Thoracoabdominal Aortic Replacement Together with Curative Oncological Surgery in Retroperitoneal and Spinal Tumours
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Acquisition
2.2. Patients
2.3. Standardised Surgical Approach
3. Results
3.1. Vascular Procedures
3.2. Tumour Resection and Adjunctive Procedures
3.3. Outcomes
3.4. Follow-Up
4. Discussion
5. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sassa, N. Retroperitoneal tumors: Review of diagnosis and management. Int. J. Urol. 2020, 27, 1058–1070. [Google Scholar] [CrossRef]
- Cody, H.S., III; Turnbull, A.D.; Fortner, J.G.; Hajdu, S.I. The continuing challenge of retroperitoneal sarcomas. Cancer 1981, 47, 2147–2152. [Google Scholar] [CrossRef]
- Shiraev, T.; Pasricha, S.S.; Choong, P.; Schlicht, S.; Van Rijswijk, C.S.; Dimmick, S.; Stuckey, S.; E Anderson, S. Retroperitoneal sarcomas: A review of disease spectrum, radiological features, characterisation and management. J. Med. Imaging Radiat. Oncol. 2013, 57, 687–700. [Google Scholar] [CrossRef]
- Gronchi, A.; Vullo, S.L.; Fiore, M.; Mussi, C.; Stacchiotti, S.; Collini, P.; Lozza, L.; Pennacchioli, E.; Mariani, L.; Casali, P.G. Aggressive Surgical Policies in a Retrospectively Reviewed Single-Institution Case Series of Retroperitoneal Soft Tissue Sarcoma Patients. J. Clin. Oncol. 2009, 27, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Walcott, B.P.; Nahed, B.V.; Mohyeldin, A.; Coumans, J.-V.; Kahle, K.T.; Ferreira, M.J. Chordoma: Current concepts, management, and future directions. Lancet Oncol. 2012, 13, e69–e76. [Google Scholar] [CrossRef]
- Bergh, P.; Kindblom, L.G.; Gunterberg, B.; Remotti, F.; Ryd, W.; Meis-Kindblom, J.M. Prognostic factors in chordoma of the sacrum and mobile spine: A study of 39 patients. Cancer 2000, 88, 2122–2134. [Google Scholar] [CrossRef]
- Hohenberger, P.; Allenberg, J.R.; Schlag, P.M.; Reichardt, P. Results of surgery and multimodal therapy for patients with soft tissue sarcoma invading to vascular structures. Cancer 1999, 85, 396–408. [Google Scholar] [CrossRef]
- Bertrand, M.M.; Carrère, S.; Delmond, L.; Mehta, S.; Rouanet, P.; Canaud, L.; Alric, P.; Quénet, F. Oncovascular compartmental resection for retroperitoneal soft tissue sarcoma with vascular involvement. J. Vasc. Surg. 2016, 64, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Radaelli, S.; Fiore, M.; Colombo, C.; Ford, S.; Palassini, E.; Sanfilippo, R.; Stacchiotti, S.; Sangalli, C.; Morosi, C.; Casali, P.G.; et al. Vascular resection en-bloc with tumor removal and graft reconstruction is safe and effective in soft tissue sarcoma (STS) of the extremities and retroperitoneum. Surg. Oncol. 2016, 25, 125–131. [Google Scholar] [CrossRef]
- Fueglistaler, P.; Gurke, L.; Stierli, P.; Obeid, T.; Koella, C.; Oertli, D.; Kettelhack, C. Major Vascular Resection and Prosthetic Replacement for Retroperitoneal Tumors. World J. Surg. 2006, 30, 1344–1349. [Google Scholar] [CrossRef] [PubMed]
- Schwarzbach, M.H.; Hormann, Y.; Hinz, U.; Leowardi, C.; Böckler, D.; Mechtersheimer, G.; Friess, H.; Büchler, M.W.; Allenberg, J.-R. Clinical results of surgery for retroperitoneal sarcoma with major blood vessel involvement. J. Vasc. Surg. 2006, 44, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Palombo, D.; Valenti, D.; Gaggiano, A.; Lupo, M.; Borin, P. Early Experience with the Minimal Extracorporeal Circulation System (MECC®) During Thoracoabdominal Aortic Aneurysm Repair. Eur. J. Vasc. Endovasc. Surg. 2004, 27, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Mohebali, J.; Carvalho, S.; Lancaster, R.T.; Ergul, E.A.; Conrad, M.F.; Clouse, W.D.; Cambria, R.P.; Patel, V.I. Use of extracorporeal bypass is associated with improved outcomes in open thoracic and thoracoabdominal aortic aneurysm repair. J. Vasc. Surg. 2018, 68, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Curtis, N.; Vohra, H.A.; Ohri, S.K. Mini extracorporeal circuit cardiopulmonary bypass system: A review. Perfusion 2010, 25, 115–124. [Google Scholar] [CrossRef]
- Balzano, G.; Maffi, P.; Nano, R.; Zerbi, A.; Venturini, M.; Melzi, R.; Mercalli, A.; Magistretti, P.; Scavini, M.; Castoldi, R.; et al. Extending Indications for Islet Autotransplantation in Pancreatic Surgery. Ann. Surg. 2013, 258, 210–218. [Google Scholar] [CrossRef]
- Casali, P.G.; Abecassis, N.; Aro, H.T.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; Brodowicz, T.; et al. Soft tissue and visceral sarcomas: ESMO–EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. S4), iv51–iv67. [Google Scholar] [CrossRef]
- Shibata, D.; Paty, P.B.; Guillem, J.G.; Wong, D.W.; Cohen, A.M. Surgical Management of Isolated Retroperitoneal Recurrences of Colorectal Carcinoma. Dis. Colon Rectum 2002, 45, 795–801. [Google Scholar] [CrossRef]
- Min, B.S.; Kim, N.K.; Sohn, S.K.; Cho, C.H.; Lee, K.Y.; Baik, S.H. Isolated paraaortic lymph-node recurrence after the curative resection of colorectal carcinoma. J. Surg. Oncol. 2007, 97, 136–140. [Google Scholar] [CrossRef]
- Awtrey, C.S.; Cadungog, M.G.; Leitao, M.M.; Alektiar, K.M.; Aghajanian, C.; Hummer, A.J.; Barakat, R.R.; Chi, D.S. Surgical resection of recurrent endometrial carcinoma. Gynecol. Oncol. 2006, 102, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Poultsides, G.A.; Tran, T.B.; Zambrano, E.; Janson, L.; Mohler, D.G.; Mell, M.W.; Avedian, R.S.; Visser, B.C.; Lee, J.T.; Ganjoo, K.; et al. Sarcoma Resection With and Without Vascular Reconstruction: A Matched Case-control Study. Ann. Surg. 2015, 262, 632–640. [Google Scholar] [CrossRef]
- Song, T.K.; Harris, E.J.; Raghavan, S.; Norton, J.A. Major Blood Vessel Reconstruction During Sarcoma Surgery. Arch. Surg. 2009, 144, 817–822. [Google Scholar] [CrossRef]
- Gösling, T.; Pichlmaier, M.A.; Länger, F.; Krettek, C.; Hüfner, T. Two-stage multilevel en bloc spondylectomy with resection and replacement of the aorta. Eur. Spine J. 2012, 22, 363–368. [Google Scholar] [CrossRef]
- Graulich, T.; Krettek, C.; Müller, C.W. Revision strategy and follow-up for implant failure in a case of combined anterior and posterior reconstruction after three-level en bloc vertebral body replacement and replacement of the aorta for chondrosarcoma of the thoracic spine. Eur. Spine J. 2018, 28, 13–17. [Google Scholar] [CrossRef]
- Homsy, P.; Blomqvist, C.; Heiskanen, I.; Vikatmaa, L.; Tukiainen, E.; Numminen, K.; Sampo, M.; Leppäniemi, A.; Albäck, A.; Kantonen, I.; et al. Multidisciplinary Oncovascular Surgery is Safe and Effective in the Treatment of Intra-abdominal and Retroperitoneal Sarcomas: A Retrospective Single Centre Cohort Study and a Comprehensive Literature Review. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 752–763. [Google Scholar] [CrossRef]
- Murakami, H.; Kawahara, N.; Tomita, K.; Demura, S.; Kato, S.; Yoshioka, K. Does Interruption of the Artery of Adamkiewicz During Total En Bloc Spondylectomy Affect Neurologic Function? Spine 2010, 35, E1187–E1192. [Google Scholar] [CrossRef]
- Abdelsattar, Z.M.; Mathis, K.L.; Colibaseanu, D.T.; Merchea, A.; Bower, T.C.; Larson, D.W.; Dozois, E.J. Surgery for Locally Advanced Recurrent Colorectal Cancer Involving the Aortoiliac Axis: Can We Achieve R0 Resection and Long-term Survival? Dis. Colon Rectum 2013, 56, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.G.; Stone, W.M.; Bower, T.C.; Fowl, R.J.; Money, S.R. Surgical Management of Tumors Invading the Aorta and Major Arterial Structures. Ann. Vasc. Surg. 2011, 25, 1026–1035. [Google Scholar] [CrossRef]
- LeMaire, S.A.; Price, M.D.; Green, S.Y.; Zarda, S.; Coselli, J.S. Results of open thoracoabdominal aortic aneurysm repair. Ann. Cardiothorac. Surg. 2012, 1, 286–292. [Google Scholar] [CrossRef]
- Gloviczki, P. Surgical repair of thoracoabdominal aneurysms: Patient selection, techniques and results. Cardiovasc. Surg. 2002, 10, 434–441. [Google Scholar] [CrossRef]
- Boriani, S.; Bandiera, S.; Donthineni, R.; Amendola, L.; Cappuccio, M.; De Iure, F.; Gasbarrini, A. Morbidity of en bloc resections in the spine. Eur. Spine J. 2009, 19, 231–241. [Google Scholar] [CrossRef]
- Sciubba, D.M.; De la Garza Ramos, R.; Goodwin, C.R.; Xu, R.; Bydon, A.; Witham, T.F.; Gokaslan, Z.L.; Wolinsky, J.P. Total en bloc spondylectomy for locally aggressive and primary malignant tumors of the lumbar spine. Eur. Spine J. 2016, 25, 4080–4087. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, J.-C.; Ball, A.B.S.; Fisher, C.; Fryatt, I.; Jones, L.; Thomas, J.M. Limitations of surgery in the treatment of retroperitoneal sarcoma. Br. J. Surg. 1991, 78, 912–916. [Google Scholar] [CrossRef] [PubMed]
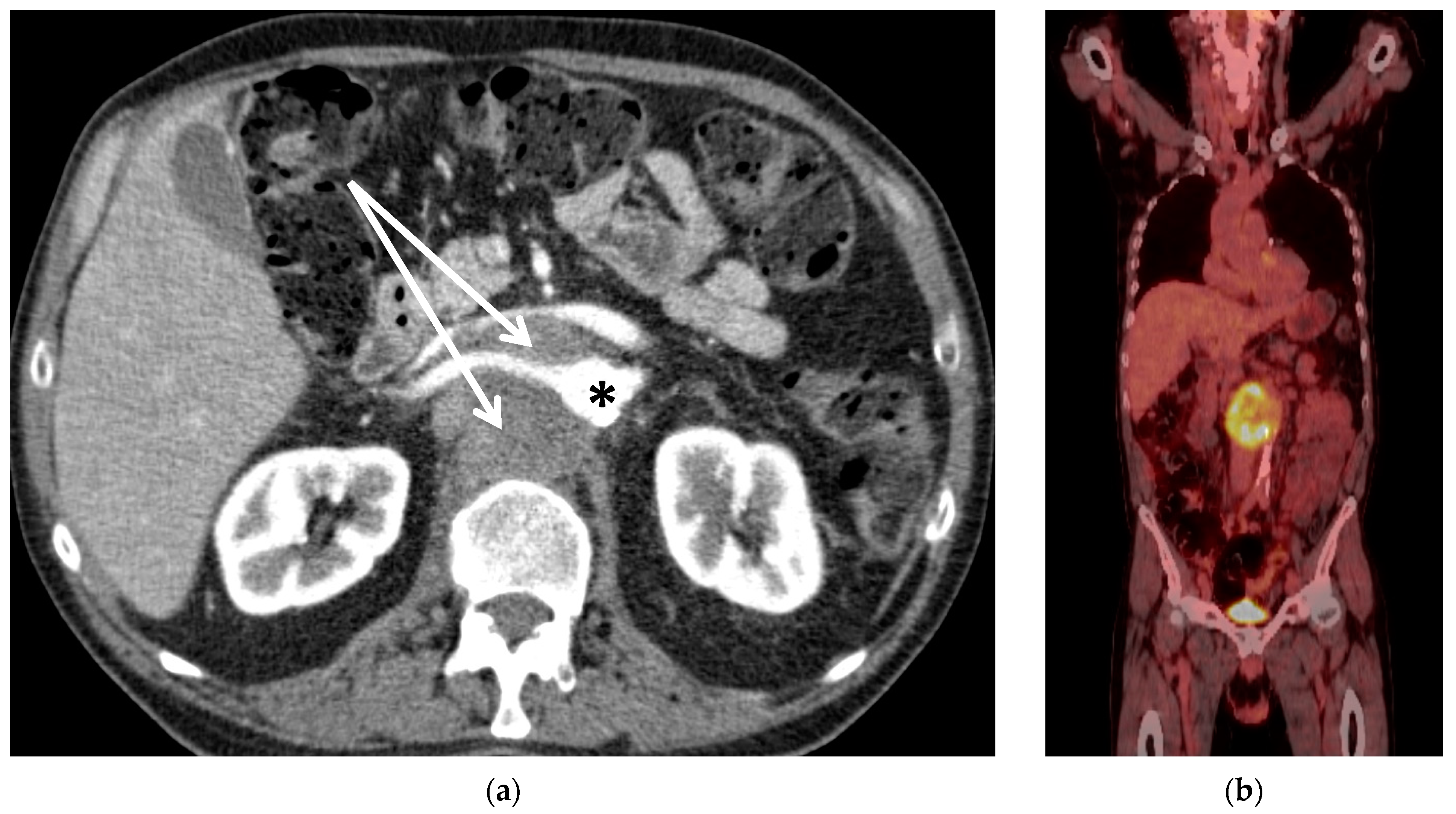
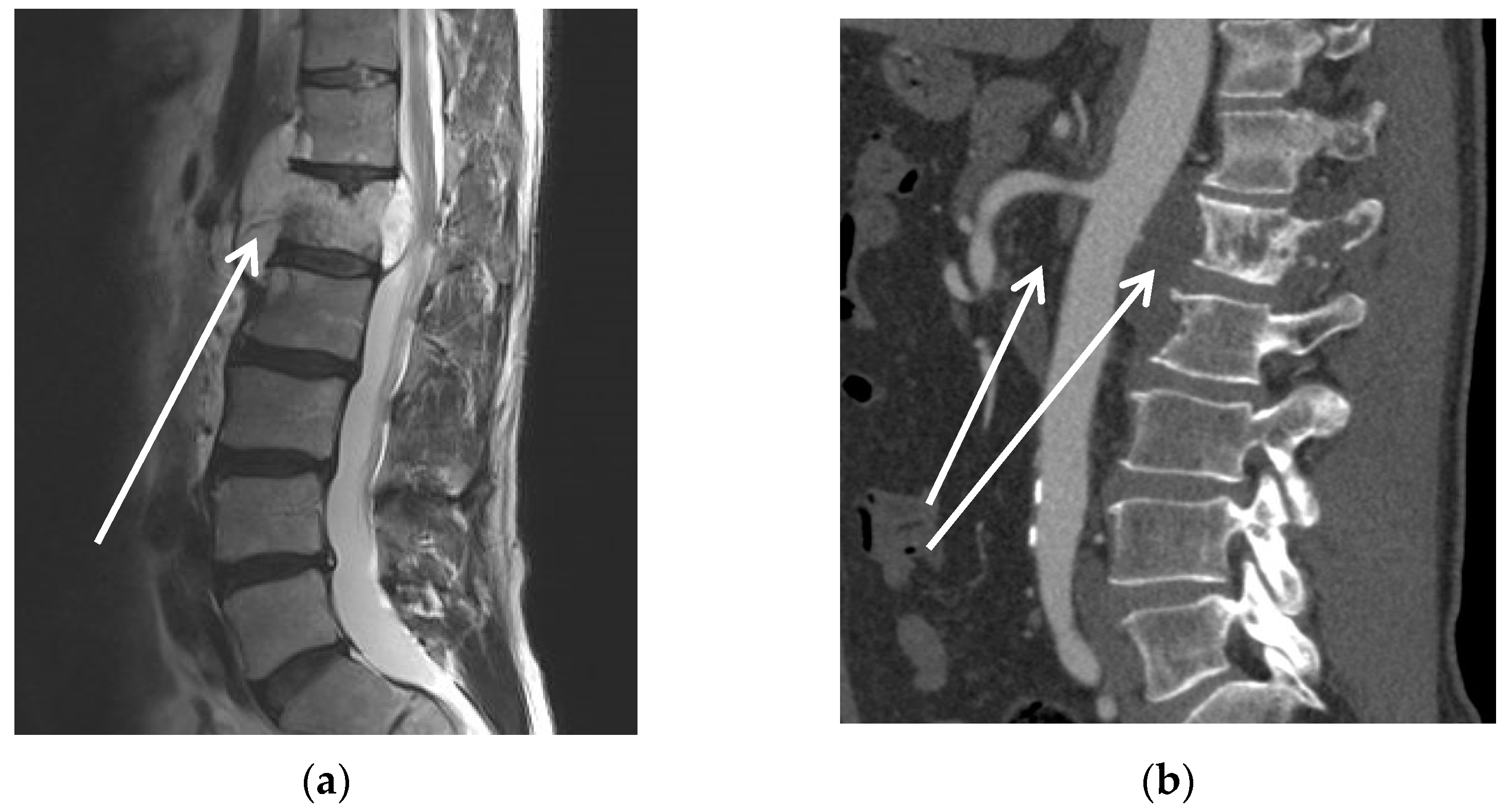
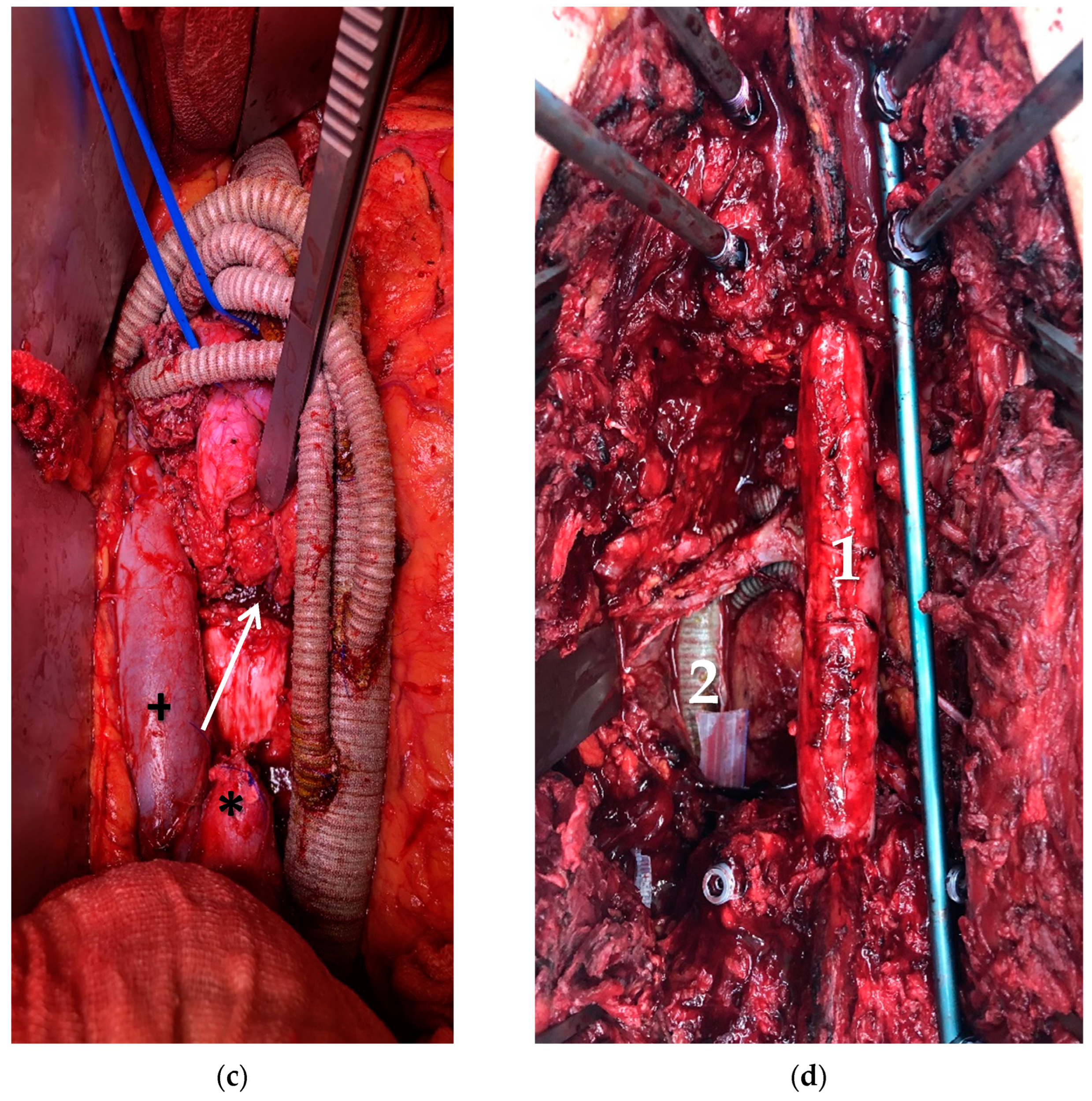
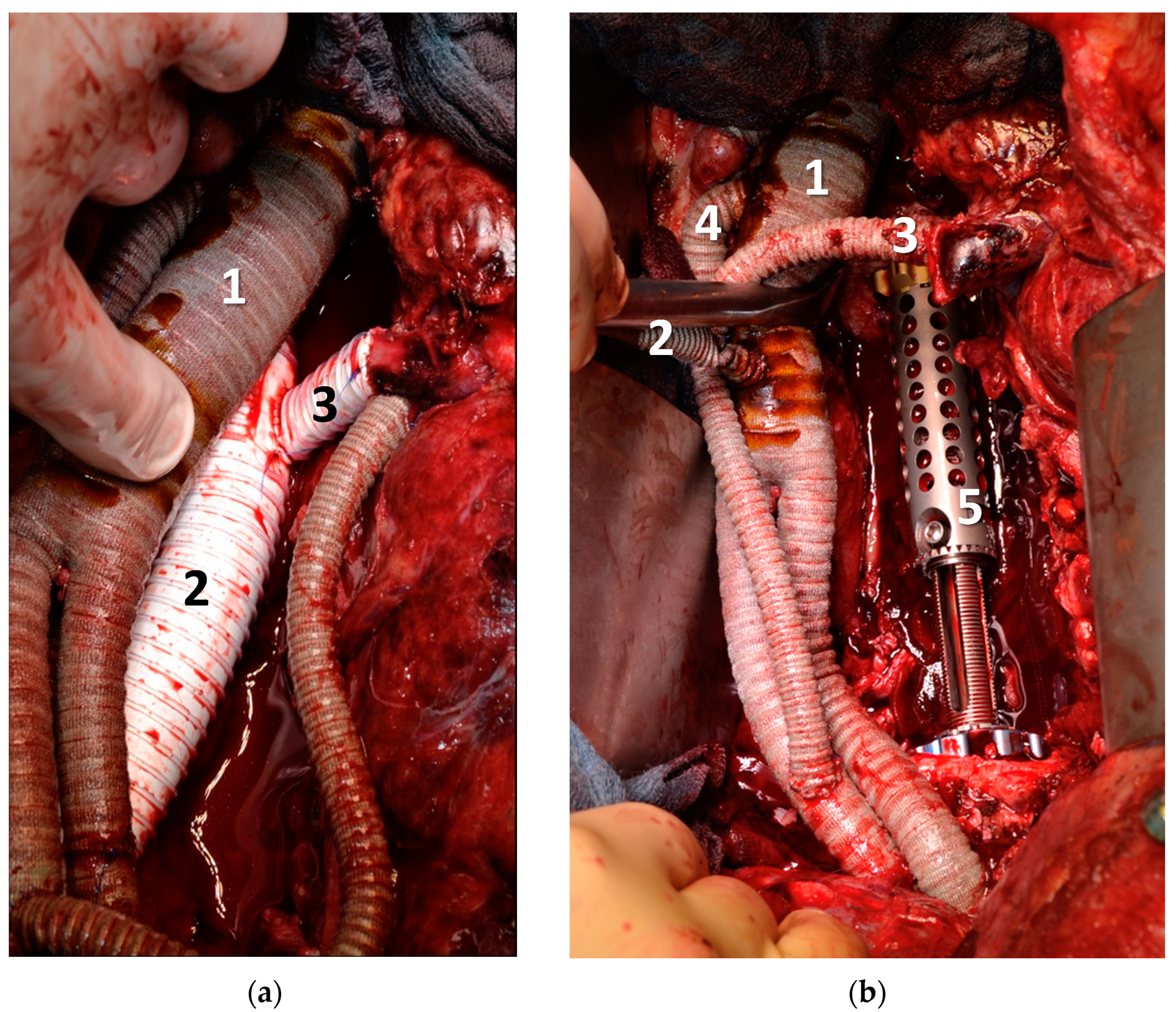
| Pat. No. | Sex | Age | Diagnosis | Presentation | Neo-Adjuvant Radiotherapy | Neo-Adj. Chemo-Therapy | ECOG | ASA |
|---|---|---|---|---|---|---|---|---|
| 1 | m | 56 | CUP with vertebral destruction L1-3 | Intolerable pain in the spine | Yes (50 Gy) | Yes | 1 | II |
| 2 | m | 28 | Retro-peritoneal pleomorphic carcinoma | Compression of abdominal organs; bowel obstruction and hydronephrosis | Yes (50 Gy) | Yes | 0 | II |
| 3 | m | 56 | Chordoma L1 | Spinal compression with impending paraplegia | Yes, proton therapy (50 Gy) | No | 1 | I |
| 4 | m | 41 | Retro-peritoneal rhabdoid sarcoma | hydronephrosis; patient’s wish for curative resection | Yes, proton therapy (50 Gy) | Yes | 0 | II |
| 5 | f | 40 | Retro-peritoneal metastasis of endometrial carcinoma | Incipient nerve infiltration at the left femoral nerve | No further radiotherapy possible | No | 1 | II |
| Patient # | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Total | |
| Vessels replaced: | ||||||
| thoraco-abdominal aorta | 1 | 1 | 1 | 1 | 1 | 5 |
| coeliac trunk | 1 | 1 | 1 | 1 | 4 | |
| common hepatic artery | 1 | 1 | ||||
| superior mesenteric artery | 1 | 1 | 1 | 1 | 1 | 5 |
| renal artery | 2 | 1 | 2 | 1 | 1 | 7 |
| inferior vena cava | 1 | 1 | 1 | 1 | 4 | |
| Resection: | ||||||
| kidney (unilateral) | 1 | 1 | 1 | 3 | ||
| 3-level spondyl-ectomy | 1 | 1 | 2 | |||
| ventral parts of the vertebral body | 1 | 1 | 2 | |||
| inferior vena cava | 1 | 1 | 1 | 1 | 4 | |
| pancreas | 1 | 1 | 2 | |||
| stomach | 1 | 1 | 2 | |||
| left hemicolon | 1 | 1 | 2 | |||
| diaphragm (partial) | 1 | 1 | 1 | 3 | ||
| femoral nerve (partial) | 1 | 1 | ||||
| aorta | 1 | 1 | 1 | 1 | 1 | 5 |
| Postoperative Course: | Patient # | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Total | |
| liquor leak | 1 | 1 | 2 | |||
| cage dislocation | 1 | 1 | ||||
| fluid collection | 1 | 1 | ||||
| biliary leak | 1 | 1 | ||||
| hematoma | 1 | 1 | 1 | 3 | ||
| bypass occlusion | 1 | 1 | ||||
| anastomotic bleeding | 1 | 1 | ||||
| vessel kinking | 1 | 1 | ||||
| open abdomen | 1 | 1 | ||||
| # | Bypass Patency | Death (Reason) | Follow Up | Recurrent Disease |
|---|---|---|---|---|
| (in Months) | ||||
| 1 | 100% primary | No | 65 | no |
| 2 | occlusion of hepatic bypass, secondary patency 100% | Yes (septicemia) | 0 | n/a |
| 3 | 100% primary | No | 44 | no |
| 4 | 100% primary | Yes (septicemia) | 0 | n/a |
| 5 | 100% primary | No | 21 | yes |
| Author | Year | Treated Malignancies | Number of All Patients | Number of Vessel Replacements: | In Hospital Mortality | Reported Patency | Resection with Negative Margin | Survival | Freedom of Recurrent Disease | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thoracoabdominal | Renal Artery | Visceral Artery | Reimplantation | |||||||||
| Schwarz- bach et al. [11] | 2006 | retroperitoneal soft-tissue sarcoma | 25 | 2 | 0 | 0 | 1 (TRC) | 4% of the whole series | primary: 88.9% of the whole series | 40% in the whole series | 2 years: 50%, 5 years: 38% | not specified |
| Song et al. [21] | 2009 | retroperitoneal or extremity sarcoma | 14 | 1 | 0 | 0 | 3 (LRA, SMA, hepatic artery) | 0 | primary 58% and assisted 83% of the whole series | 43% of Retroperitoneal Sarcomas | 5-year (overall): 68%, 5-year (disease-free): 52% | 79% |
| Goesling et al. [22] and Graulich 2019 et al. [23] (same case) | 2013/2019 | chondrosarcoma | 1 | 1 | 0 | 0 | 0 | 0 | 100% | 100% | 100% in 12 years | 100% |
| Poultsides et al. [20] | 2015 | Sarcoma, not further specified | 50 | 1 | 1 | 2 (SMA and hepatic artery) | 0 | 2% of the whole series | primary 86% and assisted 92% of the whole series | 76% | 5 year (overall): 59% | 49% |
| Homsy et. al. [24] | 2021 | miscellaneous, different locations | 17 | 1 | 1 | 2 | 0 | 0 | patency was not reported for all patients | 56% | 61% in 3 years | 46% in 3 years |
| current series, Lutz et al. | 2023 | miscellaneous, all located in retroperitoneal space | 5 | 5 | 7 (2 LRA, 5 RRA) | 9 (3 TRC, 5 SMA, 1 hepatic artery) | 1 (TRC) | 2 out of 5 | primary 96%, assisted 100% | 100% | 60% overall and disease-free, (mean follow-up 47 months) | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lutz, B.M.; Schaser, K.-D.; Weitz, J.; Kirchberg, J.; Fritzsche, H.; Disch, A.C.; Busch, A.; Wolk, S.; Reeps, C. Thoracoabdominal Aortic Replacement Together with Curative Oncological Surgery in Retroperitoneal and Spinal Tumours. Curr. Oncol. 2023, 30, 2555-2568. https://doi.org/10.3390/curroncol30030195
Lutz BM, Schaser K-D, Weitz J, Kirchberg J, Fritzsche H, Disch AC, Busch A, Wolk S, Reeps C. Thoracoabdominal Aortic Replacement Together with Curative Oncological Surgery in Retroperitoneal and Spinal Tumours. Current Oncology. 2023; 30(3):2555-2568. https://doi.org/10.3390/curroncol30030195
Chicago/Turabian StyleLutz, Brigitta M., Klaus-Dieter Schaser, Jurgen Weitz, Johanna Kirchberg, Hagen Fritzsche, Alexander C. Disch, Albert Busch, Steffen Wolk, and Christian Reeps. 2023. "Thoracoabdominal Aortic Replacement Together with Curative Oncological Surgery in Retroperitoneal and Spinal Tumours" Current Oncology 30, no. 3: 2555-2568. https://doi.org/10.3390/curroncol30030195
APA StyleLutz, B. M., Schaser, K.-D., Weitz, J., Kirchberg, J., Fritzsche, H., Disch, A. C., Busch, A., Wolk, S., & Reeps, C. (2023). Thoracoabdominal Aortic Replacement Together with Curative Oncological Surgery in Retroperitoneal and Spinal Tumours. Current Oncology, 30(3), 2555-2568. https://doi.org/10.3390/curroncol30030195







