Safety and Accuracy of Sentinel Lymph Node Biopsy Alone in Clinically Node-Positive Patients Undergoing Upfront Surgery for Invasive Breast Cancer: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Study Eligibility and Selection
2.3. Data Extraction
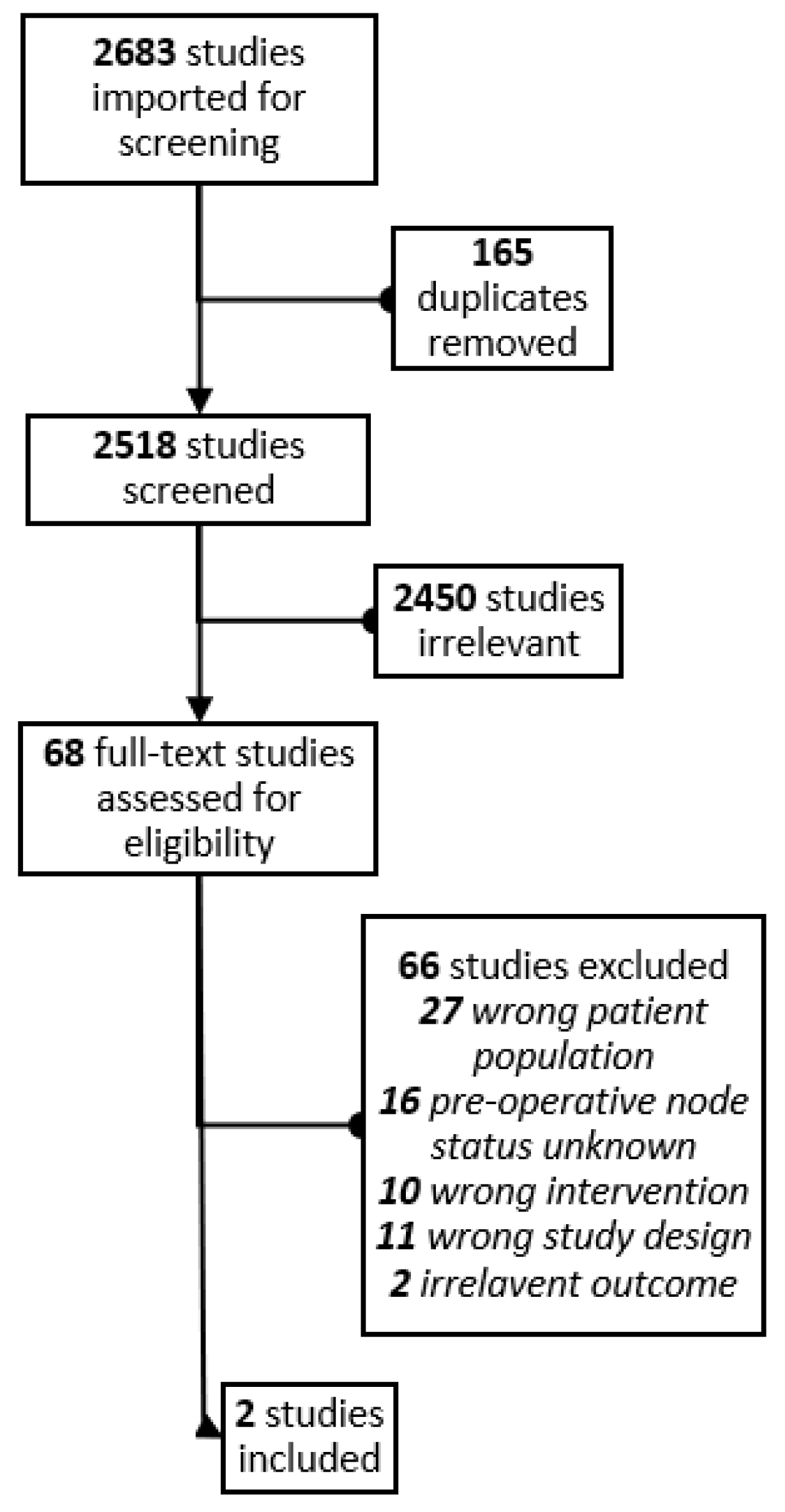
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Search Strategy
| Embase Strategy | Ovid MEDLINE Strategy |
|---|---|
|
|
References
- Naoum, G.E.; Roberts, S.; Brunelle, C.L.; Shui, A.M.; Salama, L.; Daniell, K.; Gillespie, T.; Bucci, L.; Smith, B.L.; Ho, A.Y.; et al. Quantifying the impact of axillary surgery and nodal irradiation on breast cancer-related lymphedema and local tumor control: Long-term results from a prospective screening trial. J. Clin. Oncol. 2020, 38, 3430–3438. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, M.P.; Gomberawalla, A.; Smith, M.; Boccardo, F.M.; Holmes, D.; Djohan, R.; Thiruchelvam, P.; Klimberg, S.; Dietz, J.; Feldman, S. The prevention and treatment of breast cancer—related lymphedema: A review. Front. Oncol. 2022, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, V.; Ribeiro Fontana, S.K.; Vicini, E.; Morigi, C.; Sargenti, M.; Corso, G.; Magnoni, F.; Intra, M.; Veronesi, P. “This house believes that: Sentinel node biopsy alone is better than TAD after NACT for cN+ patients”. Breast 2023, 67, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Krag, D.N.; Anderson, S.J.; Julian, T.B.; Brown, A.M.; Harlow, S.P.; Costantino, J.P.; Ashikaga, T.; Weaver, D.L.; Mamounas, E.P.; Jalovec, L.M.; et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: Overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010, 11, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Miranda, K.; Pace, D.; Cintron, R.; Rodrigues, J.C.F.; Fang, J.; Smith, A.; Rohloff, P.; Coelho, E.; De Haas, F.; Souza, D.; et al. IBCSG 23-01 randomised controlled trial comparing axillary dissection versus no axillary dissection in patients with sentinel node micrometastases. Lancet Oncol. 2011, 76, 1358–1375. [Google Scholar] [CrossRef]
- Donker, M.; van Tienhoven, G.; Straver, M.E.; Meijnen, P.; van de Velde, C.J.H.; Mansel, R.E.; Cataliotti, L.; Westenberg, A.H.; Klinkenbijl, J.H.G.; Orzalesi, L.; et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): A randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014, 15, 1303–1310. [Google Scholar] [CrossRef]
- Giuliano, A.; Hunt, K.K.; Ballman, K.V.; Beitsch, P.D.; Whitworth, P.W.; Blumencranz, P.W.; Leitch, A.M.; Mccall, L.M.; Morrow, M. Axillary Dissection vs No Axillary Dissection in Women With Invasive Breast Cancer. JAMA 2011, 305, 569–575. [Google Scholar] [CrossRef]
- Kuehn, T.; Bauerfeind, I.; Fehm, T.; Fleige, B.; Hausschild, M.; Helms, G.; Lebeau, A.; Liedtke, C.; von Minckwitz, G.; Nekljudova, V.; et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): A prospective, multicentre cohort study. Lancet Oncol. 2013, 14, 609–618. [Google Scholar] [CrossRef]
- Boileau, J.F.; Poirier, B.; Basik, M.; Holloway, C.M.B.; Gaboury, L.; Sideris, L.; Meterissian, S.; Arnaout, A.; Brackstone, M.; McCready, D.R.; et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: The SN FNAC study. J. Clin. Oncol. 2015, 33, 258–263. [Google Scholar] [CrossRef]
- Boughey, J.C.; Suman, V.J.; Mittendorf, E.A.; Ahrendt, G.M.; Wilke, L.G.; Taback, B.; Leitch, A.M.; Kuerer, H.M.; Bowling, M.; Flippo-Morton, T.S.; et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: The ACOSOG Z1071 (alliance) clinical trial. JAMA 2013, 310, 1455–1461. [Google Scholar] [CrossRef]
- Pilewskie, M.; Morrow, M. Axillary nodal management following neoadjuvant chemotherapy: A review. JAMA Oncol. 2017, 3, 549–555. [Google Scholar] [CrossRef]
- Maggi, N.; Nussbaumer, R.; Holzer, L.; Weber, W.P. Axillary surgery in node-positive breast cancer. Breast 2022, 62, S50–S53. [Google Scholar] [CrossRef] [PubMed]
- Almahariq, M.F.; Levitin, R.; Quinn, T.J.; Chen, P.Y.; Dekhne, N.; Kiran, S.; Desai, A.; Benitez, P.; Jawad, M.S.; Gustafson, G.S.; et al. Omission of Axillary Lymph Node Dissection is Associated with Inferior Survival in Breast Cancer Patients with Residual N1 Nodal Disease Following Neoadjuvant Chemotherapy. Ann. Surg. Oncol. 2021, 28, 930–940. [Google Scholar] [CrossRef]
- Kalinsky, K.; Barlow, W.E.; Gralow, J.R.; Meric-Bernstam, F.; Albain, K.S.; Hayes, D.F.; Lin, N.U.; Perez, E.A.; Goldstein, L.J.; Chia, S.K.L.; et al. 21-Gene Assay to Inform Chemotherapy Benefit in Node-Positive Breast Cancer. N. Engl. J. Med. 2021, 385, 2336–2347. [Google Scholar] [CrossRef]
- Breast Cancer Version 4. NCCN Clinical Practice Guidelines in Oncology. Published 2022. 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 26 December 2022).
- Fisher, B.; Montague, E.; Redmon, C.; Barton, B.; Borland, D.; Fisher, E.; Deutsch, M.; Schwarz, G.; Margolese, R.; Donegan, W.; et al. Comparison of radical mastectomy with alternative treatments for primary breast cancer: A first report of results from a prospective rnadomized clinical trial. Cancer 1977, 39, 2827–2839. [Google Scholar] [CrossRef]
- Riedel, F.; Schaefgen, B.; Sinn, H.P.; Feisst, M.; Hennigs, A.; Hug, S.; Binnig, A.; Gomez, C.; Harcos, A.; Stieber, A.; et al. Diagnostic accuracy of axillary staging by ultrasound in early breast cancer patients. Eur. J. Radiol. 2021, 135, 109468. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.M.; Leung, J.W.T.; Moy, L.; Ha, S.M.; Moon, W.K. Axillary nodal evaluation in breast cancer: State of the art. Radiology 2020, 295, 500–515. [Google Scholar] [CrossRef]
- Lee, J.; Jung, J.H.; Kim, W.W.; Lee, R.K.; Kim, H.J.; Kim, W.H.; Park, J.Y.; Jeong, J.Y.; Chae, Y.S.; Lee, S.J.; et al. 5-year oncological outcomes of targeted axillary sampling in pT1-2N1 breast cancer. Asian J. Surg. 2019, 42, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Covidence systematic review software. Available online: http://www.covidence.org (accessed on 29 April 2022).
- Weber, W.P.; Matrai, Z.; Hayoz, S.; Tausch, C.; Henke, G.; Zwahlen, D.R.; Gruber, G.; Zimmermann, F.; Seiler, S.; Maddox, C.; et al. Tailored axillary surgery in patients with clinically node-positive breast cancer: Pre-planned feasibility substudy of TAXIS (OPBC-03, SAKK 23/16, IBCSG 57-18, ABCSG-53, GBG 101). Breast 2021, 60, 98–110. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Montagna, G.; Sevilimedu, V.; Abbate, K.; Charyn, J.; Mehrara, B.; Morrow, M.; Barrio, A.V. Longitudinal Prospective Evaluation of Quality of Life After Axillary Lymph Node Dissection. Ann. Surg. Oncol. 2022, 29, 4127–4136. [Google Scholar] [CrossRef] [PubMed]
- Caudle, A.S.; Yang, W.T.; Krishnamurthy, S.; Mittendorf, E.A.; Black, D.M.; Gilcrease, M.Z.; Bedrosian, I.; Hobbs, B.P.; Desnyder, S.M.; Hwang, R.F.; et al. Improved axillary evaluation following neoadjuvant therapy for patientswith node-positive breast cancer using selective evaluation of clipped nodes: Implementation of targeted axillary dissection. J. Clin. Oncol. 2016, 34, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Kuemmel, S.; Heil, J.; Rueland, A.; Seiberling, C.; Harrach, H.; Schindowski, D.; Lubitz, J.; Hellerhoff, K.; Ankel, C.; Graßhoff, S.T.; et al. A Prospective, Multicenter Registry Study to Evaluate the Clinical Feasibility of Targeted Axillary Dissection (TAD) in Node-positive Breast Cancer Patients. Ann. Surg. 2022, 276, E553–E562. [Google Scholar] [CrossRef]
- Dixon, J.M.; Grewar, J.; Twelves, D.; Graham, A.; Martinez-Perez, C.; Turnbull, A. Factors affecting the number of sentinel lymph nodes removed in patients having surgery for breast cancer. Breast Cancer Res. Treat. 2020, 184, 335–343. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Mccall, L.; Beitsch, P.; Whitworth, P.W.; Blumencranz, P.; Leitch, A.M.; Saha, S.; Hunt, K.K.; Morrow, M.; Ballman, K.; et al. Locoregional Recurrence after Sentinel Lymph Node Dissection with or without Axillary Dissection in Patients with Sentinel Lymph Node Metastases: The American College of Surgeons Oncology Group Z0011 Randomized Trial. Ann. Surg. 2010, 252, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Alagoz, O.; Lowry, K.P.; Kurian, A.W.; Mandelblatt, J.S.; Ergun, M.A.; Huang, H.; Lee, S.J.; Schechter, C.B.; Tosteson, A.N.A.; Miglioretti, D.L.; et al. Impact of the COVID-19 Pandemic on Breast Cancer Mortality in the US: Estimates From Collaborative Simulation Modeling. J. Natl. Cancer Inst. 2021, 113, 1484–1494. [Google Scholar] [CrossRef]
| Authors | Year | Country/ Region | Study Design | Inclusion Criteria | Patients | Follow-Up | Outcomes |
|---|---|---|---|---|---|---|---|
| Lee et al. [19] | 2019 | South Korea | Single-center, non-randomized double arm prospective cohort study comparing TAS vs. ALND (treatment arm assigned based on patient preference | cT1-2N1 BC | n = 64 (ALND) n = 65 (TAS) | 5-years |
|
| Weber et al. [22] | 2021 | Europe | International multicenter, prospective study embedded in a randomized trial (TAXIS) comparing TAS vs. TAS + ALND (feasibility study) | cT1-3 N1-2 BC, All breast cancer subtypes | n = 166 treated with upfront surgery with TAS, of which 86 underwent subsequent ALND | Post-surgery |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lovrics, O.; Tao, B.; Parvez, E. Safety and Accuracy of Sentinel Lymph Node Biopsy Alone in Clinically Node-Positive Patients Undergoing Upfront Surgery for Invasive Breast Cancer: A Systematic Review. Curr. Oncol. 2023, 30, 3102-3110. https://doi.org/10.3390/curroncol30030235
Lovrics O, Tao B, Parvez E. Safety and Accuracy of Sentinel Lymph Node Biopsy Alone in Clinically Node-Positive Patients Undergoing Upfront Surgery for Invasive Breast Cancer: A Systematic Review. Current Oncology. 2023; 30(3):3102-3110. https://doi.org/10.3390/curroncol30030235
Chicago/Turabian StyleLovrics, Olivia, Brendan Tao, and Elena Parvez. 2023. "Safety and Accuracy of Sentinel Lymph Node Biopsy Alone in Clinically Node-Positive Patients Undergoing Upfront Surgery for Invasive Breast Cancer: A Systematic Review" Current Oncology 30, no. 3: 3102-3110. https://doi.org/10.3390/curroncol30030235
APA StyleLovrics, O., Tao, B., & Parvez, E. (2023). Safety and Accuracy of Sentinel Lymph Node Biopsy Alone in Clinically Node-Positive Patients Undergoing Upfront Surgery for Invasive Breast Cancer: A Systematic Review. Current Oncology, 30(3), 3102-3110. https://doi.org/10.3390/curroncol30030235





