2022, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Chernyshov, S.; Alexeev, M.; Rybakov, E.; Tarasov, M.; Shelygin, Y.; Zarodniuk, I.; Sukhina, M. Risk Factors and Inflammatory Predictors for Anastomotic Leakage Following Total Mesorectal Excision with Defunctioning Stoma. Pol. Przegl. Chir. 2018, 90, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wu, H.; Yang, Y.; Jiang, Y.; Yuan, J.; Tong, Q. Oxidative Stress Level as a Predictor of Anastomotic Leakage after Rectal Surgery. Mediat. Inflamm. 2021, 2021, 9968642. [Google Scholar] [CrossRef]
- Su’a, B.U.; Mikaere, H.L.; Rahiri, J.L.; Bissett, I.B.; Hill, A.G. Systematic Review of the Role of Biomarkers in Diagnosing Anastomotic Leakage Following Colorectal Surgery. Br. J. Surg. 2017, 104, 503–512. [Google Scholar] [PubMed]
- Klupp, F.; Schuler, S.; Kahlert, C.; Halama, N.; Franz, C.; Mayer, P.; Schmidt, T.; Ulrich, A. Evaluation of the Inflammatory Markers CCL8, CXCL5, and LIF in Patients with Anastomotic Leakage after Colorectal Cancer Surgery. Int. J. Colorectal Dis. 2020, 35, 1221–1230. [Google Scholar] [CrossRef]
- Shimura, T.; Toiyama, Y.; Hiro, J.; Imaoka, H.; Fujikawa, H.; Kobayashi, M.; Ohi, M.; Inoue, Y.; Mohri, Y.; Kusunoki, M. Monitoring Perioperative Serum Albumin Can Identify Anastomotic Leakage in Colorectal Cancer Patients with Curative Intent. Asian J. Surg. 2018, 41, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, K.W.; Poeze, M.; Hulsewé, K.W.E.; van Acker, B.A.; van Bijnen, A.A.; Hoofwijk, A.G.M.; Stoot, J.H.M.B.; Derikx, J.P.M. Accurate Prediction of Anastomotic Leakage after Colorectal Surgery Using Plasma Markers for Intestinal Damage and Inflammation. J. Am. Coll. Surg. 2014, 219, 744–751. [Google Scholar] [CrossRef]
- Morandi, E.; Monteleone, M.; Merlini, D.A.; Vignati, G.; D’Aponte, T.; Castoldi, M. P0071 Faecal Calprotectin as an Early Biomarker of Colorectal Anastomotic Leak. Eur. J. Cancer 2015, 51, e15. [Google Scholar] [CrossRef]
- Cikot, M.; Kones, O.; Gedikbası, A.; Kocatas, A.; Karabulut, M.; Temizgonul, K.B.; Alis, H. The Marker C-Reactive Protein Is Helpful in Monitoring the Integrity of Anastomosis: Plasma Calprotectin. Am. J. Surg. 2016, 212, 53–61. [Google Scholar] [CrossRef]
- Dusek, T.; Orhalmi, J.; Sotona, O.; Krcmova, L.K.; Javorska, L.; Dolejs, J.; Paral, J. Neopterin, Kynurenine and Tryptophan as New Biomarkers for Early Detection of Rectal Anastomotic Leakage. Wideochir. Inne Tech. Malo Inwazyjne 2018, 13, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Murr, C.; Widner, B.; Wirleitner, B.; Fuchs, D. Neopterin as a Marker for Immune System Activation. Curr. Drug Metab. 2002, 3, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Corke, C.; Glenister, K. Monitoring Intestinal Ischaemia. Crit. Care Resusc. 2001, 3, 176–180. [Google Scholar] [PubMed]
- Oikonomakis, I.; Jansson, D.; Hörer, T.M.; Skoog, P.; Nilsson, K.F.; Jansson, K. Results of Postoperative Microdialysis Intraperitoneal and at the Anastomosis in Patients Developing Anastomotic Leakage after Rectal Cancer Surgery. Scand. J. Gastroenterol. 2019, 54, 1261–1268. [Google Scholar] [CrossRef]
- Ellebaek Pedersen, M.; Qvist, N.; Bisgaard, C.; Kelly, U.; Bernhard, A.; Møller Pedersen, S. Peritoneal Microdialysis. Early Diagnosis of Anastomotic Leakage after Low Anterior Resection for Rectosigmoid Cancer. Scand. J. Surg. 2009, 98, 148–154. [Google Scholar] [CrossRef]
- Millan, M.; García-Granero, E.; Flor, B.; García-Botello, S.; Lledo, S. Early Prediction of Anastomotic Leak in Colorectal Cancer Surgery by Intramucosal PH. Dis. Colon Rectum 2006, 49, 595–601. [Google Scholar] [CrossRef]
- Yang, L.; Huang, X.-E.; Xu, L.; Zhou, X.; Zhou, J.-N.; Yu, D.-S.; Li, D.-Z.; Guan, X. Acidic Pelvic Drainage as a Predictive Factor for Anastomotic Leakage after Surgery for Patients with Rectal Cancer. Asian Pac. J. Cancer Prev. 2013, 14, 5441–5447. [Google Scholar] [CrossRef]
- Gray, M.E.; Marland, J.R.K.; Dunare, C.; Blair, E.O.; Meehan, J.; Tsiamis, A.; Kunkler, I.H.; Murray, A.F.; Argyle, D.; Dyson, A.; et al. In Vivo Validation of a Miniaturized Electrochemical Oxygen Sensor for Measuring Intestinal Oxygen Tension. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G242–G252. [Google Scholar] [CrossRef]
- Reisinger, K.W.; Schellekens, D.H.S.M.; Bosmans, J.W.A.M.; Boonen, B.; Hulsewé, K.W.E.; Sastrowijoto, P.; Derikx, J.P.M.; Grootjans, J.; Poeze, M. Cyclooxygenase-2 Is Essential for Colorectal Anastomotic Healing. Ann. Surg. 2017, 265, 547–554. [Google Scholar] [CrossRef]
- Holmgren, K.; Jonsson, P.; Lundin, C.; Matthiessen, P.; Rutegård, J.; Sund, M.; Rutegård, M. Preoperative Biomarkers Related to Inflammation May Identify High-Risk Anastomoses in Colorectal Cancer Surgery: Explorative Study. BJS Open 2022, 6, zrac072. [Google Scholar] [CrossRef]
- Xiang, S.; Yang, Y.-K.; Wang, T.-Y.; Yang, Z.-T.; Lu, Y.; Liu, S.-L. Development and Validation of a Nomogram to Predict Anastomotic Leakage in Colorectal Cancer Based on CT Body Composition. Front. Nutr. 2022, 9, 974903. [Google Scholar] [CrossRef] [PubMed]
- Mitsala, A.; Tsalikidis, C.; Pitiakoudis, M.; Simopoulos, C.; Tsaroucha, A.K. Artificial Intelligence in Colorectal Cancer Screening, Diagnosis and Treatment. A New Era. Curr. Oncol. 2021, 28, 1581–1607. [Google Scholar] [CrossRef]
- Mazaki, J.; Katsumata, K.; Ohno, Y.; Udo, R.; Tago, T.; Kasahara, K.; Kuwabara, H.; Enomoto, M.; Ishizaki, T.; Nagakawa, Y.; et al. A Novel Predictive Model for Anastomotic Leakage in Colorectal Cancer Using Auto-Artificial Intelligence. Anticancer Res. 2021, 41, 5821–5825. [Google Scholar] [CrossRef]
- Wen, R.; Zheng, K.; Zhang, Q.; Zhou, L.; Liu, Q.; Yu, G.; Gao, X.; Hao, L.; Lou, Z.; Zhang, W. Machine Learning-Based Random Forest Predicts Anastomotic Leakage after Anterior Resection for Rectal Cancer. J. Gastrointest. Oncol. 2021, 12, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Sammour, T.; Cohen, L.; Karunatillake, A.I.; Lewis, M.; Lawrence, M.J.; Hunter, A.; Moore, J.W.; Thomas, M.L. Validation of an Online Risk Calculator for the Prediction of Anastomotic Leak after Colon Cancer Surgery and Preliminary Exploration of Artificial Intelligence-Based Analytics. Tech. Coloproctol. 2017, 21, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Park, H.-M.; Baek, K.-R.; Ahn, H.-M.; Lee, I.Y.; Son, G.M. Artificial Intelligence Based Real-Time Microcirculation Analysis System for Laparoscopic Colorectal Surgery. World J. Gastroenterol. 2020, 26, 6945–6962. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsalikidis, C.; Mitsala, A.; Mentonis, V.I.; Romanidis, K.; Pappas-Gogos, G.; Tsaroucha, A.K.; Pitiakoudis, M. Predictive Factors for Anastomotic Leakage Following Colorectal Cancer Surgery: Where Are We and Where Are We Going? Curr. Oncol. 2023, 30, 3111-3137. https://doi.org/10.3390/curroncol30030236
Tsalikidis C, Mitsala A, Mentonis VI, Romanidis K, Pappas-Gogos G, Tsaroucha AK, Pitiakoudis M. Predictive Factors for Anastomotic Leakage Following Colorectal Cancer Surgery: Where Are We and Where Are We Going? Current Oncology. 2023; 30(3):3111-3137. https://doi.org/10.3390/curroncol30030236
Chicago/Turabian StyleTsalikidis, Christos, Athanasia Mitsala, Vasileios I. Mentonis, Konstantinos Romanidis, George Pappas-Gogos, Alexandra K. Tsaroucha, and Michail Pitiakoudis. 2023. "Predictive Factors for Anastomotic Leakage Following Colorectal Cancer Surgery: Where Are We and Where Are We Going?" Current Oncology 30, no. 3: 3111-3137. https://doi.org/10.3390/curroncol30030236
APA StyleTsalikidis, C., Mitsala, A., Mentonis, V. I., Romanidis, K., Pappas-Gogos, G., Tsaroucha, A. K., & Pitiakoudis, M. (2023). Predictive Factors for Anastomotic Leakage Following Colorectal Cancer Surgery: Where Are We and Where Are We Going? Current Oncology, 30(3), 3111-3137. https://doi.org/10.3390/curroncol30030236




