Correlation of High-Sensitivity Cardiac Troponin I Values and Cardiac Radiation Doses in Patients with Left-Sided Breast Cancer Undergoing Hypofractionated Adjuvant Radiotherapy with Concurrent Anti-HER2 Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Radiation Therapy
2.3. High-Sensitivity Cardiac Troponin I Analysis
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Anti-HER2 Treatments’ Characteristics
3.3. High-Sensitivity Cardiac Troponin I Values
3.4. Cardiac Doses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| hscTnI | high-sensitivity cardiac troponin I |
| Gy | Gray |
| LN | lymph nodes |
| ROC | receiver operating characteristics |
| RT | radiation therapy |
| DVH | dose volume histograms |
| LAD | left anterior descending artery |
| LV | left ventricle |
| LVEF | left ventricular ejection fraction |
| T-DM1 | trastuzumab emtansine |
| CFRT | conventional fractionated radiotherapy |
| HFRT | hypofractionated radiotherapy |
References
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Sardaro, A.; Petruzzelli, M.F.; D’Errico, M.P.; Grimaldi, L.; Pili, G.; Portaluri, M. Radiation-induced cardiac damage in early left breast cancer patients: Risk factors, biological mechanisms, radiobiology, and dosimetric constraints. Radiother. Oncol. 2012, 103, 133–142. [Google Scholar] [CrossRef]
- Van den Bogaard, V.A.; Ta, B.D.; van der Schaaf, A.; Bouma, A.B.; Middag, A.M.; Bantema-Joppe, E.J.; van Dijk, L.V.; van Dijk-Peters, F.B.; Marteijn, L.A.; de Bock, G.H.; et al. Validation and modification of a prediction model for acute cardiac events in patients with breast cancer treated with radiotherapy based on three-dimensional dose distributions to cardiac substructures. J. Clin. Oncol. 2017, 35, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Wennstig, A.-K.; Garmo, H.; Isacsson, U.; Gagliardi, G.; Rintelä, N.; Lagerqvist, B.; Holmberg, L.; Blomqvist, C.; Sund, M.; Nilsson, G. The relationship between radiation doses to coronary arteries and location of coronary stenosis requiring intervention in breast cancer survivors. Radiat. Oncol. 2019, 14, 40. [Google Scholar] [CrossRef]
- Garg, P.; Morris, P.; Fazlanie, A.L.; Vijayan, S.; Dancso, B.; Dastidar, A.G.; Plein, S.; Mueller, C.; Haaf, P. Cardiac biomarkers of acute coronary syndrome: From history to high-sensitivity cardiac troponin. Intern. Emerg. Med. 2017, 12, 147–155. [Google Scholar] [CrossRef]
- Pudil, R.; Mueller, C.; Čelutkienė, J.; Henriksen, P.A.; Lenihan, D.; Dent, S.; Barac, A.; Stanway, S.; Moslehi, J.; Suter, T.M.; et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: A position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1966–1983. [Google Scholar] [CrossRef]
- Stewart, F.A. Mechanisms and dose-response relationships for radiation-induced cardiovascular disease. Ann. ICRP 2012, 41, 72–79. [Google Scholar] [CrossRef]
- Wang, H.; Wei, J.; Zheng, Q.; Meng, L.; Xin, Y.; Yin, X.; Jiang, X. Radiation-induced heart disease: A review of classification, mechanism and prevention. Int. J. Biol. Sci. 2019, 15, 2128–2138. [Google Scholar] [CrossRef]
- Lv, X.; Pan, C.; Guo, H.; Chang, J.; Gao, X.; Wu, X.; Zhi, X.; Ren, C.; Chen, Q.; Jiang, H.; et al. Early diagnostic value of high-sensitivity cardiac troponin T for cancer treatment-related cardiac dysfunction: A meta-analysis. ESC Heart Fail. 2023, 10, 2170–2182. [Google Scholar] [CrossRef]
- Skyttä, T.; Tuohinen, S.; Boman, E.; Virtanen, V.; Raatikainen, P.; Kellokumpu-Lehtinen, P.L. Troponin T-release associates with cardiac radiation doses during adjuvant left-sided breast cancer radiotherapy. Radiat. Oncol. 2015, 10, 141. [Google Scholar] [CrossRef] [PubMed]
- Italian Cardio-Oncologic Network. Trastuzumab adjuvant chemotherapy and cardiotoxicity in real-world women with breast cancer. J. Card. Fail. 2012, 18, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Colombo, A.; Torrisi, R.; Sandri, M.T.; Civelli, M.; Salvatici, M.; Lamantia, G.; Colombo, N.; Cortinovis, S.; Dessanai, M.A.; et al. Trastuzumab-induced cardiotoxicity: Clinical and prognostic implications of troponin I evaluation. J. Clin. Oncol. 2010, 28, 3910–3916. [Google Scholar] [CrossRef] [PubMed]
- Von Minckwitz, G.; Procter, M.; de Azambuja, E.; Zardavas, D.; Benyunes, M.; Viale, G.; Suter, T.; Arahmani, A.; Rouchet, N.; Clark, E.; et al. Adjuvant pertuzumab and trastuzumab in Early HER2-positive breast cancer. N. Engl. J. Med. 2017, 377, 122–131, Erratum in N. Engl. J. Med. 2017, 377, 702. [Google Scholar] [CrossRef]
- Piccart, M.; Procter, M.; Fumagalli, D.; de Azambuja, E.; Clark, E.; Ewer, M.S.; Restuccia, E.; Jerusalem, G.; Dent, S.; Reaby, L.; et al. Abstract GS1-04: Interim overall survival analysis of APHINITY (BIG 4-11): A randomized, multicenter, double-blind, placebo-controlled trial comparing chemotherapy plus trastuzumab plus pertuzumab vs chemotherapy plus trastuzumab plus placebo as adjuvant therapy in patients with operable HER2-positive early breast cancer. Cancer Res. 2020, 80, GS1-04. [Google Scholar] [CrossRef]
- Alhussein, M.M.; Mokbel, A.; Cosman, T.; Aghel, N.; Yang, E.H.; Mukherjee, S.D.; Dent, S.; Ellis, P.M.; Dhesy-Thind, S.; Leong, D.P. Pertuzumab Cardiotoxicity in Patients with HER2-Positive Cancer: A Systematic Review and Meta-analysis. CJC Open 2021, 3, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- Von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Huang, C.-S.; Mano, M.; Mamounas, T.; Geyer, C.; Untch, M.; von Minckwitz, G.; Thery, J.-C.; Schwaner, I.; Limentani, S.; et al. Adjuvant trastuzumab emtansine (T-DM1) vs. trastuzumab (T) in patients with residual invasive disease after neoadjuvant therapy for HER2+ breast cancer: Subgroup analysis from KATHERINE. Ann. Oncol. 2020, 31 (Suppl. S2), S48. [Google Scholar] [CrossRef]
- Mamounas, E.; Untch, M.; Mano, M.; Huang, C.-S., Jr.; Geyer, C.E., Jr.; von Minckwitz, G.; Wolmark, N.; Pivot, X.; Kuemmel, S.; DiGiovanna, M.; et al. Adjuvant T-DM1 versus trastuzumab in patients with residual invasive disease after neoadjuvant therapy for HER2-positive breast cancer: Subgroup analyses from KATHERINE. Ann. Oncol. 2021, 32, 1005–1014. [Google Scholar] [CrossRef]
- Pondé, N.; Ameye, L.; Lambertini, M.; Paesmans, M.; Piccart, M.; de Azambuja, E. Trastuzumab emtansine (T-DM1)-associated cardiotoxicity: Pooled analysis in advanced HER2-positive breast cancer. Eur. J. Cancer 2020, 126, 65–73. [Google Scholar] [CrossRef]
- Lyon, A.R.; Dent, S.; Stanway, S.; Earl, H.; Brezden-Masley, C.; Cohen-Solal, A.; Tocchetti, C.G.; Moslehi, J.J.; Groarke, J.D.; Bergler-Klein, J.; et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: A position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur. J. Heart Fail. 2020, 22, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- Marinko, T.; Dolenc, J.; Bilban-Jakopin, C. Cardiotoxicity of concomitant radiotherapy and trastuzumab for early breast cancer. Radiol. Oncol. 2014, 48, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Cai, G.; Chang, C.; Yang, Z.-Z.; Feng, Y.; Yu, X.-L.; Ma, J.-L.; Wu, J.; Guo, X.-M.; Chen, J.-Y. Early cardiac toxicity following adjuvant radiotherapy of left-sided breast cancer with or without concurrent trastuzumab. Oncotarget 2016, 7, 1042–1054. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abouegylah, M.; Braunstein, L.Z.; El-Din, M.A.A.; Niemierko, A.; Salama, L.; Elebrashi, M.; Edgington, S.K.; Remillard, K.; Napolitano, B.; Naoum, G.E.; et al. Evaluation of radiation-induced cardiac toxicity in breast cancer patients treated with Trastuzumab-based chemotherapy. Breast Cancer Res. Treat. 2019, 174, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, H.; Sebag, I.A.; Plana, J.C.; Januzzi, J.L.; Ky, B.; Cohen, V.; Gosavi, S.; Carver, J.R.; Wiegers, S.E.; Martin, R.P.; et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am. J. Cardiol. 2011, 107, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Sandri, M.T.; Martinoni, A.; Borghini, E.; Civelli, M.; Lamantia, G.; Cinieri, S.; Martinelli, G.; Fiorentini, C.; Cipolla, C.M. Myocardial injury revealed by plasma troponin I in breast cancer treated with high-dose chemotherapy. Ann. Oncol. 2002, 13, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Iacopo, F.; Cipolla, C.M. Cardiotoxicity of Anthracyclines. Front. Cardiovasc. Med. 2020, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- START Trialists’ Group; Bentzen, S.M.; Agrawal, R.K.; Aird, E.G.A.; Barrett, J.M.; Barrett-Lee, P.J.; Bentzen, S.M.; Bliss, J.M.; Brown, J.; Dewar, J.A.; et al. The UK standardisation of breast radiotherapy (START) trial B of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet 2008, 371, 1098–1107. [Google Scholar] [CrossRef]
- Haviland, J.S.; Owen, J.R.; Dewar, J.A.; Agrawal, R.K.; Barrett, J.; Barrett-Lee, P.J.; Dobbs, H.J.; Hopwood, P.; Lawton, P.A.; Magee, B.J.; et al. The UK standardisation of breast radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013, 14, 1086–1094. [Google Scholar] [CrossRef]
- Whelan, T.J.; Pignol, J.-P.; Levine, M.N.; Julian, J.A.; MacKenzie, R.; Parpia, S.; Shelley, W.; Grimard, L.; Bowen, J.; Lukka, H.; et al. Long-term results of hypofractionated radiation therapy for breast cancer. N. Engl. J. Med. 2010, 362, 513–520. [Google Scholar] [CrossRef]
- Liu, L.; Yang, Y.; Guo, Q.; Ren, B.; Peng, Q.; Zou, L.; Zhu, Y.; Tian, Y. Comparing hypofractionated to conventional fractionated radiotherapy in postmastectomy breast cancer: A meta-analysis and systematic review. Radiat. Oncol. 2020, 15, 17. [Google Scholar] [CrossRef]
- James, M.; Swadi, S.; Yi, M.; Johansson, L.; Robinson, B.; Dixit, A. Ischaemic heart disease following conventional and hypofractionated radiation treatment in a contemporary breast cancer series. J. Med. Imaging Radiat. Oncol. 2018, 62, 425–431. [Google Scholar] [CrossRef]

| All N = 61 | Group 1 N = 17 | Group 2 N = 44 | p-Value | |
|---|---|---|---|---|
| Age (x +/− SD) | 58 ± 11 | 55 ± 12.3 | 59 ± 10.5 | 0.2767 |
| Premenopausal | 16 (26%) | 7 (41%) | 9 (20%) | 0.1018 |
| hscTnI (ng/L) baseline (M,IQR) | 4 (2–7) | 5 (3–7) | 4 (2–7) | 0.8336 |
| Anthracycline use | 30 (49%) | 11 (65%) | 19 (43%) | 0.1811 |
| Time between anthracycline and RT in days (M,IQR) | 208.5 (188–227) | 216 (190–245) | 208 (185–219) | 0.3015 |
| Hormonal therapy | 44 (72%) | 13 (76.5%) | 31 (70%) | 0.1811 |
| Tamoxifen | 11 (25%) | 4 (30.8%) | 7 (22.6%) | 0.7221 |
| AI (anastrozole, letrozole) | 31 (70%) | 8 (61.5%) | 23 (74.2%) | 0.7979 |
| Goserelin + tamoxifen | 2 (5%) | 1 (7.7%) | 1 (3.2%) | 0.5208 |
| Clinical target volume | ||||
| Left breast | 29 (47.5%) | 6 (35.4%) | 23 (52.3%) | 0.6069 |
| Left breast/thoracic wall + lymph nodes | 31 (51%) | 10 (58.8%) | 21 (47.7%) | 0.8099 |
| Both breasts | 1 (1.55%) | 1 (5.8%) | 0 | - |
| Cardiac therapy | 26 (43%) | 8 (47%) | 18 (41%) | 0.6658 |
| ACE inhibitors | 17/61 (28%) | 5/17 (29%) | 12/44 (27%) | 0.8684 |
| Anti-HER2 Therapy | All N = 61 | Group 1 N = 17 | Group 2 N = 44 | p-Value |
|---|---|---|---|---|
| Before radiotherapy | ||||
| Trastuzumab | 17 (28%) | 4 (23%) | 13 (30%) | 1.0000 |
| Trastuzumab/pertuzumab | 30 (49%) | 9 (53%) | 21 (48%) | 1.0000 |
| T-DM1 | 1 (2%) | - | 1 (2%) | - |
| Trastuzumab/pertuzumab T-DM1 | 13 (21%) | 4 (23%) | 9 (20%) | 1.0000 |
| Number of cycles of anti-HER2 therapy before RT (M,IQR) | 7 (5–8) | 7 (5–8.25) | 7 (5.25–8) | 0.9934 |
| During radiotherapy | ||||
| Trastuzumab | 19 (31%) | 4 (23.5%) | 15 (34%) | 0.7665 |
| Trastuzumab/pertuzumab | 28 (46%) | 9 (53%) | 19 (43%) | 0.8024 |
| T-DM1 | 14 (23%) | 4 (23.5%) | 10 (23%) | 1.0000 |
| RT fraction with anti-HER2 therapy application (M,IQR) | 9 (5–12) | 10 (4.75–12) | 8 (5.5–12) | 0.8590 |
| All N = 61 | Group 1 N = 17 | Group 2 N = 44 | p-Value | |
|---|---|---|---|---|
| hscTnI (ng/L) baseline (M,IQR) | 4 (2–7) | 5 (3–7) | 4 (2–7) | 0.8336 |
| hscTnI (ng/L) after RT (M,IQR) | 5 (3–10) | 8 (5–11) | 3 (2–6) | 0.0053 |
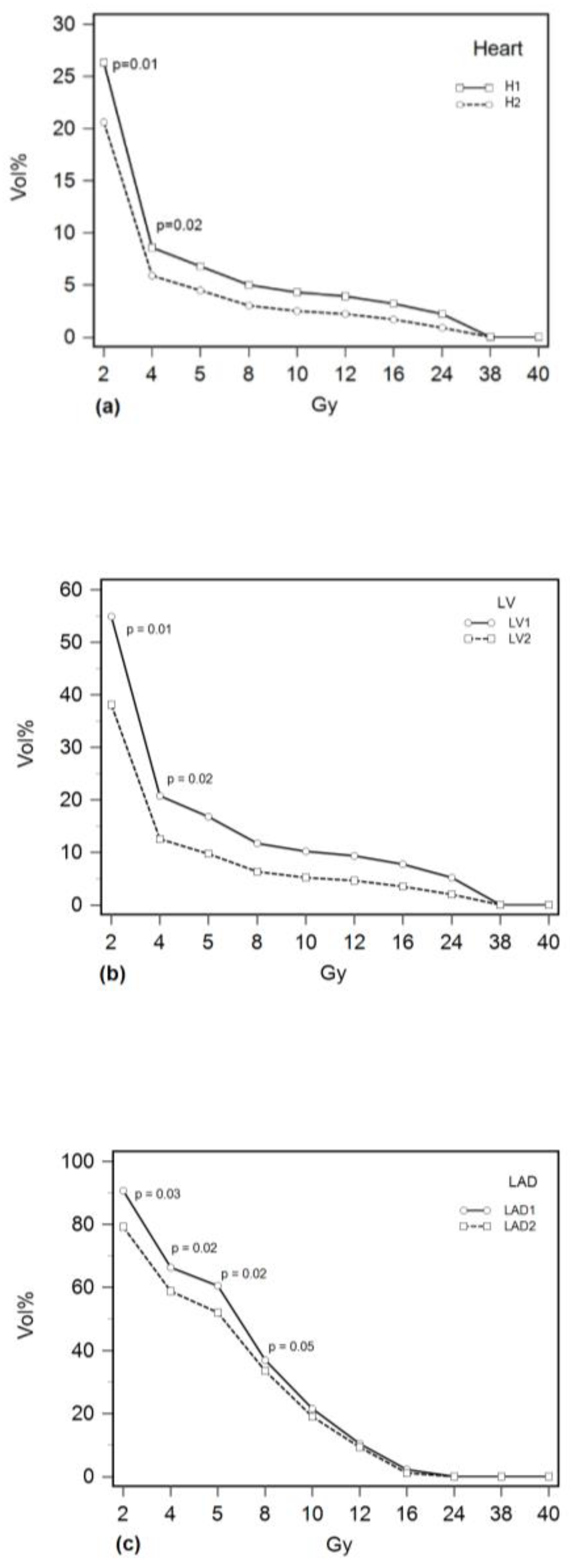
| Cardiac Structure | Group 1 (N = 17) Median (IQR) | Group 2 (N = 44) Median (IQR) | p Mann–Whitney U |
|---|---|---|---|
| Heart | |||
| Dmean (Gy) | 2.4 (1.9–3.9) | 1.8 (1.5–2.5) | 0.02 |
| Dmax (Gy) | 38.3 (35.3–39.6) | 37.4 (33.8–38.6) | 0.22 |
| V2 (%) | 26.3 (21.1–35.2) | 20.5 (18.9–23.6) | 0.01 |
| V4 (%) | 8.6 (6–16) | 5.8 (4.4–8.9) | 0.02 |
| V5 (%) | 6.8 (4–13.8) | 4.5 (3.2–7.1) | 0.07 |
| V8 (%) | 5 (2.3–10.7) | 3 (1.5–5) | 0.08 |
| V10 (%) | 4.3 (1.7–9.5) | 2.5 (1–4.2) | 0.08 |
| V12 (%) | 3.9 (1.3–8.5) | 2.1 (0.8–3.7) | 0.08 |
| V16 (%) | 3.2 (0.8–7) | 1.6 (0.5–3) | 0.09 |
| V24 (%) | 2.2 (0.3–4.4) | 0.8 (0.1–4.7) | 0.10 |
| V38 (%) | 0 (0–0.3) | 0 (0–0) | 0.27 |
| V40 (%) | 0 (0–0.3) | 0 (0–0) | 0.74 |
| AUC (%) | 72.9 (72.1–74.3) | 51.4 (51.4–54.3) | 0.01 |
| LV | |||
| Dmean (Gy) | 4.7 (2.8–6.1) | 2.9 (2.3–4.2) | 0.03 |
| Dmax (Gy) | 38.4 (35.3–39.6) | 37.2 (33.5–38.5) | 0.13 |
| V2 (%) | 54.9 (41.3–62.8) | 34.8 (32.3–48.3) | 0.01 |
| V4 (%) | 20.8 (12.7–27.7) | 12.5 (10.4–16.1) | 0.02 |
| V5 (%) | 16.8 (9–22.8) | 9.7 (6.3–15.6) | 0.06 |
| V8 (%) | 11.7 (5.2–17.5) | 6.3 (3.2–10.8) | 0.08 |
| V10 (%) | 10.2 (3.9–15.7) | 5.2 (2.1–9.2) | 0.08 |
| V12 (%) | 9.3 (3.1–14.4) | 4.6 (16–8.1) | 0.08 |
| V16 (%) | 7.7 (1.8–12.3) | 3.7 (0.8–6.5) | 0.08 |
| V24 (%) | 5.2 (0.7–8.7) | 2 (0.2–4.2) | 0.09 |
| V38 (%) | 0.1 (0–0.6) | 0 (0–0) | 0.03 |
| V40 (%) | 0 (0–0) | 0 (0–0) | 0.8 |
| AUC (%) | 67.8 (67.1–72.9) | 51.4 (51.4–52.9) | 0.01 |
| LAD | |||
| Dmean (Gy) | 6.8 (6.3–19.2) | 6.2 (5.1–6.9) | 0.04 |
| Dmax (Gy) | 22.4 (16–37.8) | 19.1 (17.2–25) | 0.21 |
| V2 (%) | 90.7 (82.2–99.9) | 79.2 (71.8–88.9) | 0.03 |
| V4 (%) | 66.3 (61.8–74.1) | 58.8 (48–67.8) | 0.02 |
| V5 (%) | 60.5 (55.6–70.6) | 52 (41.3–63) | 0.02 |
| V8 (%) | 37 (31.1–55.7) | 33.5 (16.3–38.4) | 0.05 |
| V10 (%) | 21.5 (16.1–53.3) | 19.1 (6.6–23.2) | 0.07 |
| V12 (%) | 10.4 (6.1–51.3) | 9.2 (2.5–16.1) | 0.08 |
| V16 (%) | 2.2 (0–48.2) | 1.1 (0.1–3.5) | 0.24 |
| V24 (%) | 0 (0–44.3) | 0 (0 –0) | 0.16 |
| V38 (%) | 0 (0–0) | 0 (0–0) | 0.19 |
| V40 (%) | 0 (0–0) | 0 (0–0) | 0.54 |
| AUC (%) | 63.2 (58.6–68.6) | 60 (57.1–63.6) | 0.12 |
| Radiation Dose (Gy) | Heart Dose-Volume Constraint (%) | LV Dose-Volume Constraint (%) | LAD Dose-Volume Constraint (%) |
|---|---|---|---|
| 2 | >19.7 | >38.7 | >86.4 |
| 4 | >10.2 | >12.1 | >59.6 |
| 5 | >8.6 | >14.5 | >52.4 |
| 8 | >4.7 | >10 | >16.7 |
| 10 | >3.9 | >8.2 | >7.1 |
| 16 | >2.6 | >7.3 | >1.6 |
| 38 | >0.1 | >0.1 | >0 |
| 40 | >0.1 | >0.2 | ≤0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antunac, K.; Mayer, L.; Banovic, M.; Beketic-Oreskovic, L. Correlation of High-Sensitivity Cardiac Troponin I Values and Cardiac Radiation Doses in Patients with Left-Sided Breast Cancer Undergoing Hypofractionated Adjuvant Radiotherapy with Concurrent Anti-HER2 Therapy. Curr. Oncol. 2023, 30, 9049-9062. https://doi.org/10.3390/curroncol30100654
Antunac K, Mayer L, Banovic M, Beketic-Oreskovic L. Correlation of High-Sensitivity Cardiac Troponin I Values and Cardiac Radiation Doses in Patients with Left-Sided Breast Cancer Undergoing Hypofractionated Adjuvant Radiotherapy with Concurrent Anti-HER2 Therapy. Current Oncology. 2023; 30(10):9049-9062. https://doi.org/10.3390/curroncol30100654
Chicago/Turabian StyleAntunac, Katarina, Ljiljana Mayer, Marija Banovic, and Lidija Beketic-Oreskovic. 2023. "Correlation of High-Sensitivity Cardiac Troponin I Values and Cardiac Radiation Doses in Patients with Left-Sided Breast Cancer Undergoing Hypofractionated Adjuvant Radiotherapy with Concurrent Anti-HER2 Therapy" Current Oncology 30, no. 10: 9049-9062. https://doi.org/10.3390/curroncol30100654
APA StyleAntunac, K., Mayer, L., Banovic, M., & Beketic-Oreskovic, L. (2023). Correlation of High-Sensitivity Cardiac Troponin I Values and Cardiac Radiation Doses in Patients with Left-Sided Breast Cancer Undergoing Hypofractionated Adjuvant Radiotherapy with Concurrent Anti-HER2 Therapy. Current Oncology, 30(10), 9049-9062. https://doi.org/10.3390/curroncol30100654





