Mitigating Breast-Cancer-Related Lymphedema—A Calgary Program for Immediate Lymphatic Reconstruction (ILR)
Abstract
1. Introduction and Rationale for Performing Immediate Lymphatic Reconstruction
1.1. Spotlight on Survivorship and the Burden of Lymphedema
1.2. Surgical Options for Lymphedema Prevention
1.3. What Is the Current Place of Axillary Lymph Node Dissection?
1.4. Neoadjuvant Therapy and the Axilla—What to Do?
1.5. Maybe This Is Where ILR Finds a Home
1.6. Why Forming a Team and Getting Ready to Perform ILR Makes Sense
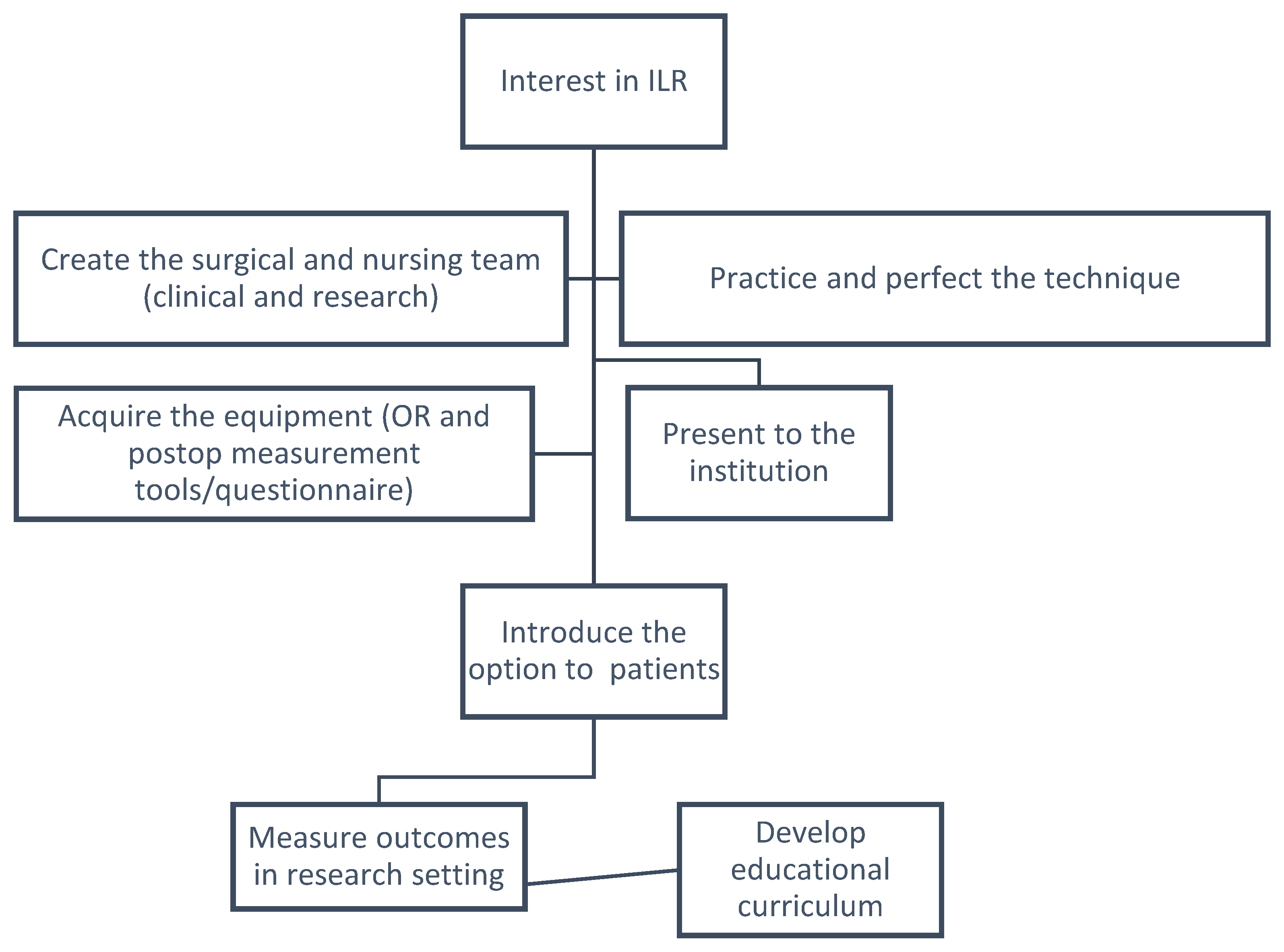
2. Initial Steps
2.1. First Thing’s First—Fostering the Team
2.2. Selecting the Patients
2.3. The Technique
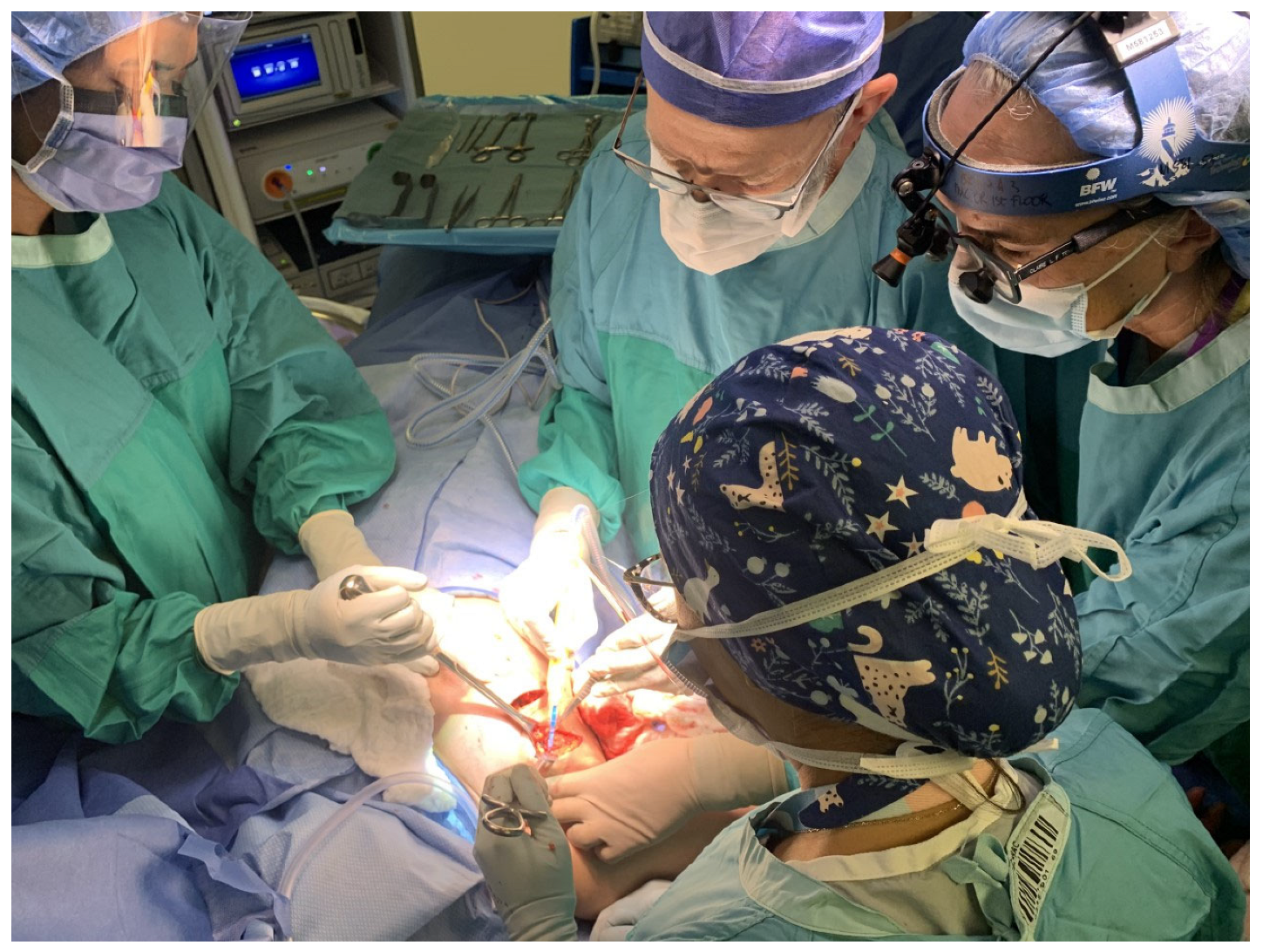
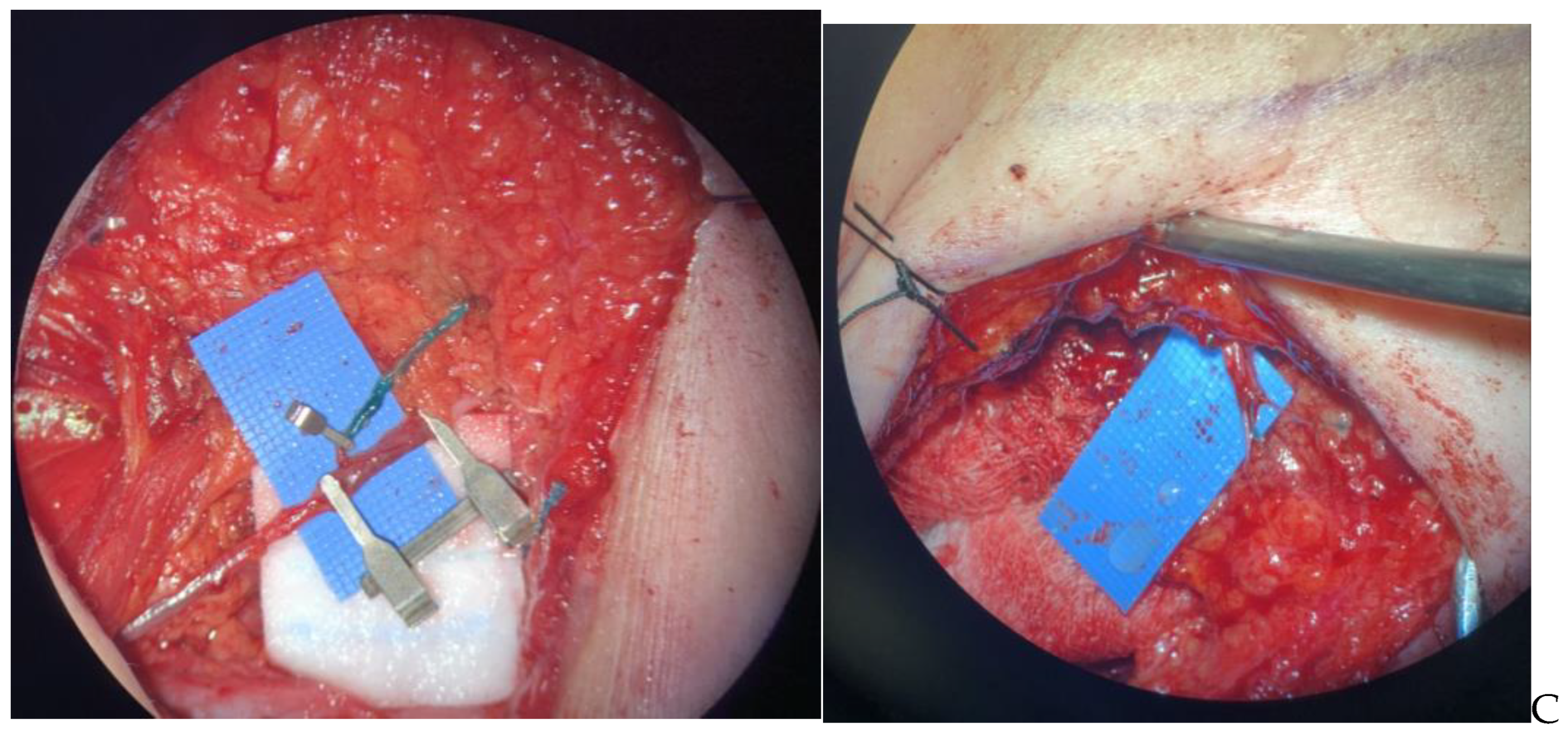
2.3.1. Part 1: Macrosurgery
2.3.2. Part 2: Microsurgery
2.4. Learning the Intraoperative Dance
2.5. All the Extra Stuff
2.6. Operating Room Size and Time
2.7. Important for Patients to Know
2.8. Who Else Needs to Be on the Team?
3. Postoperative Follow-Up
3.1. Measuring Lymphedema
4. Education and Research
4.1. Establishing a Research Setting
4.2. Development of an Educational Curriculum
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Canadian Cancer Society. Survival Statistics for Breast Cancer. Available online: https://cancer.ca/en/cancer-information/cancer-types/breast/prognosis-and-survival/survival-statistics (accessed on 10 December 2022).
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef] [PubMed]
- Al-Rashdan, A.; Deban, M.; Quan, M.L.; Cao, J.Q. Locoregional Management of Breast Cancer: A Chronological Review. Curr. Oncol. 2022, 29, 4647–4664. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, P.; Ballardini, B.; De Lorenzi, F.; Magnoni, F.; Lissidini, G.; Caldarella, P.; Galimberti, V. Immediate breast recon-struction after mastectomy. Breast 2011, 20 (Suppl. 3), S104–S107. [Google Scholar] [CrossRef] [PubMed]
- Voss, R.K.; Cromwell, K.D.; Chiang, Y.-J.; Armer, J.M.; Ross, M.I.; Lee, J.E.; Gershenwald, J.E.; Stewart, B.R.; Shaitelman, S.F.; Cormier, J.N. The long-term risk of upper-extremity lymphedema is two-fold higher in breast cancer patients than in melanoma patients. J. Surg. Oncol. 2015, 112, 834–840. [Google Scholar] [CrossRef]
- Bowman, C.; Piedalue, K.-A.; Baydoun, M.; Carlson, L.E. The Quality of Life and Psychosocial Implications of Cancer-Related Lower-Extremity Lymphedema: A Systematic Review of the Literature. J. Clin. Med. 2020, 9, 3200. [Google Scholar] [CrossRef]
- Dean, L.T.; Moss, S.L.; Ransome, Y.; Frasso-Jaramillo, L.; Zhang, Y.; Visvanathan, K.; Nicholas, L.H.; Schmitz, K.H. “It still affects our economic situation”: Long-term economic burden of breast cancer and lymphedema. Support. Care Cancer 2018, 27, 1697–1708. [Google Scholar] [CrossRef]
- Taghian, N.R.; Miller, C.L.; Jammallo, L.S.; O’Toole, J.; Skolny, M.N. Lymphedema following breast cancer treatment and impact on quality of life: A review. Crit. Rev. Oncol. 2014, 92, 227–234. [Google Scholar] [CrossRef]
- Woodruff, T.K.; Ataman-Millhouse, L.; Acharya, K.S.; Almeida-Santos, T.; Anazodo, A.; Anderson, R.A.; Appiah, L.; Bader, J.; Becktell, K.; Brannigan, R.E.; et al. A view from the past into our collective future: The oncofertility consortium vision statement. J. Assist. Reprod. Genet. 2021, 38, 3–15. [Google Scholar] [CrossRef]
- Gnant, M.; Harbeck, N.; Thomssen, C. St. Gallen/Vienna 2017: A Brief Summary of the Consensus Discussion about Escalation and De-Escalation of Primary Breast Cancer Treatment. Breast Care 2017, 12, 101–106. [Google Scholar] [CrossRef]
- Clough, K.B.; Kaufman, G.J.; Nos, C.; Buccimazza, I.; Sarfati, I.M. Improving Breast Cancer Surgery: A Classification and Quadrant per Quadrant Atlas for Oncoplastic Surgery. Ann. Surg. Oncol. 2010, 17, 1375–1391. [Google Scholar] [CrossRef]
- Rockson, S.G. Lymphedema after Breast Cancer Treatment. N. Engl. J. Med. 2018, 379, 1937–1944. [Google Scholar] [CrossRef]
- Nos, C.; Kaufmann, G.; Clough, K.B.; Collignon, M.-A.; Zerbib, E.; Cusumano, P.; Lecuru, F. Combined Axillary Reverse Mapping (ARM) Technique for Breast Cancer Patients Requiring Axillary Dissection. Ann. Surg. Oncol. 2008, 15, 2550–2555. [Google Scholar] [CrossRef]
- Nos, C.; Lesieur, B.; Clough, K.B.; Lecuru, F. Blue dye injection in the arm in order to conserve the lymphatic drainage of the arm in breast cancer patients requiring an axillary sissection. Ann. Surg. Oncol. 2007, 14, 2490–2496. [Google Scholar] [CrossRef]
- Thompson, M.; Korourian, S.; Henry-Tillman, R.; Adkins, L.; Mumford, S.; Westbrook, K.C.; Klimberg, V.S. Axillary Reverse Mapping (ARM): A New Concept to Identify and Enhance Lymphatic Preservation. Ann. Surg. Oncol. 2007, 14, 1890–1895. [Google Scholar] [CrossRef]
- Boccardo, F.; Casabona, F.; De Cian, F.; Friedman, D.; Villa, G.; Bogliolo, S.; Ferrero, S.; Murelli, F.; Campisi, C. Lymphedema Microsurgical Preventive Healing Approach: A New Technique for Primary Prevention of Arm Lymphedema After Mastectomy. Ann. Surg. Oncol. 2009, 16, 703–708. [Google Scholar] [CrossRef]
- Boccardo, F.; Casabona, F.; De Cian, F.; Friedman, D.; Murelli, F.; Puglisi, M.; Corrado, C.C.; Molinari, L.; Spinaci, S.; Dessalvi, S.; et al. Lymphatic microsurgical preventing healing approach (LYMPHA) for primary surgical prevention of breast cancer-related lymphedema: Over 4 years follow-up. Microsurgery 2014, 34, 421–424. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Ballman, K.V.; McCall, L.; Beitsch, P.D.; Brennan, M.B.; Kelemen, P.R.; Ollila, D.W.; Hansen, N.M.; Whitworth, P.W.; Blumencranz, P.W.; et al. Effect of axillary dissection vs. no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The acosog Z0011 (alliance) randomized clinical trial. JAMA 2017, 318, 918–926. [Google Scholar] [CrossRef]
- Donker, M.; van Tienhoven, G.; Straver, M.E.; Meijnen, P.; van de Velde, C.J.; Mansel, R.E.; Cataliotti, L.; Westenberg, A.H.; Klinkenbijl, J.H.; Orzalesi, L. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (Eortc 10981–22023 Amaros): A randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014, 15, 1303–1310. [Google Scholar] [CrossRef]
- Boughey, J.C.; Suman, V.J.; Mittendorf, E.A.; Ahrendt, G.M.; Wilke, L.G.; Taback, B.; Leitch, A.M.; Kuerer, H.M.; Bowling, M.; Flippo-Morton, T.S. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: The Acosog Z1071 (Alliance) clinical trial. JAMA 2013, 310, 1455–1461. [Google Scholar] [CrossRef]
- Boughey, J.C.; Ballman, K.V.; Le-Petross, H.T.; McCall, L.M.; Mittendorf, E.A.; Ahrendt, G.M.; Wilke, L.G.; Taback, B.; Feliberti, E.C.; Hunt, K.K. Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0-T4, N1-N2) who receive neoadjuvant chemo-therapy: Results from Acosog Z1071 (Alliance). Ann. Surg. 2016, 263, 802–807. [Google Scholar] [CrossRef]
- Kuehn, T.; Bauerfeind, I.; Fehm, T.; Fleige, B.; Hausschild, M.; Helms, G.; Lebeau, A.; Liedtke, C.; von Minckwitz, G.; Nekljudova, V.; et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (Sentina): A prospective, multicentre chort study. Lancet Oncol. 2013, 14, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Boileau, J.F.; Poirier, B.; Basik, M.; Holloway, C.M.; Gaboury, L.; Sideris, L.; Meterissian, S.; Arnaout, A.; Brackstone, M.; McCready, D.R.; et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: The SN FNAC study. J. Clin. Oncol. 2015, 33, 258–264. [Google Scholar] [CrossRef]
- Caudle, A.S.; Yang, W.T.; Krishnamurthy, S.; Mittendorf, E.A.; Black, D.M.; Gilcrease, M.Z.; Bedrosian, I.; Hobbs, B.P.; DeSnyder, S.M.; Hwang, R.F.; et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: Implementation of targeted axillary dissection. J. Clin. Oncol. 2016, 34, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Lustig, D.B.; Temple-Oberle, C.; Bouchard-Fortier, A.; Quan, M.L. Incorporating lymphovenous anastomosis in clinically node positive women receiving neoadjuvant chemotherapy: A shared decision-making model and nuanced approached to the axilla. Curr. Oncol. 2023, submitted.
- Morrow, M.; Khan, A.J. Locoregional management after neoadjuvant chemotherapy. J. Clin. Oncol. 2020, 38, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, V.; Ribeiro Fontana, S.K.; Maisonneuve, P.; Steccanella, F.; Vento, A.R.; Intra, M.; Naninato, P.; Caldarella, P.; Iorfida, M.; Colleoni, M.; et al. Sentinel node biopsy after neoadjuvant treatment in breast cancer: Five-year follow-up of patients with clinically node-negative or node-positive disease before treatment. Eur. J. Surg. Oncol. 2016, 42, 361–368. [Google Scholar] [CrossRef]
- Boughey, J.C.; McCall, L.M.; Ballman, K.V.; Mittendorf, E.A.; Ahrendt, G.M.; Wilke, L.G.; Taback, B.; Leitch, A.M.; Flip-po-Morton, T.; Hunt, K.K. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: Findings from the Acosog Z1071 (Alliance) prospective multicenter clinical trial. Ann. Surg. 2014, 260, 608–614. [Google Scholar] [CrossRef]
- Samiei, S.; Simons, J.M.; Engelen, S.M.E.; Beets-Tan, R.G.H.; Classe, J.M.; Smidt, M.L.; Eubreast Group. Axillary pathologic complete response after neoadjuvant systemic therapy by breast cancer subtype in patients with initially clinically node-positive disease: A systematic review and meta-analysis. JAMA Surg. 2021, 156, e210891. [Google Scholar] [CrossRef]
- Hill, W.K.F.; Deban, M.; Platt, A.M.; Rojas-Garcia, P.; Jost, E.M.; Temple-Oberle, C.M. Immediate lymphatic reconstruction during axillary node dissection for breast cancer: A systematic review and meta-analysis. Plast. Reconstr. Surg. 2022, 10, e4291. [Google Scholar] [CrossRef]
- Scaglioni, M.F.; Fontein, D.B.Y.; Arvanitakis, M.; Giovanoli, P. Systematic review of lymphovenous anastomosis (LVA) for the treatment of lymphedema. Microsurgery 2017, 37, 947–953. [Google Scholar] [CrossRef]
- Jørgensen, M.G.; Toyserkani, N.M.; Sørensen, J.A. The effect of prophylactic lymphovenous anastomosis and shunts for preventing cancer-related lymphedema: A systematic review and meta-analysis. Microsurgery 2017, 38, 576–585. [Google Scholar] [CrossRef]
- Johnson, A.R.; Fleishman, A.; Granoff, M.D.; Shillue, K.; Houlihan, M.J.; Sharma, R.; Kansal, K.J.; Teller, P.; James, T.A.; Lee, B.T.; et al. Evaluating the impact of immediate lymphatic reconstruction for the surgical prevention of lymphedema. Plast. Reconstr. Surg. 2021, 147, 373e–381e. [Google Scholar] [CrossRef]
- University of Calgary. Advanced Technical Skills Simulation Laboratory. Available online: https://cumming.ucalgary.ca/atssl (accessed on 10 December 2022).
- Bittar, S.; Simman, R.; Lurie, F. Lymphedema: A practical approach and clinical update. Wounds 2020, 32, 86–92. [Google Scholar]
- Casabona, F.; Bogliolo, S.; Menada, M.V.; Sala, P.; Villa, G.; Ferrero, S. Feasibility of axillary reverse mapping during sentinel lymph node biopsy in breast cancer patients. Ann. Surg. Oncol. 2009, 16, 2459–2463. [Google Scholar] [CrossRef]
- Feldman, S.; Bansil, H.; Ascherman, J.; Grant, R.; Borden, B.; Henderson, P.; Ojo, A.; Taback, B.; Chen, M.; Ananthakrishnan, P.; et al. Single institution experience with lymphatic microsurgical preventive healing approach (lympha) for the primary prevention of lymphedema. Ann. Surg. Oncol. 2015, 22, 3296–3301. [Google Scholar] [CrossRef]
- Schwarz, G.S.; Grobmyer, S.R.; Djohan, R.S.; Cakmakoglu, C.; Bernard, S.L.; Radford, D.; Al-Hilli, Z.; Knackstedt, R.; Djohan, M.; Valente, S.A. Axillary reverse mapping and lymphaticovenous bypass: Lymphedema prevention through enhanced lymphatic visualization and restoration of flow. J. Surg. Oncol. 2019, 120, 160–167. [Google Scholar] [CrossRef]
- Johnson, A.R.; Asban, A.; Granoff, M.D.; Kang, C.O.; Lee, B.T.; Chatterjee, A.; Singhal, D. Is immediate lymphatic reconstruction cost-effective? Ann. Surg. 2021, 274, e581–e588. [Google Scholar] [CrossRef]
- Armer, J.M.; Stewart, B.R. A comparison of four diagnostic criteria for lymphedema in a post-breast cancer population. Lymphat. Res. Biol. 2005, 3, 208–217. [Google Scholar] [CrossRef]
- Executive Committee. The diagnosis and treatment of peripheral lymphedema: 2016 consensus document of the International Society of Lymphology. Lymphology 2016, 49, 170–184. [Google Scholar]
- Hidding, J.T.; Viehoff, P.B.; Beurskens, C.H.; van Laarhoven, H.W.; Nijhuis-van der Sanden, M.W.; van der Wees, P.J. Measurement properties of instruments for measuring of lymphedema: Systematic review. Phys. Ther. 2016, 96, 1965–1981. [Google Scholar] [CrossRef]
- Lawenda, B.D.; Mondry, T.E.; Johnstone, P.A.S. Lymphedema: A primer on the identification and management of a chronic condition in oncologic treatment. CA Cancer J. Clin. 2009, 59, 8–24. [Google Scholar] [CrossRef]
- Levenhagen, K.; Davies, C.; Perdomo, M.; Ryans, K.; Gilchrist, L. Diagnosis of upper quadrant lymphedema secondary to cancer: Clinical practice guideline from the oncology section of the American Physical Therapy Association. Phys. Ther. 2017, 97, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Warren, A.G.; Brorson, H.; Borud, L.J.; Slavin, S.A. Lymphedema: A comprehensive review. Ann. Plast. Surg. 2007, 59, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.; Montero, E.C.; Hagenström, K.; Herberger, K.; Blome, C. Validation of a short-form of the freiburg life quality assessment for lymphoedema (FLQA-LS) instrument. Br. J. Dermatol. 2018, 179, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Devoogdt, N.; Van Kampen, M.; Geraerts, I.; Coremans, T.; Christiaens, M.R. Lymphoedema functioning, disability and health questionnaire (Lymph-ICF): Reliability and validity. Phys. Ther. 2011, 91, 944–957. [Google Scholar] [CrossRef]
- Monticone, M.; Ferriero, G.; Keeley, V.; Brunati, R.; Liquori, V.; Maggioni, S.; Restelli, M.; Giordano, A.; Franchignoni, F. Lymphedema quality of life questionnaire (Lymqol): Cross-cultural adaptation and validation in Italian women with upper limb lymphedema after breast cancer. Disabil. Rehabil. 2021, 44, 4075–4080. [Google Scholar] [CrossRef]
- University of Aberdeen. The Non-Technical Skills for Surgeons (Notss) System Handbook V1.2. Available online: https://www.iscp.ac.uk/static/help/NOTSS_Handbook_2012.pdf (accessed on 10 December 2022).




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deban, M.; McKinnon, J.G.; Temple-Oberle, C. Mitigating Breast-Cancer-Related Lymphedema—A Calgary Program for Immediate Lymphatic Reconstruction (ILR). Curr. Oncol. 2023, 30, 1546-1559. https://doi.org/10.3390/curroncol30020119
Deban M, McKinnon JG, Temple-Oberle C. Mitigating Breast-Cancer-Related Lymphedema—A Calgary Program for Immediate Lymphatic Reconstruction (ILR). Current Oncology. 2023; 30(2):1546-1559. https://doi.org/10.3390/curroncol30020119
Chicago/Turabian StyleDeban, Melina, J. Gregory McKinnon, and Claire Temple-Oberle. 2023. "Mitigating Breast-Cancer-Related Lymphedema—A Calgary Program for Immediate Lymphatic Reconstruction (ILR)" Current Oncology 30, no. 2: 1546-1559. https://doi.org/10.3390/curroncol30020119
APA StyleDeban, M., McKinnon, J. G., & Temple-Oberle, C. (2023). Mitigating Breast-Cancer-Related Lymphedema—A Calgary Program for Immediate Lymphatic Reconstruction (ILR). Current Oncology, 30(2), 1546-1559. https://doi.org/10.3390/curroncol30020119




