CD8+ T Cells and PD-L1 Expression as Prognostic Indicators in a Low Prevalence of HPV-Associated Oropharyngeal Squamous Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Immunohistochemical Method
2.3. Tumor Microenvironment
2.4. Statistical Analysis
3. Results
3.1. Immunohistochemical Analysis
3.2. Patient Characteristics
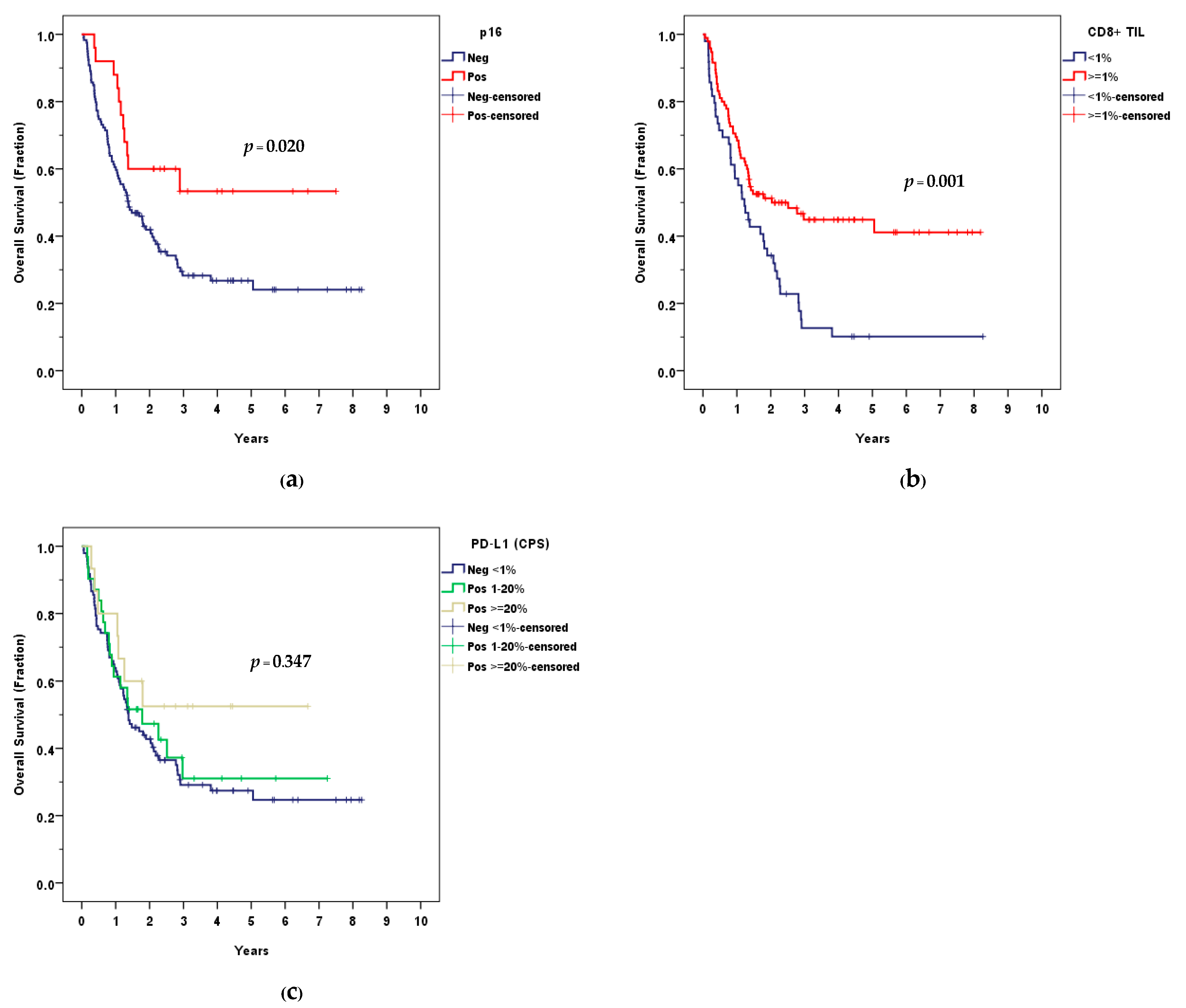
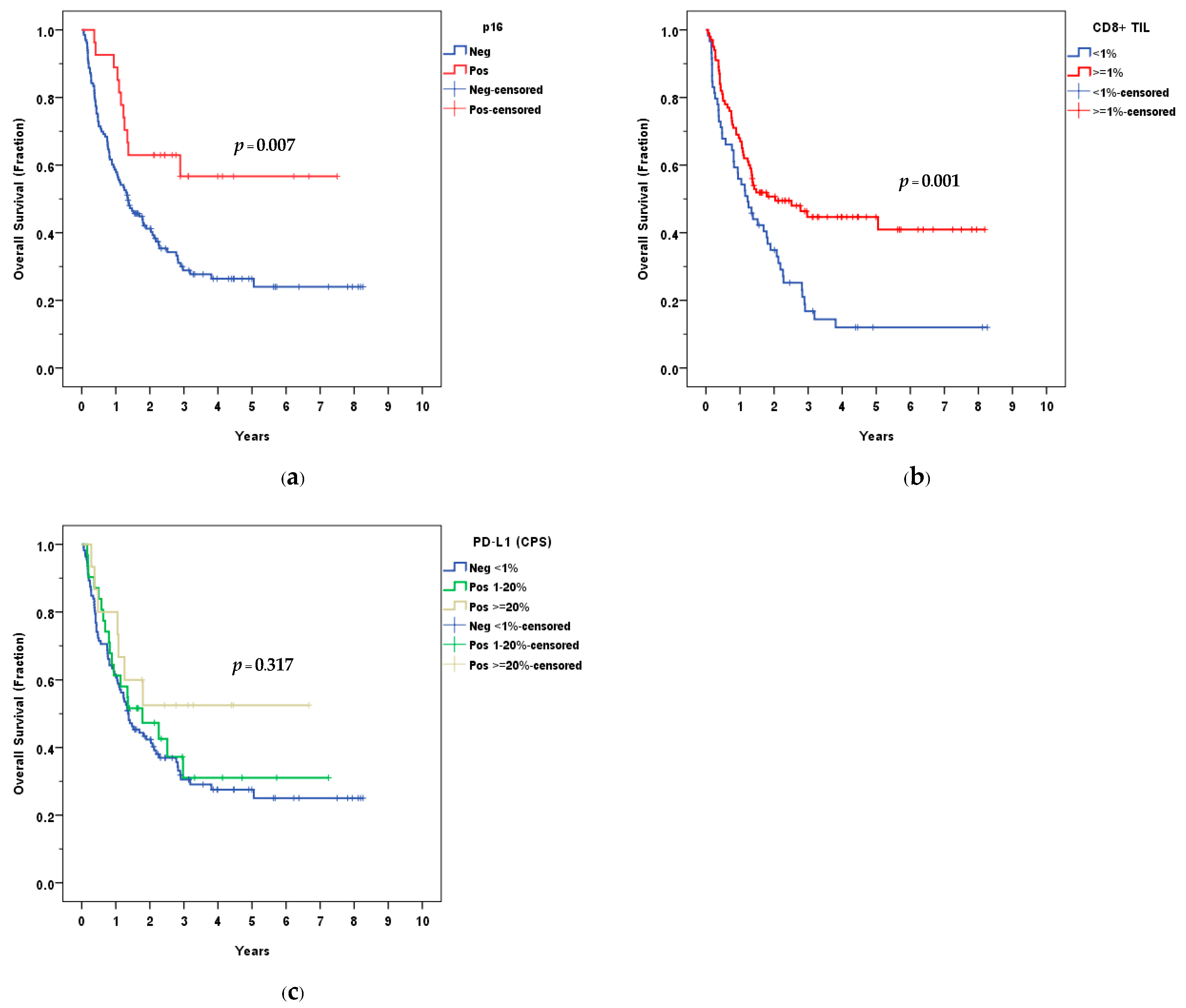
3.3. Survival Outcomes
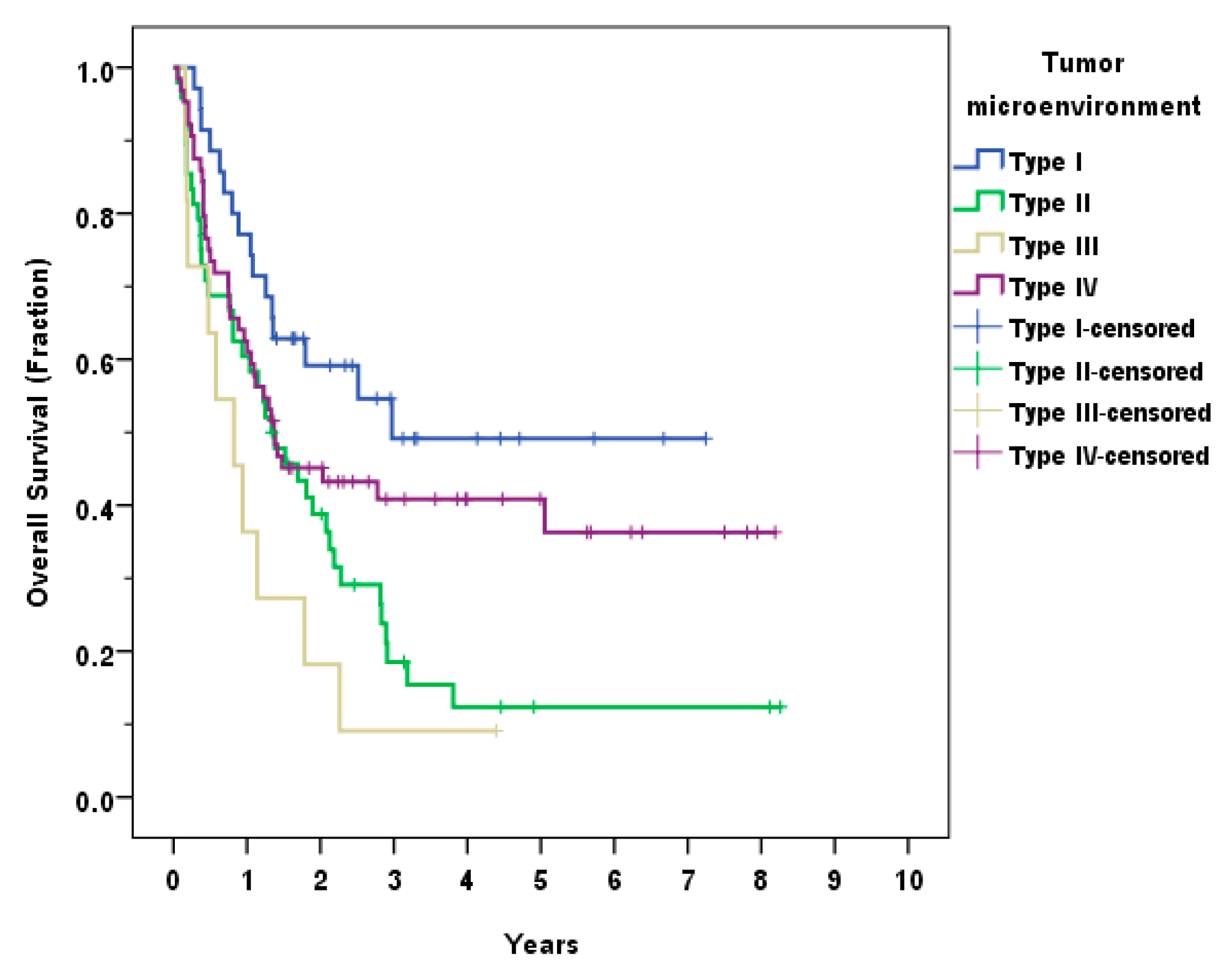
3.4. Tumor Microenvironment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Wang, M.B.; Liu, I.Y.; Gornbein, J.A.; Nguyen, C.T. HPV-Positive Oropharyngeal Carcinoma: A Systematic Review of Treatment and Prognosis. Otolaryngol.-Head Neck Surg. Off. J. Am. Acad. Otolaryngol.-Head Neck Surg. 2015, 153, 758–769. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Head and Neck Cancers (Version 2.2018). Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed on 22 June 2018).
- Mehanna, H.; Beech, T.; Nicholson, T.; El-Hariry, I.; McConkey, C.; Paleri, V.; Roberts, S. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer--systematic review and meta-analysis of trends by time and region. Head Neck 2013, 35, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Nopmaneepaisarn, T.; Tangjaturonrasme, N.; Rawangban, W.; Vinayanuwattikun, C.; Keelawat, S.; Bychkov, A. Low preva-lence of p16-positive HPV-related head-neck cancers in Thailand: Tertiary referral center experience. BMC Cancer 2019, 19, 1050. [Google Scholar] [CrossRef] [PubMed]
- Chotipanich, A.; Siriarechakul, S.; Mungkung, O.O. Role of high-risk human papillomavirus in the etiology of oral and oro-pharyngeal cancers in Thailand: A case-control study. SAGE Open Med. 2018, 6, 2050312118765604. [Google Scholar] [CrossRef]
- Pongsapich, W.; Jotikaprasardhna, P.; Lianbanchong, C.; Phumchan, A.; Siritantikorn, S.; Chongkolwatana, C. Human Papil-lomavirus Infection in Oral Cavity and Oropharyngeal Cancers: Are They the Same Story? J. Med. Assoc. Thail. Chotmaihet Thangphaet 2016, 99, 684–690. [Google Scholar]
- Arsa, L.; Siripoon, T.; Trachu, N.; Foyhirun, S.; Pangpunyakulchai, D.; Sanpapant, S.; Jinawath, N.; Pattaranutaporn, P.; Jinawath, A.; Ngamphaiboon, N. Discrepancy in p16 expression in patients with HPV-associated head and neck squamous cell carcinoma in Thailand: Clinical characteristics and survival outcomes. BMC Cancer 2021, 21, 504. [Google Scholar] [CrossRef]
- Ferris, R.L. Immunology and Immunotherapy of Head and Neck Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 3293–3304. [Google Scholar] [CrossRef]
- Lechien, J.R.; Descamps, G.; Seminerio, I.; Furgiuele, S.; Dequanter, D.; Mouawad, F.; Badoual, C.; Journe, F.; Saussez, S. HPV Involvement in the Tumor Microenvironment and Immune Treatment in Head and Neck Squamous Cell Carcinomas. Cancers 2020, 12, 1060. [Google Scholar] [CrossRef]
- Welters, M.J.P.; Santegoets, S.J.; van der Burg, S.H. The Tumor Microenvironment and Immunotherapy of Oropharyngeal Squamous Cell Carcinoma. Front. Oncol. 2020, 10, 545385. [Google Scholar] [CrossRef]
- Fukushima, Y.; Someya, M.; Nakata, K.; Hori, M.; Kitagawa, M.; Hasegawa, T.; Tsuchiya, T.; Gocho, T.; Ikeda, H.; Hirohashi, Y.; et al. Influence of PD-L1 expression in immune cells on the response to radiation therapy in patients with oropharyngeal squamous cell carcinoma. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2018, 129, 409–414. [Google Scholar] [CrossRef]
- Ou, D.; Adam, J.; Garberis, I.; Blanchard, P.; Nguyen, F.; Levy, A.; Casiraghi, O.; Gorphe, P.; Breuskin, I.; Janot, F.; et al. Clinical relevance of tumor infiltrating lymphocytes, PD-L1 expression and correlation with HPV/p16 in head and neck cancer treated with bio- or chemo-radiotherapy. Oncoimmunology 2017, 6, e1341030. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.; Angell, T.; Lechner, M.; Liebertz, D.; Correa, A.; Sinha, U.; Kokot, N.; Epstein, A. Immune cell infiltration patterns and survival in head and neck squamous cell carcinoma. Head Neck Oncol. 2013, 5, 24. [Google Scholar] [PubMed]
- Solomon, B.; Young, R.J.; Rischin, D. Head and neck squamous cell carcinoma: Genomics and emerging biomarkers for im-munomodulatory cancer treatments. Semin. Cancer Biol. 2018, 52 Pt 2, 228–240. [Google Scholar] [CrossRef] [PubMed]
- De Meulenaere, A.; Vermassen, T.; Aspeslagh, S.; Huvenne, W.; Van Dorpe, J.; Ferdinande, L.; Rottey, S. Turning the tide: Clinical utility of PD-L1 expression in squamous cell carcinoma of the head and neck. Oral Oncol. 2017, 70, 34–42. [Google Scholar] [CrossRef]
- Tang, H.; Zhou, X.; Ye, Y.; Zhou, Y.; Wu, C.; Xu, Y. The different role of PD-L1 in head and neck squamous cell carcinomas: A meta-analysis. Pathol. Res. Pract. 2020, 216, 152768. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Ha, S.J.; Hong, M.H.; Heo, S.J.; Koh, Y.W.; Choi, E.C.; Kim, E.K.; Pyo, K.H.; Jung, I.; Seo, D.; et al. PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor for head and neck cancer pa-tients. Sci. Rep. 2016, 6, 36956. [Google Scholar] [CrossRef]
- Yi, M.; Niu, M.; Xu, L.; Luo, S.; Wu, K. Regulation of PD-L1 expression in the tumor microenvironment. J. Hematol. Oncol. 2021, 14, 10. [Google Scholar] [CrossRef]
- Zerdes, I.; Matikas, A.; Bergh, J.; Rassidakis, G.Z.; Foukakis, T. Genetic, transcriptional and post-translational regulation of the programmed death protein ligand 1 in cancer: Biology and clinical correlations. Oncogene 2018, 37, 4639–4661. [Google Scholar] [CrossRef]
- Lyford-Pike, S.; Peng, S.; Young, G.D.; Taube, J.M.; Westra, W.H.; Akpeng, B.; Bruno, T.C.; Richmon, J.D.; Wang, H.; Bishop, J.A.; et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013, 73, 1733–1741. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Lewis, J.S., Jr.; Beadle, B.; Bishop, J.A.; Chernock, R.D.; Colasacco, C.; Lacchetti, C.; Moncur, J.T.; Rocco, J.W.; Schwartz, M.R.; Seethala, R.R.; et al. Human Papillomavirus Testing in Head and Neck Carcinomas: Guideline From the College of American Pathologists. Arch. Pathol. Lab. Med. 2018, 142, 559–597. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.W.; Ngiow, S.F.; Ribas, A.; Smyth, M.J. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015, 75, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef]
- Ragin, C.C.; Taioli, E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: Review and meta-analysis. Int. J. Cancer 2007, 121, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Argiris, A.; Li, S.; Ghebremichael, M.; Egloff, A.M.; Wang, L.; Forastiere, A.A.; Burtness, B.; Mehra, R. Prognostic significance of human papillomavirus in recurrent or metastatic head and neck cancer: An analysis of Eastern Cooperative Oncology Group trials. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Näsman, A.; Romanitan, M.; Nordfors, C.; Grün, N.; Johansson, H.; Hammarstedt, L.; Marklund, L.; Munck-Wikland, E.; Da-lianis, T.; Ramqvist, T. Tumor infiltrating CD8+ and Foxp3+ lymphocytes correlate to clinical outcome and human papilloma-virus (HPV) status in tonsillar cancer. PLoS ONE 2012, 7, e38711. [Google Scholar] [CrossRef]
- Saber, C.N.; Grønhøj Larsen, C.; Dalianis, T.; von Buchwald, C. Immune cells and prognosis in HPV-associated oropharyngeal squamous cell carcinomas: Review of the literature. Oral Oncol. 2016, 58, 8–13. [Google Scholar] [CrossRef]
- Nordfors, C.; Grün, N.; Tertipis, N.; Ährlund-Richter, A.; Haeggblom, L.; Sivars, L.; Du, J.; Nyberg, T.; Marklund, L.; Munck-Wikland, E.; et al. CD8+ and CD4+ tumour infiltrating lymphocytes in relation to human papillomavirus status and clinical outcome in tonsillar and base of tongue squamous cell carcinoma. Eur. J. Cancer 2013, 49, 2522–2530. [Google Scholar] [CrossRef]
- Wansom, D.; Light, E.; Thomas, D.; Worden, F.; Prince, M.; Urba, S.; Chepeha, D.; Kumar, B.; Cordell, K.; Eisbruch, A.; et al. Infiltrating lymphocytes and human papilloma-virus-16--associated oropharyngeal cancer. Laryngoscope 2012, 122, 121–127. [Google Scholar] [CrossRef]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef]
- Hecht, M.; Eckstein, M.; Rutzner, S.; von der Grün, J.; Illmer, T.; Klautke, G.; Laban, S.; Hautmann, M.G.; Brunner, T.B.; Ta-maskovics, B.; et al. Induction chemoimmunotherapy followed by CD8+ immune cell-based patient selection for chemotherapy-free radioimmunotherapy in locally advanced head and neck cancer. J. Immunother. Cancer 2022, 10, e003747. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.F.; Wong, M.C.M.; Thomson, P.J.; Li, K.Y.; Su, Y.X. The prognostic role of PD-L1 expression for survival in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol. 2018, 86, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Balermpas, P.; Rödel, F.; Krause, M.; Linge, A.; Lohaus, F.; Baumann, M.; Tinhofer, I.; Budach, V.; Sak, A.; Stuschke, M.; et al. The PD-1/PD-L1 axis and human papilloma virus in patients with head and neck cancer after adjuvant chemoradiotherapy: A multicentre study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Int. J. Cancer 2017, 141, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Canteli, M.; Granda-Díaz, R.; Del Rio-Ibisate, N.; Allonca, E.; López-Alvarez, F.; Agorreta, J.; Garmendia, I.; Montuenga, L.M.; García-Pedrero, J.M.; Rodrigo, J.P. PD-L1 expression correlates with tumor-infiltrating lymphocytes and better prognosis in patients with HPV-negative head and neck squamous cell carcinomas. Cancer Immunol. Immunother. CII 2020, 69, 2089–2100. [Google Scholar] [CrossRef]
- Huang, Z.; Zheng, S.; Ding, S.; Wei, Y.; Chen, C.; Liu, X.; Li, H.; Xia, Y. Prognostic role of programmed cell death ligand-1 ex-pression in head and neck cancer treated with programmed cell death protein-1/programmed cell death ligand-1 inhibitors: A meta-analysis based on clinical trials. J. Cancer Res. Ther. 2021, 17, 676–687. [Google Scholar] [PubMed]
- De Meulenaere, A.; Vermassen, T.; Aspeslagh, S.; Deron, P.; Duprez, F.; Laukens, D.; Van Dorpe, J.; Ferdinande, L.; Rottey, S. Tumor PD-L1 status and CD8(+) tumor-infiltrating T cells: Markers of improved prognosis in oropharyngeal cancer. Oncotarget 2017, 8, 80443–80452. [Google Scholar] [CrossRef]
- Sittel, C.; Ruiz, S.; Volling, P.; Kvasnicka, H.M.; Jungehülsing, M.; Eckel, H.E. Prognostic significance of Ki-67 (MIB1), PCNA and p53 in cancer of the oropharynx and oral cavity. Oral Oncol. 1999, 35, 583–589. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, M.; Rose, B.; Veillard, A.S.; Jones, D.; Zhang, X.; Soon Lee, C.; Milross, C.; Hong, A. Ki67 Expression has Prognostic Significance in Relation to Human Papillomavirus Status in Oropharyngeal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2015, 22, 1893–1900. [Google Scholar] [CrossRef]
- Macluskey, M.; Chandrachud, L.M.; Pazouki, S.; Green, M.; Chisholm, D.M.; Ogden, G.R.; Schor, S.L.; Schor, A.M. Apoptosis, proliferation, and angiogenesis in oral tissues. Possible relevance to tumour progression. J. Pathol. 2000, 191, 368–375. [Google Scholar] [CrossRef]
- Mallick, S.; Breta, M.; Gupta, S.D.; Dinda, A.K.; Mohanty, B.K.; Singh, M.K. Angiogenesis, Proliferative Activity and DNA Ploidy in Oral Verrucous Carcinoma: A Comparative Study Including Verrucous Hyperplasia and Squamous Cell Carcinoma. Pathol. Oncol. Res. POR 2015, 21, 1249–1257. [Google Scholar] [CrossRef]
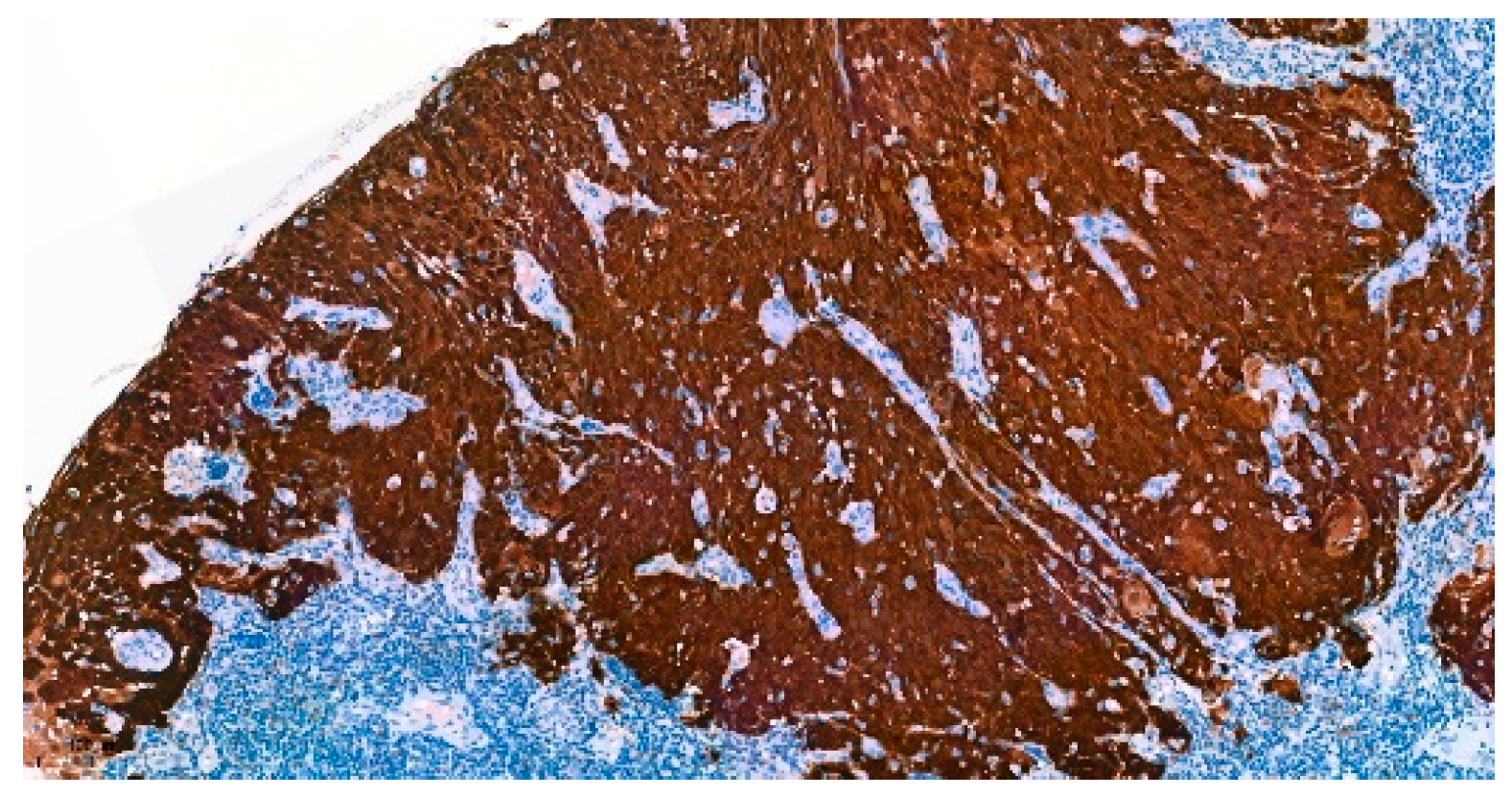
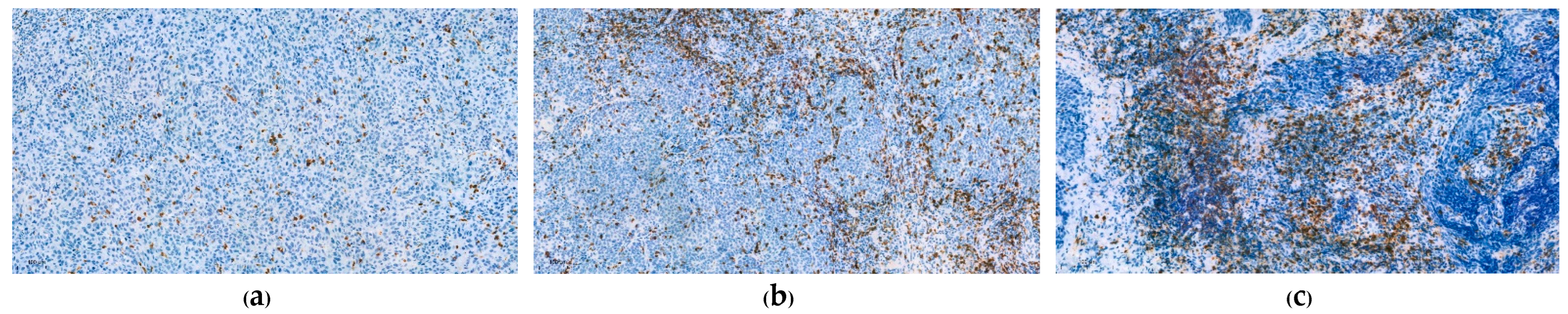

| CD8+ TIL, n(%) | (n = 159) |
| <1% | 59 (37.1) |
| ≥1%, <5% | 54 (34.0) |
| ≥5%, <10% | 19 (11.9) |
| ≥10% | 27 (17.0) |
| PD-L1: CPS, n(%) | (n = 158) |
| CPS < 1 | 112 (70.9) |
| CPS ≥ 1, < 20 | 31 (19.6) |
| CPS ≥ 20 | 15 (9.5) |
| PD-L1: Intensity, n(%) | (n = 46) |
| 1+ (weak) | 24 (52.2) |
| 2+ (moderate) | 19 (41.3) |
| 3+ (strong) | 3 (6.5) |
| p16− | p16+ | p-Value | PD-L1, CPS < 1 | PD-L1, CPS ≥ 1 | p-Value | |
|---|---|---|---|---|---|---|
| CD8+ TIL, n(%) | 0.005 * | 0.039 * | ||||
| <1% | 51 (38.6) | 8 (29.6) | 48 (42.9) | 11 (23.9) | ||
| ≥1%, <5% | 50 (37.9) | 4 (14.8) | 39 (34.8) | 15 (32.6) | ||
| ≥5%, <10% | 14 (10.6) | 5 (18.5) | 11 (9.8) | 8 (17.4) | ||
| ≥10% | 17 (12.9) | 10 (37.0) | 14 (12.5) | 12 (26.1) | ||
| PD-L1, n(%) | 0.596 | |||||
| CPS < 1 | 94 (71.8) | 18 (66.7) | ||||
| CPS ≥ 1 | 37 (28.2) | 9 (33.3) |
| p16− (n = 133) | p16+ (n = 27) | p-Value | CD8+ TIL < 1% (n = 59) | CD8+ TIL ≥ 1% (n = 100) | p-Value | PD-L1; CPS < 1 (n = 112) | PD-L1; CPS ≥ 1 (n = 46) | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| Age | 0.045 * | 0.754 | 0.445 | ||||||
| Mean ± SD | 61.12 ± 11.18 | 56.19 ± 13.42 | 60.58 ± 11.87 | 59.97 ± 11.61 | 59.73 ± 12.03 | 61.30 ± 10.94 | |||
| Sex, n (%) | 0.066 | 0.325 | 0.156 | ||||||
| Male | 127 (95.5) | 23 (85.2) | 57 (96.6) | 92 (92.0) | 107 (95.5) | 41 (89.1) | |||
| Female | 6 (4.5) | 4 (14.8) | 2 (3.4) | 8 (8.0) | 5 (4.5) | 5 (10.9) | |||
| Smoking status, n (%) | 0.058 | 0.015 * | 0.223 | ||||||
| Yes | 122 (93.1) | 21 (80.8) | 57 (98.3) | 85 (86.7) | 103 (92.8) | 38 (86.4) | |||
| No | 9 (6.9) | 5 (19.2) | 1 (1.7) | 13 (13.3) | 8 (7.2) | 6 (13.6) | |||
| Alcohol use, n (%) | 0.094 | 0.28 | 0.302 | ||||||
| Yes | 84 (66.7) | 11 (44.0) | 39 (70.9) | 55 (57.9) | 71 (67.0) | 23 (53.5) | |||
| Social drinking | 16 (12.7) | 6 (24.0) | 6 (10.9) | 16 (16.8) | 14 (13.2) | 8 (18.6) | |||
| Never | 26 (20.6) | 8 (32.0) | 10 (18.2) | 24 (25.3) | 21 (19.8) | 12 (27.9) | |||
| Subsite, n (%) | 0.081 | 0.139 | 0.132 | ||||||
| Base of tongue | 60 (45.1) | 11 (40.7) | 27 (45.8) | 43 (43.0) | 44 (39.3) | 26 (56.5) | |||
| Tonsil | 49 (36.8) | 15 (55.6) | 19 (32.2) | 45 (45.0) | 48 (42.9) | 15 (32.6) | |||
| Others | 24 (18.0) | 1 (3.7) | 13 (22.0) | 12 (12.0) | 20 (17.9) | 5 (10.9) | |||
| Pathological grading, n (%) | 0.698 | 0.815 | 0.069 | ||||||
| Well diff. | 18 (13.6) | 3 (13.6) | 9 (15.3) | 12 (12.8) | 16 (15.1) | 5 (10.9) | |||
| Moderately diff. | 88 (66.7) | 13 (59.1) | 39 (66.1) | 61 (64.9) | 73 (68.9) | 26 (56.5) | |||
| Poorly diff. | 26 (19.7) | 6 (27.3) | 11 (18.6) | 21 (22.3) | 17 (16.0) | 15 (32.6) | |||
| T stage, n (%) | 0.144 | 0.317 | 0.89 | ||||||
| T1 | 10 (7.5) | 6 (22.2) | 6 (10.2) | 10 (10.0) | 12 (10.7) | 4 (8.7) | |||
| T2 | 28 (21.1) | 5 (18.5) | 8 (13.6) | 25 (25.0) | 22 (19.6) | 10 (21.7) | |||
| T3 | 52 (39.1) | 9 (33.3) | 23 (39.0) | 37 (37.0) | 41 (36.6) | 19 (41.3) | |||
| T4 | 43 (32.3) | 7 (25.9) | 22 (37.3) | 28 (28.0) | 37 (33.0) | 13 (28.3) | |||
| N stage, n (%) | 0.007 * | 0.304 | 0.554 | ||||||
| N0 | 27 (20.3) | 4 (14.8) | 16 (27.1) | 15 (15.0) | 23 (20.5) | 8 (17.4) | |||
| N1 | 19 (14.3) | 11 (40.7) | 11 (18.6) | 19 (19.0) | 24 (21.4) | 6 (13.0) | |||
| N2 | 62 (46.6) | 6 (22.2) | 22 (37.3) | 45 (45.0) | 44 (39.3) | 22 (47.8) | |||
| N3 | 25 (18.8) | 6 (22.2) | 10 (16.9) | 21 (21.0) | 21 (18.8) | 10 (21.7) | |||
| M stage, n (%) | 0.213 | 0.040 * | 0.035 * | ||||||
| M0 | 122 (91.7) | 27 (100.0) | 52 (88.1) | 97 (97.0) | 102 (91.1) | 46 (100.0) | |||
| M1 | 11 (8.3) | 0 (0.0) | 7 (11.9) | 3 (3.0) | 10 (8.9) | 0 (0.0) | |||
| Stage (AJCC 8th), n (%) | <0.001 * | 0.954 | 0.578 | ||||||
| I | 3 (2.3) | 6 (22.2) | 4 (6.8) | 5 (5.0) | 8 (7.1) | 1 (2.2) | |||
| II | 9 (6.8) | 10 (37.0) | 7 (11.9) | 12 (12.0) | 12 (10.7) | 7 (15.2) | |||
| III | 24 (18.0) | 11 (40.7) | 12 (20.3) | 23 (23.0) | 25 (22.3) | 10 (21.7) | |||
| IV | 97 (72.9) | 0 (0.0) | 36 (61.0) | 60 (60.0) | 67 (59.8) | 28 (60.9) | |||
| Stage (AJCC 7th), n (%) | 0.368 | 0.461 | 0.62 | ||||||
| I | 3 (2.3) | 2 (7.4) | 3 (5.1) | 2 (2.0) | 5 (4.5) | 0 (0.0) | |||
| II | 9 (6.8) | 1 (3.7) | 5 (8.5) | 5 (5.0) | 7 (6.3) | 3 (6.5) | |||
| III | 24 (18.0) | 3 (11.1) | 8 (13.6) | 19 (19.0) | 20 (17.9) | 7 (15.2) | |||
| IV | 97 (72.9) | 21 (77.8) | 43 (72.9) | 74 (74.0) | 80 (71.4) | 36 (78.3) |
| Univariate HR (95% CI) | p-Value | Adjusted HR (95% CI) | p-Value | |
|---|---|---|---|---|
| Smoking status: yes (former/current) | 3.46 (1.27–9.41) | 0.015 * | 2.48 (0.90–6.82) | 0.079 |
| Stage: III-IV | 2.18 (1.01–4.70) | 0.047 * | 2.43 (1.11–5.32) | 0.026 * |
| p16: negative | 2.32 (1.24–4.33) | 0.009 * | 1.98 (1.05–3.71) | 0.034 * |
| CD8+ TIL: low density | 1.89 (1.28–2.78) | 0.001 * | 1.77 (1.18–2.67) | 0.006 * |
| PD-L1: negative | 1.33 (0.85–2.07) | 0.211 | 1.13 (0.71–1.80) | 0.603 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atipas, K.; Laokulrath, N.; Petsuksiri, J.; Ratanaprasert, N.; Pongsapich, W. CD8+ T Cells and PD-L1 Expression as Prognostic Indicators in a Low Prevalence of HPV-Associated Oropharyngeal Squamous Cell Carcinoma. Curr. Oncol. 2023, 30, 1450-1460. https://doi.org/10.3390/curroncol30020111
Atipas K, Laokulrath N, Petsuksiri J, Ratanaprasert N, Pongsapich W. CD8+ T Cells and PD-L1 Expression as Prognostic Indicators in a Low Prevalence of HPV-Associated Oropharyngeal Squamous Cell Carcinoma. Current Oncology. 2023; 30(2):1450-1460. https://doi.org/10.3390/curroncol30020111
Chicago/Turabian StyleAtipas, Kawita, Natthawadee Laokulrath, Janjira Petsuksiri, Narin Ratanaprasert, and Warut Pongsapich. 2023. "CD8+ T Cells and PD-L1 Expression as Prognostic Indicators in a Low Prevalence of HPV-Associated Oropharyngeal Squamous Cell Carcinoma" Current Oncology 30, no. 2: 1450-1460. https://doi.org/10.3390/curroncol30020111
APA StyleAtipas, K., Laokulrath, N., Petsuksiri, J., Ratanaprasert, N., & Pongsapich, W. (2023). CD8+ T Cells and PD-L1 Expression as Prognostic Indicators in a Low Prevalence of HPV-Associated Oropharyngeal Squamous Cell Carcinoma. Current Oncology, 30(2), 1450-1460. https://doi.org/10.3390/curroncol30020111





