Association between Dynamic Contrast-Enhanced MRI Parameters and Prognostic Factors in Patients with Primary Rectal Cancer
Abstract
1. Introduction
2. Materials and Methods
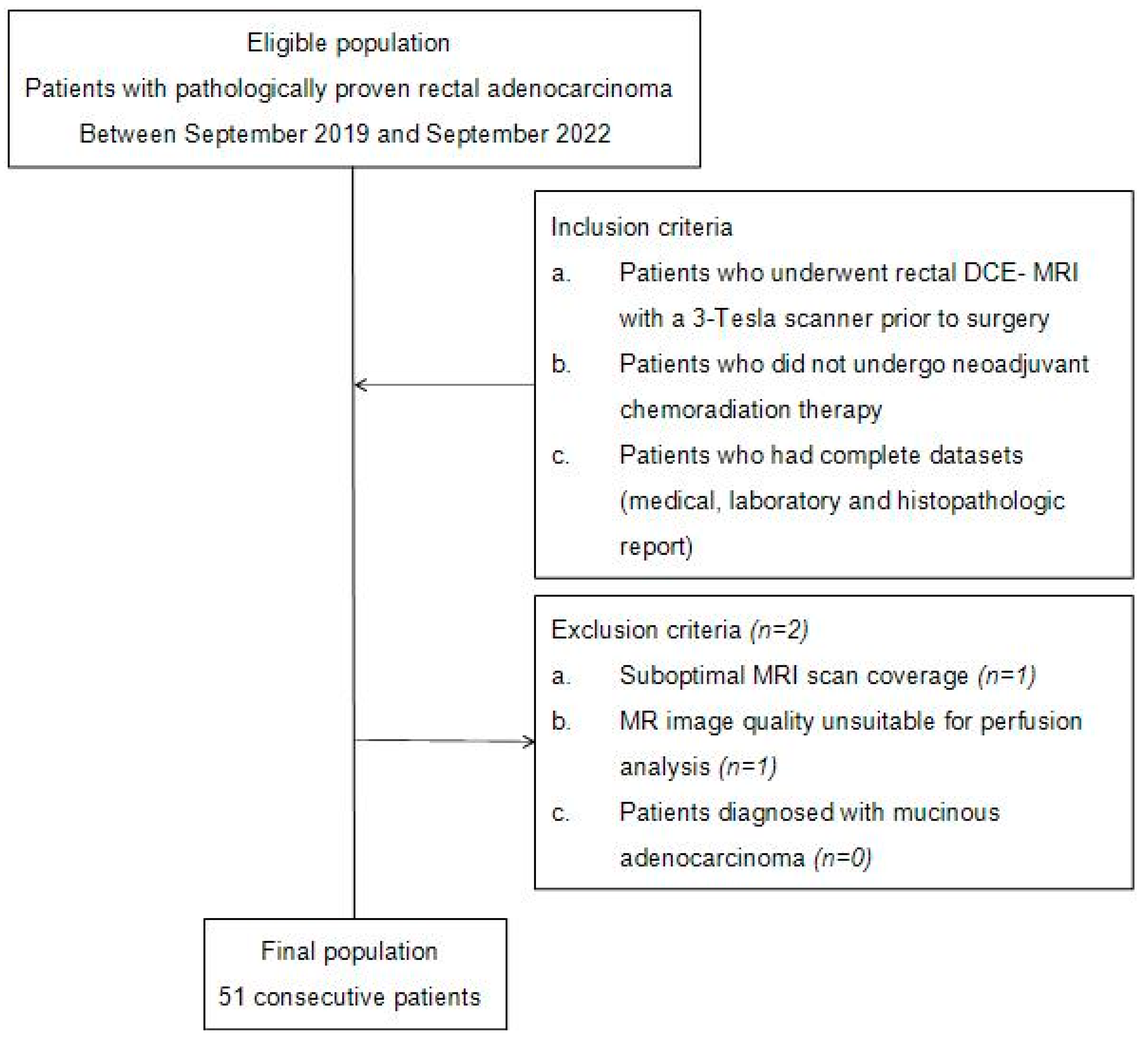
2.1. Patients and Patient Selection Criteria
2.2. MRI Technique
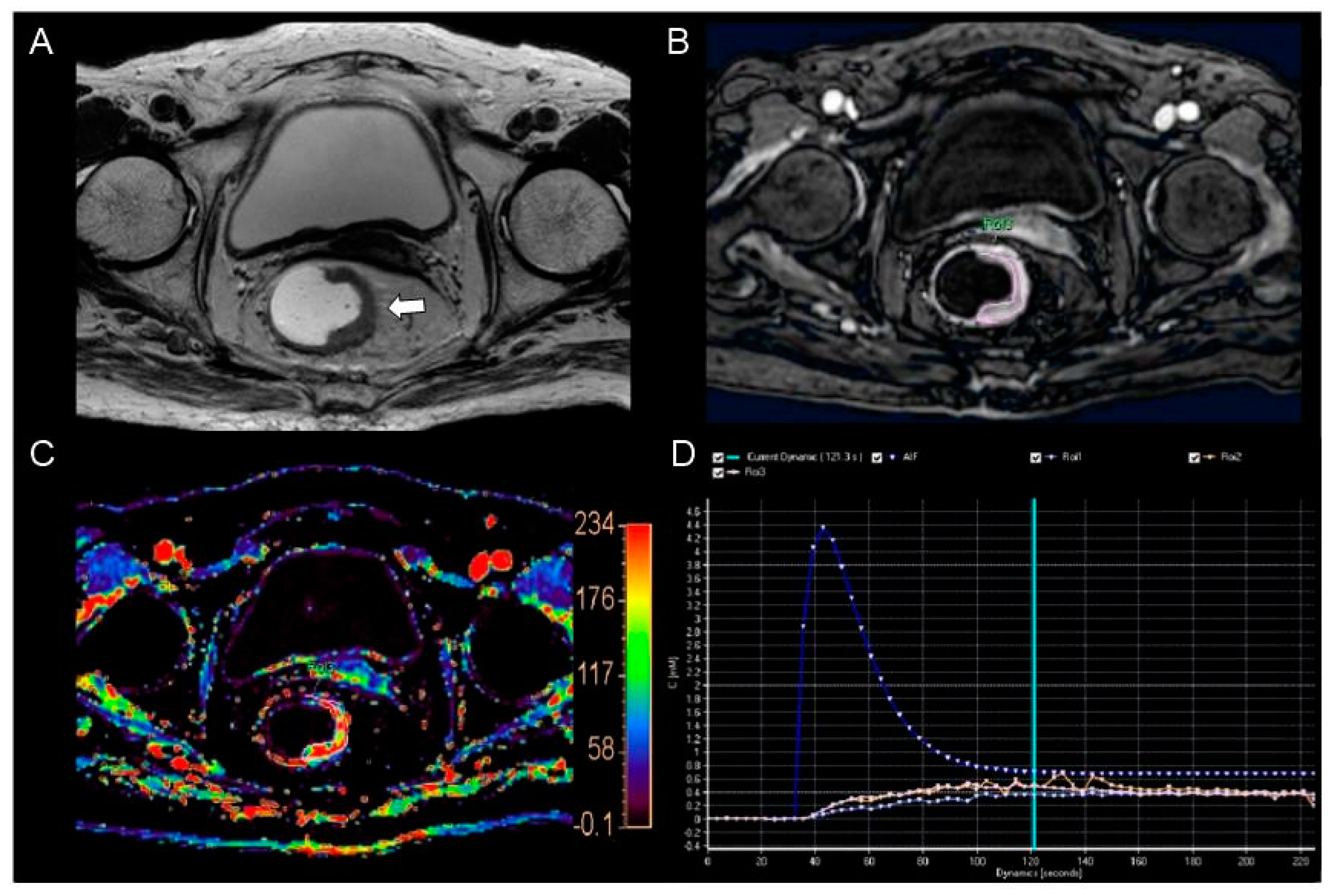
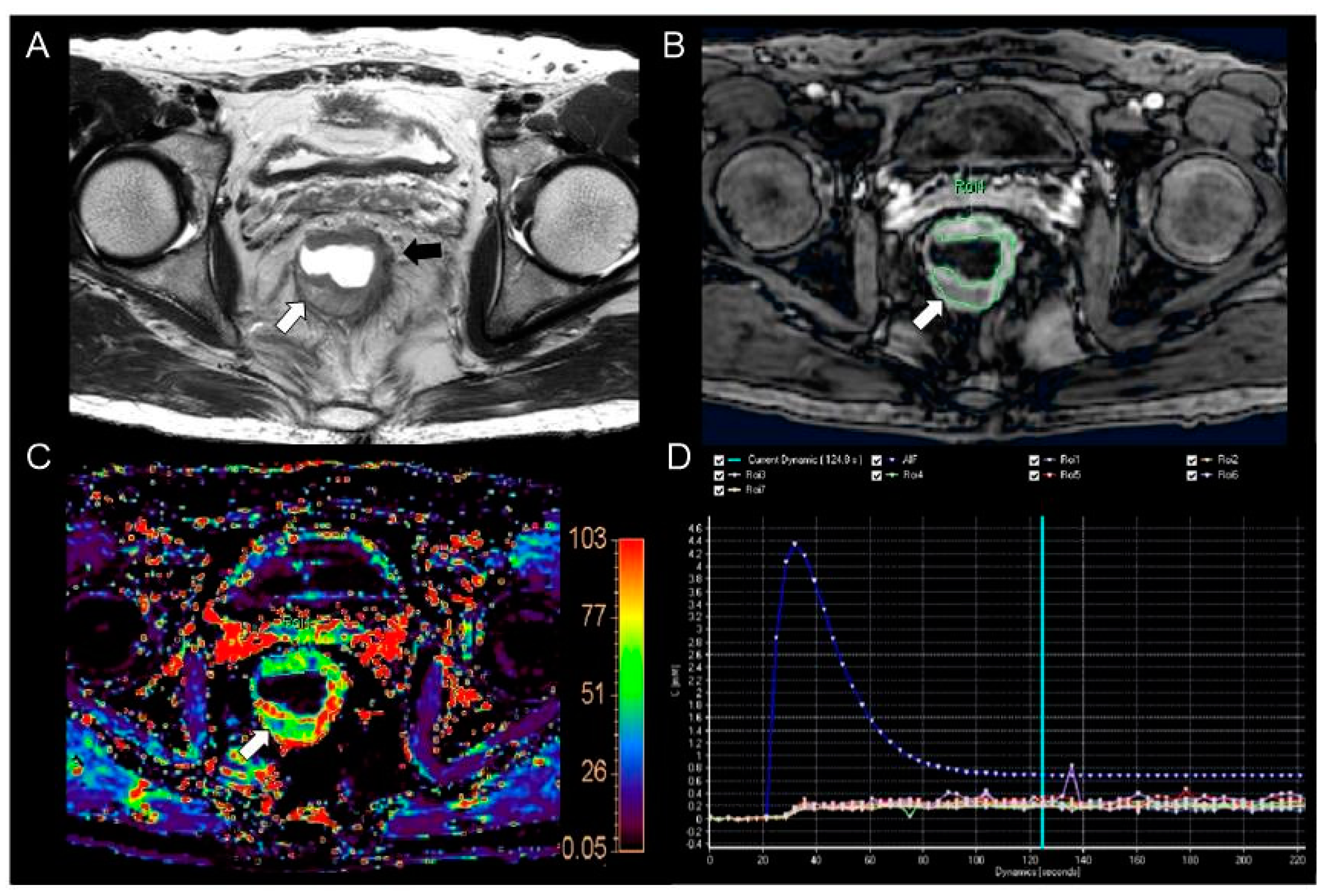
2.3. Perfusion Parameter Measurement
2.4. Reference Standard for Prognostic Factors
2.5. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. Comparison of Perfusion Parameters According to Prognostic Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 681, 7–30. [Google Scholar] [CrossRef]
- Shen, F.U.; Lu, J.; Chen, L.; Wang, Z.; Chen, Y. Diagnostic value of dynamic contrast-enhanced magnetic resonance imaging in rectal cancer and its correlation with tumor differentiation. Mol. Clin. Oncol. 2016, 4, 500–506. [Google Scholar] [CrossRef]
- Rocca, A.; Cipriani, F.; Belli, G.; Berti, S.; Boggi, U.; Bottino, V.; Cillo, U.; Cescon, M.; Cimino, M.; Corcione, F.; et al. The Italian Consensus on minimally invasive simultaneous resections for synchronous liver metastasis and primary colorectal cancer: A Delphi methodology. Updates Surg. 2021, 73, 1247–1265. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Nordlinger, B.; Adam, R.; Köhne, C.H.; Pozzo, C.; Poston, G.; Ychou, M.; Rougier, P. European Colorectal Metastases Treatment Group. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur. J. Cancer 2006, 42, 2212–2221. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Gao, J.; Mao, Z.; Wang, J.; Li, J.; Li, W.; Lei, Y.; Li, S.; Wu, Z.; Tang, C.; et al. Genetic mutations in human rectal cancers detected by targeted sequencing. J. Hum. Genet. 2015, 60, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Tong, G.J.; Zhang, G.Y.; Liu, J.; Zheng, Z.Z.; Chen, Y.; Niu, P.P.; Xu, X.T. Comparison of the eighth version of the American Joint Committee on Cancer manual to the seventh version for colorectal cancer: A retrospective review of our data. World J. Clin. Oncol. 2018, 9, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Weiser, M.R. AJCC 8th Edition: Colorectal Cancer. Ann. Surg. Oncol. 2018, 25, 1454–1455. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, C.; Zhang, Z.; Qin, Q.; Sun, X. Value of 3Tesla MRI in the preoperative staging of mid-low rectal cancer and its impact on clinical strategies. Asia Pac. J. Clin. Oncol. 2020, 16, e216–e222. [Google Scholar] [CrossRef]
- Inoue, A.; Sheedy, S.P.; Heiken, J.P.; Mohammadinejad, P.; Graham, R.P.; Lee, H.E.; Kelley, S.R.; Hansel, S.L.; Bruining, D.H.; Fidler, J.L.; et al. MRI-detected extramural venous invasion of rectal cancer: Multimodality performance and implications at baseline imaging and after neoadjuvant therapy. Insights Imaging 2021, 12, 110. [Google Scholar] [CrossRef]
- Jo, S.J.; Kim, S.H.; Park, S.J.; Lee, Y.; Son, J.H. Association between Texture Analysis Parameters and Molecular Biologic KRAS Mutation in Non-Mucinous Rectal Cancer. Taehan Yongsang Uihakhoe Chi 2021, 82, 406–416. [Google Scholar] [CrossRef]
- Seo, N.; Kim, H.; Cho, M.S.; Lim, J.S. Response Assessment with MRI after Chemoradiotherapy in Rectal Cancer: Current Evidences. Korean J. Radiol. 2019, 20, 1003–1018. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, F.; Soliman, A.; El-Baz, A.; Abou El-Ghar, M.; El-Diasty, T.; Gimel'farb, G.; Ouseph, R.; Dwyer, A.C. Models and methods for analyzing DCE-MRI: A review. Med. Phys. 2014, 41, 124301. [Google Scholar] [CrossRef] [PubMed]
- Curvo-Semedo, L.; Lambregts, D.M.; Maas, M.; Thywissen, T.; Mehsen, R.T.; Lammering, G.; Beets, G.L.; Caseiro-Alves, F.; Beets-Tan, R.G. Rectal Cancer: Assessment of Complete Response to Preoperative Combined Radiation Therapy with Chemotherapy—Conventional MR Volumetry versus Diffusion-weighted MR Imaging. Radiology 2011, 260, 734–743. [Google Scholar] [CrossRef]
- Nougaret, S.; Rouanet, P.; Molinari, N.; Pierredon, M.A.; Bibeau, F.; Azria, D.; Lemanski, C.; Assenat, E.; Duffour, J.; Ychou, M.; et al. MR Volumetric Measurement of Low Rectal Cancer Helps Predict Tumor Response and Outcome after Combined Chemotherapy and Radiation Therapy. Radiology 2012, 263, 409–418. [Google Scholar] [CrossRef]
- Aggarwal, S. Targeted cancer therapies. Nat. Rev. Drug Discov. 2010, 9, 427–428. [Google Scholar] [CrossRef]
- Kim, Y.E.; Lim, J.S.; Choi, J.; Kim, D.; Myoung, S.; Kim, M.J.; Kim, K.W. Perfusion parameters of dynamic contrast-enhanced magnetic resonance imaging in patients with rectal cancer: Correlation with microvascular density and vascular endothelial growth factor expression. Korean J. Radiol. 2013, 14, 878–885. [Google Scholar] [CrossRef]
- Yeo, D.M.; Oh, S.N.; Jung, C.K.; Lee, M.A.; Oh, S.T.; Rha, S.E.; Jung, S.E.; Byun, J.Y.; Gall, P.; Son, Y. Correlation of dynamic contrast-enhanced MRI perfusion parameters with angiogenesis and biologic aggressiveness of rectal cancer: Preliminary results. J. Magn. Reason. Imaging 2015, 41, 474–480. [Google Scholar] [CrossRef]
- Ye, Z.M.; Dai, S.J.; Yan, F.Q.; Wang, L.; Fang, J.; Fu, Z.F.; Wang, Y.Z. DCE-MRI-Derived Volume Transfer Constant (Ktrans) and DWI Apparent Diffusion Coefficient as Predictive Markers of Short- and Long-Term Efficacy of Chemoradiotherapy in Patients With Esophageal Cancer. Technol. Cancer Res. Treat. 2018, 17, 1533034618765254. [Google Scholar] [CrossRef]
- Skinner, J.T.; Moots, P.L.; Ayers, G.D.; Quarles, C.C. On the Use of DSC-MRI for Measuring Vascular Permeability. AJNR Am. J. Neuroradiol. 2016, 37, 80–87. [Google Scholar] [CrossRef]
- DeVries, A.F.; Piringer, G.; Kremser, C.; Judmaier, W.; Saely, C.H.; Lukas, P.; Öfner, D. Pretreatment evaluation of microcirculation by dynamic contrast-enhanced magnetic resonance imaging predicts survival in primary rectal cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Sun, Y.; Gollub, M.J.; Peng, W.; Cai, S.; Zhang, Z.; Gu, Y. Dynamic contrast-enhanced MRI: Use in predicting pathological complete response to neoadjuvant chemoradiation in locally advanced rectal cancer. J. Magn. Reason. Imaging 2015, 42, 673–680. [Google Scholar] [CrossRef]
- Intven, M.; Reerink, O.; Philippens, M.E. Dynamic contrast enhanced MR imaging for rectal cancer response assessment after neo-adjuvant chemoradiation. J. Magn. Reason. Imaging 2015, 41, 1646–1653. [Google Scholar] [CrossRef]
- Intven, M.; Monninkhof, E.M.; Reerink, O.; Philippens, M.E. Combined T2w volumetry, DW-MRI and DCE-MRI for response assessment after neo-adjuvant chemoradiation in locally advanced rectal cancer. Acta Oncol. 2015, 54, 1729–1736. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.S.; Kim, D.; Baek, S.E.; Myoung, S.; Choi, J.; Shin, S.J.; Kim, M.J.; Kim, N.K.; Suh, J.; Kim, K.W.; et al. Perfusion MRI for the prediction of treatment response after preoperative chemoradiotherapy in locally advanced rectal cancer. Eur. Radiol. 2012, 22, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Song, W.; Guo, D.; Liu, H.; Zhang, H.; He, X.; Song, J.; Zhou, J.; Liu, X. Preoperative Prediction of Extramural Venous Invasion in Rectal Cancer: Comparison of the Diagnostic Efficacy of Radiomics Models and Quantitative Dynamic Contrast-Enhanced Magnetic Resonance Imaging. Front. Oncol. 2020, 10, 459. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, X.; Xia, K.; Jiang, H.; Jiang, J.; Sun, J.; Lu, Z. Comparison of Diagnostic Performance between Perfusion-Related Intravoxel Incoherent Motion DWI and Dynamic Contrast-Enhanced MRI in Rectal Cancer. Comput. Math. Methods Med. 2021, 2021, 5095940. [Google Scholar] [CrossRef]
- Yao, W.W.; Zhang, H.; Ding, B.; Fu, T.; Jia, H.; Pang, L.; Song, L.; Xu, W.; Song, Q.; Chen, K.; et al. Rectal cancer: 3D dynamic contrast-enhanced MRI; correlation with microvascular density and clinicopathological features. Radiol. Med. 2011, 116, 366–374. [Google Scholar] [CrossRef]
- Park, H.; Kim, S.H.; Kim, J.Y. Dynamic contrast-enhanced magnetic resonance imaging for risk stratification in patients with prostate cancer. Quant. Imaging Med. Surg. 2022, 12, 742–751. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, J.M.; Gupta, S.N.; Han, J.K.; Choi, B.I. Dynamic contrast-enhanced MRI to evaluate the therapeutic response to neoadjuvant chemoradiation therapy in locally advanced rectal cancer. J. Magn. Reason. Imaging 2014, 40, 730–737. [Google Scholar] [CrossRef]
- Tofts, P.S. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J. Magn. Reason. Imaging 1997, 7, 91–101. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Kim, S.H.; Son, J.H.; Jo, S.J. Added value of diffusion-weighted imaging for evaluation of extramural venous invasion in patients with primary rectal cancer. Br. J. Radiol. 2019, 92, 20180821. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.E.; Kim, M.J.; Lee, J.; Hur, B.Y.; Kim, B.; Kim, D.Y.; Baek, J.Y.; Chang, H.J.; Park, S.C.; Oh, J.H.; et al. Magnetic Resonance-Based Texture Analysis Differentiating KRAS Mutation Status in Rectal Cancer. Cancer Res. Treat. 2020, 52, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Pollheimer, M.J.; Kornprat, P.; Lindtner, R.A.; Harbaum, L.; Schlemmer, A.; Rehak, P.; Langner, C. Tumor necrosis is a new promising prognostic factor in colorectal cancer. Hum. Pathol. 2010, 41, 1749–1757. [Google Scholar] [CrossRef]
- Langner, C.; Harbaum, L.; Pollheimer, M.J.; Kornprat, P.; Lindtner, R.A.; Schlemmer, A.; Vieth, M.; Rehak, P. Mucinous differentiation in colorectal cancer--indicator of poor prognosis? Histopathology 2012, 60, 1060–1072. [Google Scholar] [CrossRef]
- Newton, K.F.; Newman, W.; Hill, J. Review of biomarkers in colorectal cancer. Colorectal. Dis. 2012, 14, 3–17. [Google Scholar] [CrossRef]
- Ao, W.; Zhang, X.; Yao, X.; Zhu, X.; Deng, S.; Feng, J. Preoperative prediction of extramural venous invasion in rectal cancer by dynamic contrast-enhanced and diffusion weighted MRI: A preliminary study. BMC Med. Imaging 2022, 22, 78. [Google Scholar] [CrossRef]
- Beets-Tan, R.G.H.; Lambregts, D.M.J.; Maas, M.; Bipat, S.; Barbaro, B.; Curvo-Semedo, L.; Fenlon, H.M.; Gollub, M.J.; Gourtsoyianni, S.; Halligan, S.; et al. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur. Radiol. 2018, 28, 1465–1475. [Google Scholar] [CrossRef]
- Mirbagheri, N.; Kumar, B.; Deb, S.; Poh, B.R.; Dark, J.G.; Leow, C.C.; Teoh, W.M. Lymph node status as a prognostic indicator after preoperative neoadjuvant chemoradiotherapy of rectal cancer. Colorectal. Dis. 2014, 16, O339–O346. [Google Scholar] [CrossRef]
- Dinapoli, N.; Barbaro, B.; Gatta, R.; Chiloiro, G.; Casà, C.; Masciocchi, C.; Damiani, A.; Boldrini, L.; Gambacorta, M.A.; Dezio, M.; et al. Magnetic Resonance, Vendor-independent, Intensity Histogram Analysis Predicting Pathologic Complete Response After Radiochemotherapy of Rectal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2018, 15, 765–774. [Google Scholar] [CrossRef]
- Zhou, X.; Yi, Y.; Liu, Z.; Cao, W.; Lai, B.; Sun, K.; Li, L.; Zhou, Z.; Feng, Y.; Tian, J. Radiomics-Based Pretherapeutic Prediction of Non-response to Neoadjuvant Therapy in Locally Advanced Rectal Cancer. Ann. Surg. Oncol. 2019, 26, 1676–1684. [Google Scholar] [CrossRef]
- Lambregts, D.M.; Beets, G.L.; Maas, M.; Curvo-Semedo, L.; Kessels, A.G.; Thywissen, T.; Beets-Tan, R.G. Tumour ADC measurements in rectal cancer: Effect of ROI methods on ADC values and interobserver variability. Eur. Radiol. 2011, 21, 2567–2574. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.S.; Waterton, J.C.; Kundra, V.; Brammer, D.; Ravoori, M.; Han, L.; Wei, W.; Klumpp, S.; Johnson, V.E.; Jackson, E.F. Reproducibility and comparison of DCE-MRI and DCE-CT perfusion parameters in a rat tumor model. Technol. Cancer Res. Treat. 2012, 11, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Lankester, K.J.; Taylor, N.J.; Stirling, J.J.; Boxall, J.; d’Arcy, J.A.; Collins, D.J.; Walker-Samuel, S.; Leach, M.O.; Rustin, G.J.; Padhani, A.R. Dynamic MRI for imaging tumor microvasculature: Comparison of susceptibility and relaxivity techniques in pelvic tumors. J. Mag. Reason. Imaging 2007, 25, 796–805. [Google Scholar] [CrossRef] [PubMed]
| Parameters | T2-Weighted Axial, Sagittal, and Coronal | Pre-Contrast Axial 3D-Spoiled Gradient Echo | Post-Contrast Axial 3D-Spoiled Gradient Echo |
|---|---|---|---|
| TR (msec) | 2500–3782 | 10.0 | 3.5 |
| TE (msec) | 90 | 1.6 | 1.2 |
| Slice thickness (mm) | 3, 5, 4 | 4 | 4 |
| Slice gap (mm) | 0.3, 1, 0.4 | 0 | 0 |
| Matrix size | 316 × 281, 356 × 331, 332 × 317 | 216 × 166 | 216 × 166 |
| Flip angle (degree) | - | 5, 15 | 8 |
| FOV (mm × mm) | 220 × 220 | 300 × 300 | 300 × 300 |
| Acquisition time | 2 min 15–25 s | 10 s | 4 min 12 s |
| Number of slices | 26–30 | 18 | 18 |
| Characteristic | Study Population (n = 51) |
|---|---|
| Tumor location | |
| Upper rectum | 15 (29%) |
| Mid rectum | 20 (39%) |
| Lower rectum | 16 (31%) |
| Histological grade | |
| Well-differentiated | 6 (12%) |
| Moderately differentiated | 45 (88%) |
| Histologic tumor stage | |
| pT1 | 8 (16%) |
| pT2 | 13 (25%) |
| pT3 | 25 (49%) |
| pT4 | 5 (10%) |
| Pathologic lymph node stage | |
| N0 | 33 (65%) |
| N1 | 12 (23%) |
| N2 | 6 (12%) |
| EMVI | |
| Positive | 8 (16%) |
| Negative | 43 (84%) |
| CRM status | |
| Positive | 8 (16%) |
| Negative | 43 (84%) |
| KRAS mutation | |
| Positive | 22 (43%) |
| Negative | 29 (56%) |
| Perfusion Parameters Prognostic Factors | Ktrans (min−1, Mean ± SD) | p Value | kep (min−1, Mean ± SD) | p Value | ve (Mean ± SD) | p Value | vp (Mean ± SD) | p Value |
|---|---|---|---|---|---|---|---|---|
| T stage | 0.7525 | 0.4767 | 0.3517 | 0.8902 | ||||
| pT1, pT2 (n = 21) | 0.093 ± 0.046 | 0.457 ± 0.201 | 0.224 ± 0.148 | 0.018 ± 0.016 | ||||
| pT3, pT4 (n = 30) | 0.101 ± 0.067 | 0.421 ± 0.160 | 0.264 ± 0.152 | 0.018 ± 0.015 | ||||
| N stage | 0.5373 | 0.4179 | 0.3010 | 0.4043 | ||||
| N0 (n = 33) | 0.102 ± 0.069 | 0.450 ± 0.194 | 0.231 ± 0.147 | 0.019 ± 0.018 | ||||
| N1-2 (n = 18) | 0.092 ± 0.038 | 0.408 ± 0.141 | 0.278 ± 0.155 | 0.016 ± 0.009 | ||||
| EMVI | 0.7254 | 0.5961 | 0.4213 | 0.5178 | ||||
| Positive (n = 8) | 0.094 ± 0.028 | 0.405 ± 0.146 | 0.291 ± 0.160 | 0.016 ± 0.007 | ||||
| Negative (n = 43) | 0.099 ± 0.064 | 0.441 ± 0.183 | 0.239 ± 0.149 | 0.018 ± 0.017 | ||||
| KRAS mutation | 0.2793 | 0.6904 | 0.6747 | 0.6253 | ||||
| Positive (n = 22) | 0.087 ± 0.040 | 0.418 ± 0.170 | 0.237 ± 0.177 | 0.016 ± 0.016 | ||||
| Negative (n = 29) | 0.105 ± 0.071 | 0.438 ± 0.178 | 0.256 ± 0.132 | 0.019 ± 0.016 | ||||
| Tumor differentiation | 0.0360 | 0.0053 | 0.3333 | 0.7004 | ||||
| Well (n = 6) | 0.127 ± 0.032 | 0.623 ± 0.252 | 0.327 ± 0.206 | 0.021 ± 0.018 | ||||
| Moderately (n = 45) | 0.084 ± 0.036 | 0.415 ± 0.151 | 0.235 ± 0.142 | 0.018 ± 0.016 | ||||
| CRM status | 0.9337 | 0.3737 | 0.4742 | 0.8316 | ||||
| Positive (n = 8) | 0.097 ± 0.045 | 0.384 ± 0.099 | 0.287 ± 0.168 | 0.017 ± 0.010 | ||||
| Negative (n = 43) | 0.099 ± 0.062 | 0.445 ± 0.187 | 0.240 ± 0.148 | 0.018 ± 0.016 |
| Prognostic Factors | Well-Differentiated Group (n = 6) (Mean ± SD) | Moderately Differentiated Group (n = 45) (Mean ± SD) | p Value |
|---|---|---|---|
| CEA level (ng/mL) | 1.86 ± 0.47 | 7.90 ± 7.67 | 0.2243 |
| Tumor size (cm) | 2.82 ± 1.78 | 4.72 ± 2.26 | 0.0496 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.R.; Kim, S.H.; Nam, K.H. Association between Dynamic Contrast-Enhanced MRI Parameters and Prognostic Factors in Patients with Primary Rectal Cancer. Curr. Oncol. 2023, 30, 2543-2554. https://doi.org/10.3390/curroncol30020194
Kim HR, Kim SH, Nam KH. Association between Dynamic Contrast-Enhanced MRI Parameters and Prognostic Factors in Patients with Primary Rectal Cancer. Current Oncology. 2023; 30(2):2543-2554. https://doi.org/10.3390/curroncol30020194
Chicago/Turabian StyleKim, Hye Ri, Seung Ho Kim, and Kyung Han Nam. 2023. "Association between Dynamic Contrast-Enhanced MRI Parameters and Prognostic Factors in Patients with Primary Rectal Cancer" Current Oncology 30, no. 2: 2543-2554. https://doi.org/10.3390/curroncol30020194
APA StyleKim, H. R., Kim, S. H., & Nam, K. H. (2023). Association between Dynamic Contrast-Enhanced MRI Parameters and Prognostic Factors in Patients with Primary Rectal Cancer. Current Oncology, 30(2), 2543-2554. https://doi.org/10.3390/curroncol30020194





