Capecitabine-Induced Ileitis during Neoadjuvant Pelvic Radio-Chemotherapy for Locally Advanced Rectal Cancer: A Case Report with Literature Review
Abstract
:1. Introduction
2. Detailed Case Description
2.1. Clinical Case Presentation
2.2. Search Strategy and Data Extraction
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rodel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus Postoperative Chemoradiotherapy for Rectal Cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Sauer, R.; Liersch, T.; Merkel, S.; Fietkau, R.; Hohenberger, W.; Hess, C.; Becker, H.; Raab, H.-R.; Villanueva, M.-T.; Witzigmann, H.; et al. Preoperative Versus Postoperative Chemoradiotherapy for Locally Advanced Rectal Cancer: Results of the German CAO/ARO/AIO-94 Randomized Phase III Trial After a Median Follow-Up of 11 Years. J. Clin. Oncol. 2012, 30, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Bonnetain, F.; Bosset, J.; Gerard, J.; Calais, G.; Conroy, T.; Mineur, L.; Bouché, O.; Maingon, P.; Chapet, O.; Radosevic-Jelic, L.; et al. What is the clinical benefit of preoperative chemoradiotherapy with 5FU/leucovorin for T3-4 rectal cancer in a pooled analysis of EORTC 22921 and FFCD 9203 trials: Surrogacy in question? Eur. J. Cancer 2012, 48, 1781–1790. [Google Scholar] [CrossRef]
- Roh, M.S.; Colangelo, L.H.; O’Connell, M.J.; Yothers, G.; Deutsch, M.; Allegra, C.J.; Kahlenberg, M.S.; Baez-Diaz, L.; Ursiny, C.S.; Petrelli, N.J.; et al. Preoperative Multimodality Therapy Improves Disease-Free Survival in Patients With Carcinoma of the Rectum: NSABP R-03. J. Clin. Oncol. 2009, 27, 5124–5130. [Google Scholar] [CrossRef]
- Walko, C.M.; Lindley, C. Capecitabine: A review. Clin. Ther. 2005, 27, 23–44. [Google Scholar] [CrossRef]
- Ruiz-Tovar, J.; Morales, V.; Hervás, A.; Sanjuanbenito, A.; Lobo, E.; Martínez-Molina, E. Late gastrointestinal complications after pelvic radiotherapy: Radiation enteritis. Clin. Transl. Oncol. 2009, 11, 539–543. [Google Scholar] [CrossRef]
- Saif, M.W.; Katirtzoglou, N.A.; Syrigos, K.N. Capecitabine: An overview of the side effects and their management. Anti-Cancer Drugs 2008, 19, 447–464. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Meyer, L.R.; et al. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: Cham, Switzerland; American Joint Commission on Cancer: Chicago, IL, USA, 2017. [Google Scholar]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Rectal Cancer, V.4.2023; National Comprehensive Cancer Network, Inc.: Plymouth Meeting, PA, USA, 2023.
- Valentini, V.; Gambacorta, M.A.; Barbaro, B.; Chiloiro, G.; Coco, C.; Das, P.; Fanfani, F.; Joye, I.; Kachnic, L.; Maingon, P.; et al. International consensus guidelines on Clinical Target Volume delineation in rectal cancer. Radiother. Oncol. 2016, 120, 195–201. [Google Scholar] [CrossRef]
- Bisello, S.; Cilla, S.; Benini, A.; Cardano, R.; Nguyen, N.P.; Deodato, F.; Macchia, G.; Buwenge, M.; Cammelli, S.; Wondemagegnehu, T.; et al. Dose–Volume Constraints for Organs at Risk in Radiotherapy (CORSAIR): An “All-in-One” Multicenter–Multidisciplinary Practical Summary. Curr. Oncol. 2022, 29, 7021–7050. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events, Version 5.0; U.S. Department of Health and Human Services: Washington, DC, USA, 2017. [Google Scholar]
- Letschert, J.G.; Lebesque, J.V.; de Boer, R.W.; Hart, A.A.; Bartelink, H. Dose-volume correlation in radiation-related late small-bowel complications: A clinical study. Radiother. Oncol. 1990, 18, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Shadad, A.K.; Sullivan, F.J.; Martin, J.D.; Egan, L.J. Gastrointestinal radiation injury: Symptoms, risk factors and mechanisms. World J. Gastroenterol. 2013, 19, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Bosset, J.-F.; Collette, L.; Calais, G.; Mineur, L.; Maingon, P.; Radosevic-Jelic, L.; Daban, A.; Bardet, E.; Beny, A.; Ollier, J.-C.; et al. Chemotherapy with Preoperative Radiotherapy in Rectal Cancer. N. Engl. J. Med. 2006, 355, 1114–1123. [Google Scholar] [CrossRef]
- Theis, V.; Sripadam, R.; Ramani, V.; Lal, S. Chronic Radiation Enteritis. Clin. Oncol. (R. Coll. Radiol.) 2010, 22, 70–83. [Google Scholar] [CrossRef]
- Gérard, J.-P.; Conroy, T.; Bonnetain, F.; Bouché, O.; Chapet, O.; Closon-Dejardin, M.-T.; Untereiner, M.; LeDuc, B.; Francois, É.; Maurel, J.; et al. Preoperative Radiotherapy With or Without Concurrent Fluorouracil and Leucovorin in T3-4 Rectal Cancers: Results of FFCD 9203. J. Clin. Oncol. 2006, 24, 4620–4625. [Google Scholar] [CrossRef] [PubMed]
- Mosseri, M.; Fingert, H.J.; Varticovski, L.; Chokshi, S.; Isner, J.M. In vitro evidence that myocardial ischemia resulting from 5-fluorouracil chemotherapy is due to protein kinase C-mediated vasoconstriction of vascular smooth muscle. Cancer Res. 1993, 53, 3028–3033. [Google Scholar]
- Kakinuma, S.; Ohwada, S. Gastric mucosal blood flow and gastric secretion following intravenous administration of 5-fluorouracil in anesthetized rats. Cancer Chemother. Pharmacol. 1997, 39, 357–360. [Google Scholar] [CrossRef]
- Barton, D. Ulcerative Ileitis Secondary to Adjuvant Capecitabine for Colon Cancer: A Case Report; UICC World Cancer Congress: Washington, DC, USA, 2006. [Google Scholar]
- Bouma, G.; Imholz, A.L. Ileïtis bij gebruik van capecitabine [Ileitis following capecitabine use]. Ned. Tijdschr. Geneeskd. 2011, 155, A3064. [Google Scholar]
- Radwan, R.; Namelo, W.C.; Robinson, M.; Brewster, A.E.; Williams, G.L. Ileitis Secondary to Oral Capecitabine Treatment? Case Rep. Med. 2012, 2012, 154981. [Google Scholar] [CrossRef]
- Al-Gahmi, A.M.; Kerr, I.G.; Zekri, J.M.; Zagnoon, A.A. Capecitabine-induced terminal ileitis. Ann. Saudi Med. 2012, 32, 661–662. [Google Scholar] [CrossRef]
- Mokrim, M.; Aftimos, P.G.; Errihani, H.; Piccart-Gebhart, M. Breast cancer, DPYD mutations and capecitabine-related ileitis: Description of two cases and a review of the literature. BMJ Case Rep. 2014, 2014, bcr2014203647. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.F.; Chiang, C.L.; Lee, A.S.; Wong, F.C.S.; Tung, S.Y. Severe ileitis associated with capecitabine: Two case reports and review of the literature. Mol. Clin. Oncol. 2015, 3, 1398–1400. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, L.; Russo, I.; De Sanctis, V.; Minniti, G.; Valeriani, M.; Osti, M.F. Two Cases of Capecitabine-Induced Ileitis in Patients Treated with Radiochemotherapy to the Pelvis and Review of the Literature. J. Gastrointest. Cancer 2018, 49, 538–542. [Google Scholar] [CrossRef]
- van Hellemond, I.E.; Thijs, A.M.; Creemers, G.-J. Capecitabine-Associated Terminal Ileitis. Case Rep. Oncol. 2018, 11, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Dao, A.E.; Hsu, A.; Nakshabandi, A.; Mandaliya, R.; Nadella, S.; Sivaraman, A.; Mattar, M.; Charabaty, A. Role of colonoscopy in diagnosis of capecitabine associated ileitis: Two case reports. World J. Gastrointest. Endosc. 2019, 11, 383–388. [Google Scholar] [CrossRef]
- Klimko, A.; Tieranu, C.G.; Olteanu, A.O.; Preda, C.M.; Ionescu, E.M. Capecitabine-Induced Terminal Ileitis: Case Report and Literature Review. Cureus 2021, 13, e14621. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, S.; Wu, J.; Sun, Z. Severe ileum bleeding following adjuvant capecitabine chemotherapy for locally advanced colon cancer: A case report and review of the literature. World J. Surg. Oncol. 2021, 19, 332. [Google Scholar] [CrossRef]
- Gomez-Paz, S.; Yeroushalmi, K.; Desai, J.; Kagolanu, D.; Rizvon, K.; Khan, N.; Mustacchia, P. S2678 A Case of Capecitabine-Induced Gastrointestinal Hemorrhage. Am. J. Gastroenterol. 2022, 117, e1767. [Google Scholar] [CrossRef]
- Sinha, A.; Desai, J.; Gomez-Paz, S.; Yeroushalmi, K.; Kagolanu, D.; Subramani, K.; Khan, N.; Rizvon, K. S3379 Capecitabine-Induced Rapid Onset Terminal Ileitis in Patient With Stage III Sigmoid Adenocarcinoma. Am. J. Gastroenterol. 2022, 117, e2139–e2140. [Google Scholar] [CrossRef]
- Shao, T.; Zhang, Y.; Liu, J.; Chen, J.; Shu, Q.; Shou, L. Capecitabine-induced enterocolitis: A case report and pharmacogenetic profile. Pharmacogenomics 2022, 23, 953–959. [Google Scholar] [CrossRef]
- Hauer-Jensen, M. Late Radiation Injury of the Small Intestine Clinical, pathophysiologic and radiobiologic aspects: A review. Acta Oncol. 1990, 29, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Diefenhardt, M.; Martin, D.; Ludmir, E.B.; Fleischmann, M.; Hofheinz, R.-D.; Ghadimi, M.; Kosmala, R.; Polat, B.; Friede, T.; Minsky, B.D.; et al. Development and Validation of a Predictive Model for Toxicity of Neoadjuvant Chemoradiotherapy in Rectal Cancer in the CAO/ARO/AIO-04 Phase III Trial. Cancers 2022, 14, 4425. [Google Scholar] [CrossRef] [PubMed]
- Arcadipane, F.; Franco, P.; Ceccarelli, M.; Furfaro, G.; Rondi, N.; Trino, E.; Martini, S.; Iorio, G.C.; Mistrangelo, M.; Cassoni, P.; et al. Image-guided IMRT with simultaneous integrated boost as per RTOG 0529 for the treatment of anal cancer. Asia-Pac. J. Clin. Oncol. 2018, 14, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Arcadipane, F.; Silvetti, P.; Olivero, F.; Gastino, A.; De Luca, V.; Mistrangelo, M.; Cassoni, P.; Racca, P.; Gallio, E.; Lesca, A.; et al. Bone Marrow-Sparing IMRT in Anal Cancer Patients Undergoing Concurrent Chemo-Radiation: Results of the First Phase of a Prospective Phase II Trial. Cancers 2020, 12, 3306. [Google Scholar] [CrossRef]
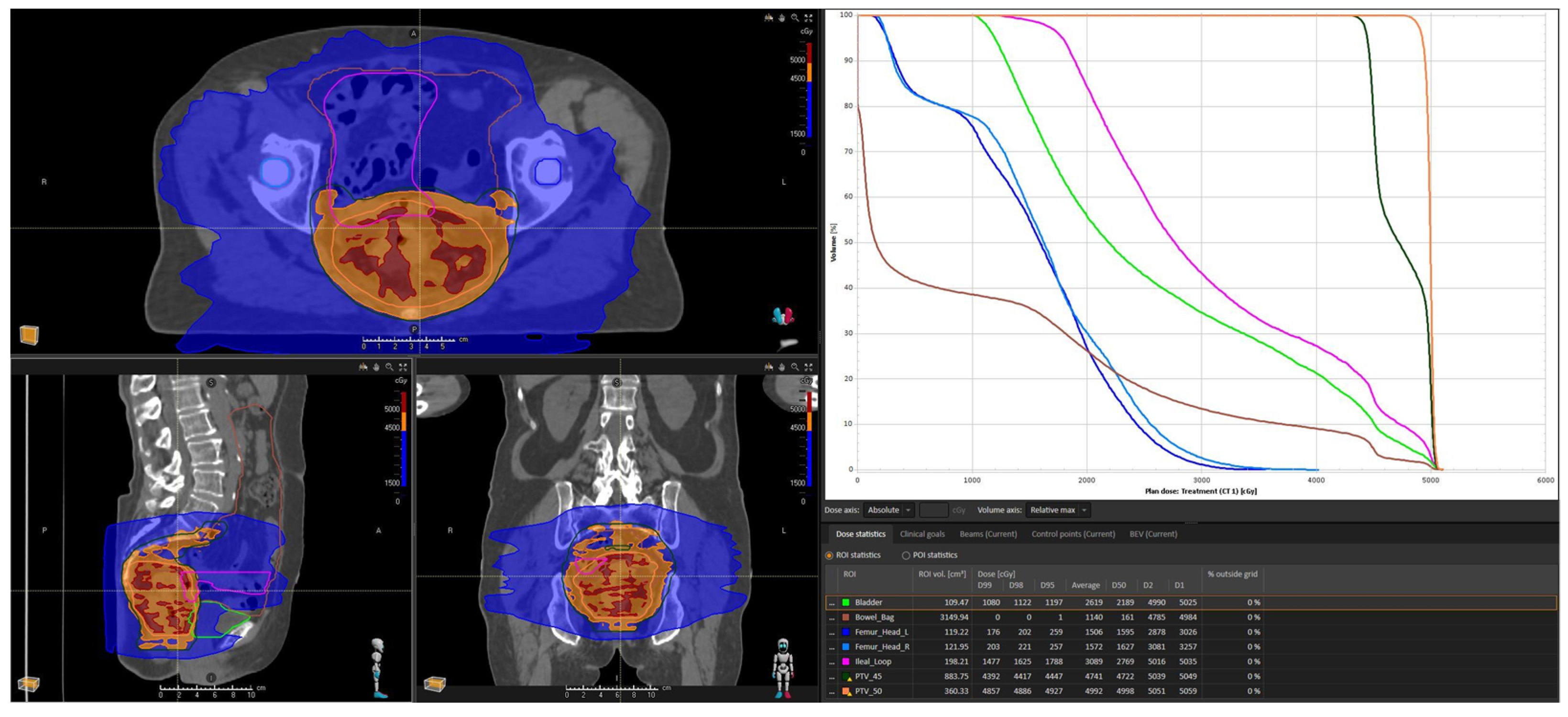


| Constraints | Our Case | |
|---|---|---|
| Bowel space | V45 ≤ 195 cc | 141.7 cc |
| Small bowel | -Dmax ≤ 55 Gy -V15 ≤ 120 cc (optimal) -V45 ≤ 15% -V50 ≤ 10 cc (optimal) or ≤ 10% (mandatory) | 50.98 Gy 195.93 cc 16% 5.9 cc |
| Reference | Patient | Treatment | Capecitabine Dose | RT Doses/Volumes | Clinical Features | Symptoms Onset | Diagnostic Procedures | DPD Testing | Management | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Barton, 2006 [21] | 54 y.o. man with locally advanced colon cancer | Adjuvant capecitabine | N.R. | / | Diarrhea, abdominal pain | After 3rd cycle | Colonoscopy w/biopsy: ulcerative ileitis with eosinophilic infiltrate | N.R. | Bowel rest with IV nutrition and broad-spectrum antibiotics | N.R. |
| Bouma, 2011 [22] | 73 y.o. man with stage IV colon cancer | Palliative capecitabine/oxaliplatin/bevacizumab | N.R. | / | Abdominal pain, diarrhea, nausea | After 3rd cycle | CT: ileal walls thickening | Not performed | Hydration and capecitabine interruption (restarted at reduced dose after 4 weeks) | Clinical recovery after supportive therapy (duration of treatment not reported) |
| Radwan, 2012 [23] | 67 y.o. man with pT4N0 transverse colon cancer | Adjuvant capecitabine | 1000 mg/m2 twice daily for the first cycle (increased to 1250 mg/m2 from the second cycle) | / | Abdominal pain, reduced appetite, diarrhea and giddiness | After 2nd cycle | Abdominal X-ray: small bowel distension. CT: fluid distended loops and distal ileum walls thickening | N.R. | Broad-spectrum antibiotics, symptomatic therapy and permanent capecitabine discontinuation | Clinical recovery after 2 weeks of supportive therapy |
| Al-Gahmi, 2012 [24] | 65 y.o. man with stage IV rectal carcinoma | Palliative pelvic radiotherapy + sequential capecitabine/oxaliplatin | 1500 mg/m2 twice daily d1-14q21 | 30 Gy/10 fx on gross rectal disease | Abdominal pain, diarrhea, vomit, fever | 12 days after CHT start | Colonoscopy w/biopsy: terminal ileum ulceration with eosinophilic infiltrate | Negative (tested afterwards) | Hydration, broad-spectrum antibiotics and capecitabine interruption (restarted at reduced dose after 5 weeks) | Clinical recovery after CHT discontinuation and supportive therapy. (duration of treatment not reported) |
| Mokrim, 2014 (case 1) [25] | 66 y.o. woman with stage IV breast cancer | Palliative capecitabine | 1250 mg/m2 twice daily | / | Diarrhea, fever, vomit, fatigue | During 2nd cycle | CT: ileal walls thickening. Colonoscopy w/biopsy: eosinophilic infiltrates | Positive for (DPYD *5,6) mutation (tested afterwards) | Broad-spectrum antibiotics, hydration and permanent capecitabine discontinuation | Full recovery after a few days of hydration and antibiotics |
| Mokrim, 2014 (case 2) [25] | 67 y.o. woman with stage IV breast cancer | Palliative capecitabine | N.R. | / | Diarrhea, fever, nausea, fatigue | After 2nd cycle | CT: ileal walls thickening | Negative (tested afterwards) | Broad-spectrum antibiotics, hydration, bowel rest and permanent capecitabine discontinuation | Full recovery after a few days of hydration and antibiotics |
| Lee, 2015 (case 1) [26] | 61 y.o. woman with stage IV colon cancer | Palliative capecitabine/irinotecan/bevacizumab | N.R. | / | Abdominal pain, diarrhea, vomit, G3 neutropenia, hypokalemia | After 4th cycle | CT: submucosal ileal edema, increased fat stranding | N.R. | Broad-spectrum antibiotics, hydration and permanent capecitabine discontinuation | Clinical recovery after 12 days of supportive therapy and dietary modifications |
| Lee, 2015 (case 2) [26] | 59 y.o. woman with pT3N0 sigmoid colon cancer | Adjuvant capecitabine | 2500 mg/m2 d1-14q21 | / | Mucositis, hand-foot syndrome, diarrhea, abdominal pain, febrile neutropenia. | At 1st cycle, worsened after 3rd cycle | CT: submucosal ileal edema with fat stranding, pneumatosis intestinalis | N.R. | Broad-spectrum antibiotics, IV nutrition, electrolyte replacement and capecitabine discontinuation (not reported if restarted) | Clinical recovery after 29 days of supportive therapy and IV nutrition |
| Nicosia, 2017 (case 1) [27] | 71 y.o. woman with cT3N1 lower rectal cancer | Neoadjuvant capecitabine + concurrent pelvic radiotherapy | 825 mg/m2 twice daily | 45 Gy/25 fx to pelvic nodal stations (bilateral common/internal iliac, presacral and obturator) 55 Gy/25 fx to rectum + mesorectum | Abdominal pain, diarrhea, vomit, hand-foot syndrome | After 16th fraction | CT: distal ileal edema with lumen reduction and small bowel distension | N.R. | Broad-spectrum antibiotics, hydration and permanent capecitabine discontinuation | Clinical recovery after 15 days of supportive therapy and antibiotics. Neoadjuvant treatment restarted with sole RT |
| Nicosia, 2017 (case 2) [27] | 54 y.o. woman with cT3N0 lower rectal cancer | Neoadjuvant capecitabine + concurrent pelvic radiotherapy | 825 mg/m2 twice daily | N.R. | Abdominal pain, dehydration, sub-occlusion | 3 days after completion of RT-CHT | CT: ileal walls thickening with bowel loops distension and perivisceral effusion | N.R. | Broad-spectrum antibiotics, bowel rest with IV hydration and nutrition | Clinical recovery after 12 days of supportive therapy and antibiotics |
| Van Hellemond, 2018 [28] | 69 y.o. woman with pT3N2 sigmoid colon cancer | Adjuvant capecitabine/oxaliplatin | N.R. | / | Nausea, appetite reduction, diarrhea and increased CRP | At CHT start | Colonoscopy w/biopsy: terminal ileitis with extensive inflammation. MR enterography; colic distension and thickening of the terminal ileal loop | Negative | Hydration and electrolyte replacement, antidiarrheal therapy, anti-inflammatory therapy and switch to FOLFOX | Clinical recovery with symptomatic therapy (duration of treatment not reported) |
| Dao, 2019 (case 1) [29] | 72 y.o. woman with stage IIIC ascending colon cancer | Adjuvant capecitabine | N.R. | / | Diarrhea and G3 leuko-neutropenia | N.R. | CT: mild ileal loops dilation with vasa recta engorgement and mesenteric edema. Colonoscopy w/biopsy: granular erythematous mucosa and mucosal erosion | N.R. | Hydration and electrolyte replacement, broad-spectrum antibiotics, IV nutrition, anti-inflammatory therapy, antidiarrheal therapy and permanent capecitabine discontinuation | Persistence of symptoms for a total of four weeks after CHT discontinuation and supportive therapy initiation |
| Dao, 2019 (case 2) [29] | 42 y.o. woman with recurrent breast cancer | Palliative capecitabine | N.R. | / | Abdominal pain, fever and bloody diarrhea with anemia and hypokalemia | N.R. | CT: ileal walls thickening and fluid filled bowel loops. Colonoscopy w/biopsy: terminal ileum with diffuse pseudomembranes, inflammatory exudates and spontaneous bleeding | N.R. | Hydration, broad-spectrum antibiotics, antidiarrheal therapy and permanent capecitabine discontinuation | Clinical resolution after four weeks of supportive therapy and antibiotics |
| Klimko, 2021 [30] | 68 y.o. man with locally advanced colon cancer | Adjuvant capecitabine | N.R. | / | Diarrhea, nausea, vomit and malaise | 10 days after CHT start | CT: ileal walls thickening. Colonscopy: large ileal ulcers and erythematous mucosa | (DPYD) *2A heterozygous mutation (tested afterward) | Hydration, antidiarrheal drugs and permanent capecitabine discontinuation | Clinical improvement three days after CHT discontinuation and symptomatic treatment |
| Zou, 2021 [31] | 63 y.o. man with pT4N0 colon cancer | Adjuvant capecitabine/oxaliplatin | 1000 mg/m2 twice daily | / | Bloody diarrhea with anemia and hypovolemic shock, fatigue | After 1st cycle | Colonoscopy: large amount of ileal bloody fluid. CT: submucosal ileal edema and fat stranding | Not performed | Emergency terminal ileal resection and permanent capecitabine discontinuation | Bloody diarrhea resolved after surgery |
| Gomez-Paz, 2022 [32] | 69 y.o. man with colon cancer (stage N.R.) | Adjuvant capecitabine | N.R. | / | Watery diarrhea, pallor, abdominal pain and haematochezia. BTs: severe normocytic anemia, increased WBC count, hypokalemia, high lactate and increased INR | After 3rd cycle | Colonoscopy w/biopsy: erythematous mucosa and non-bleeding ulcerations from terminal ileum to ileo-colonic anastomosis | N.R. | Supportive care and capecitabine discontinuation (not reported if restarted) | Symptoms improvement with supportive care (duration of treatment not reported) |
| Sinha, 2022 [33] | 42 y.o. woman with stage III (pT3N2) sigmoid colon cancer | Adjuvant capecitabine | N.R. | / | Abdominal pain and watery, bloody-tinged diarrhea | 2 days after CHT start | CT: small bowel walls thickening most prominent in ileum with reactive edema. Colonoscopy w/biopsy: erythematous and friable mucosa with ulceration and exudate | Negative | IV antibiotics and permanent capecitabine discontinuation. Switch to different CHT agent (not specified) after discharge | N.R. |
| Shao, 2022 [34] | 68 y.o. man with stage IIIB (pT3N1c) rectal cancer | Adjuvant capecitabine/oxaliplatin (switched to capecitabine monotherapy after 3 cycles due to recurrent G3 thrombocytopenia) | 1500 mg twice daily d1-14q21 | / | G3 diarrhea | During 2nd monotherapy cycle | CT/MRI: ileum and colon walls thickening and edema. Colonoscopy w/biopsy: hyperemia, patchy erosions and scattered ulcers | Variants of DPYD *5, DPYD *9A, TYMP and ABCB1 | Symptomatic treatment and progressive capecitabine dose reduction. CHT discontinuation after 6 monotherapy cycles | Clinical resolution 2 weeks after capecitabine discontinuation |
| Our case | 71 y.o. woman with cT3N2 rectal cancer | Neoadjuvant capecitabine + concurrent pelvic radiotherapy | 825 mg/m2 twice daily | 45 Gy/25 fx to pelvic nodal stations (bilateral internal iliac, presacral and obturator). 50 Gy/25 fx to rectum + mesorectum | Diarrhea, nausea, abdominal pain | After 17th fraction | CT: ileal walls thickening, mucosal hyperemia and vascular enhancement | Negative | Broad-spectrum antibiotics, bowel rest with IV nutrition and hydration, anti-inflammatory therapy, antidiarrheal therapy and permanent radiochemotherapy discontinuation. Capecitabine re-initiated at reduced dose in the post-operative setting | Clinical resolution after 20 days of supportive therapy and RT-CHT discontinuation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brignoli, A.; Ferrara, E.; Zannetti, M.; Loi, G.; Forti, L.; Socci, C.; Carriero, A.; Gennari, A.; Krengli, M.; Franco, P. Capecitabine-Induced Ileitis during Neoadjuvant Pelvic Radio-Chemotherapy for Locally Advanced Rectal Cancer: A Case Report with Literature Review. Curr. Oncol. 2023, 30, 9063-9077. https://doi.org/10.3390/curroncol30100655
Brignoli A, Ferrara E, Zannetti M, Loi G, Forti L, Socci C, Carriero A, Gennari A, Krengli M, Franco P. Capecitabine-Induced Ileitis during Neoadjuvant Pelvic Radio-Chemotherapy for Locally Advanced Rectal Cancer: A Case Report with Literature Review. Current Oncology. 2023; 30(10):9063-9077. https://doi.org/10.3390/curroncol30100655
Chicago/Turabian StyleBrignoli, Andrea, Eleonora Ferrara, Micol Zannetti, Gianfranco Loi, Laura Forti, Carlo Socci, Alessandro Carriero, Alessandra Gennari, Marco Krengli, and Pierfrancesco Franco. 2023. "Capecitabine-Induced Ileitis during Neoadjuvant Pelvic Radio-Chemotherapy for Locally Advanced Rectal Cancer: A Case Report with Literature Review" Current Oncology 30, no. 10: 9063-9077. https://doi.org/10.3390/curroncol30100655
APA StyleBrignoli, A., Ferrara, E., Zannetti, M., Loi, G., Forti, L., Socci, C., Carriero, A., Gennari, A., Krengli, M., & Franco, P. (2023). Capecitabine-Induced Ileitis during Neoadjuvant Pelvic Radio-Chemotherapy for Locally Advanced Rectal Cancer: A Case Report with Literature Review. Current Oncology, 30(10), 9063-9077. https://doi.org/10.3390/curroncol30100655






