Do Histology and Primary Tumor Location Influence Metastatic Patterns in Bladder Cancer?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Data Extraction
2.2. Definition of Organotropic Metastasis Rate
2.3. Statistical Analysis
3. Results
3.1. Comparison of Metastatic Behavior in Different Histological Types of Bladder Cancer
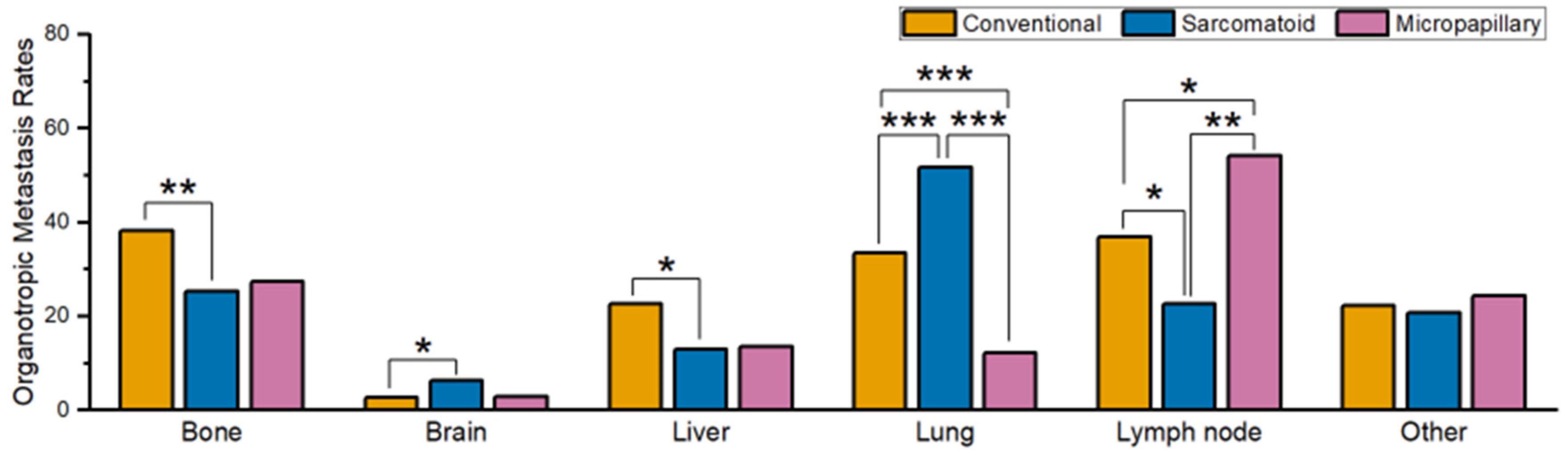
3.2. Comparison of Metastatic Behavior in Different Histological Subtypes of Bladder Primary UC
3.3. Comparison of Metastatic Behavior in Patients with Conventional UC of Bladder, Ureter, and Renal Pelvis Primary
3.4. Comparison of Metastatic Behavior between Conventional UC Patients Originating from Different Regions of the Bladder
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Aveta, A.; Cacciapuoti, C.; Barone, B.; Di Zazzo, E.; Del Giudice, F.; Maggi, M.; Ferro, M.; Terracciano, D.; Busetto, G.M.; Lucarelli, G.; et al. The Impact of Meat Intake on Bladder Cancer Incidence: Is It Really a Relevant Risk? Cancers 2022, 14, 4775. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, V.; Chin, J.L.; Izawa, J.I. Histologic Variants of Urothelial Bladder Cancer and Nonurothelial Histology in Bladder Cancer. Can. Urol. Assoc. J. 2009, 3, S193–S198. [Google Scholar] [CrossRef] [PubMed]
- Flammia, R.S.; Tufano, A.; Chierigo, F.; Würnschimmel, C.; Hoeh, B.; Sorce, G.; Tian, Z.; Anceschi, U.; Leonardo, C.; Del Giudice, F.; et al. The Effect of Sex on Disease Stage and Survival after Radical Cystectomy in Non-Urothelial Variant-Histology Bladder Cancer. J. Clin. Med. 2023, 12, 1776. [Google Scholar] [CrossRef]
- Hu, X.; Xue, Y.; Zhu, G. Clinical Characteristics and Current Status of Treatment for Recurrent Bladder Cancer after Surgeries on Upper Tract Urothelial Carcinoma. Diagnostics 2023, 13, 1004. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. Urinary and Male Genital Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2022; Volume 8. [Google Scholar]
- Chung, J.-H.; Lee, C.-U.; Lee, D.-H.; Song, W. Expression and Prognostic Implication of PD-L1 in Patients with Urothelial Carcinoma with Variant Histology (Squamous Differentiation or Micropapillary) Undergoing Radical Cystectomy. Biomedicines 2022, 10, 910. [Google Scholar] [CrossRef]
- Ogbue, O.; Haddad, A.; Almassi, N.; Lapinski, J.; Daw, H. Overview of Histologic Variants of Urothelial Carcinoma: Current Trends and Narrative Review on Treatment Outcomes. Transl. Androl. Urol. 2022, 11, 877–901. [Google Scholar] [CrossRef]
- Mori, K.; Abufaraj, M.; Mostafaei, H.; Quhal, F.; Karakiewicz, P.I.; Briganti, A.; Kimura, S.; Egawa, S.; Shariat, S.F. A Systematic Review and Meta-Analysis of Variant Histology in Urothelial Carcinoma of the Bladder Treated with Radical Cystectomy. J. Urol. 2020, 204, 1129–1140. [Google Scholar] [CrossRef]
- Alvarado-Cabrero, I.; Sierra-Santiesteban, F.I.; Mantilla-Morales, A.; Hernández-Hernandez, D.M. Micropapillary Carcinoma of the Urothelial Tract: A Clinicopathologic Study of 38 Cases. Ann. Diagn. Pathol. 2005, 9, 1–5. [Google Scholar] [CrossRef]
- Dobruch, J.; Oszczudłowski, M. Bladder Cancer: Current Challenges and Future Directions. Medicina 2021, 57, 749. [Google Scholar] [CrossRef] [PubMed]
- The Surveillance, Epidemiology, and End Results (SEER) Program. Cancer Stat Facts: Bladder Cancer. Available online: https://seer.cancer.gov/statfacts/html/urinb.html (accessed on 4 September 2023).
- Shinagare, A.B.; Ramaiya, N.H.; Jagannathan, J.P.; Fennessy, F.M.; Taplin, M.E.; Van Den Abbeele, A.D. Metastatic Pattern of Bladder Cancer: Correlation with the Characteristics of the Primary Tumor. Am. J. Roentgenol. 2011, 196, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.; Hasnain, Z.; Miranda, G.; Gill, K.; Djaladat, H.; Desai, M.; Newton, P.K.; Gill, I.S.; Kuhn, P. Prediction of Metastatic Patterns in Bladder Cancer: Spatiotemporal Progression and Development of a Novel, Web-Based Platform for Clinical Utility. Eur. Urol. Open Sci. 2021, 32, 8–18. [Google Scholar] [CrossRef]
- Shou, J.; Zhang, Q.; Zhang, D. The Prognostic Effect of Metastasis Patterns on Overall Survival in Patients with Distant Metastatic Bladder Cancer: A SEER Population-Based Analysis. World J. Urol. 2021, 39, 4151–4158. [Google Scholar] [CrossRef]
- Yoo, Y.; Lee, J.; Park, H.S.; Cho, M.S.; Sung, S.H.; Park, S.; Choi, E. Histologically Confirmed Distant Metastatic Urothelial Carcinoma from the Urinary Bladder: A Retrospective Review of One Institution’s 20-Year Experience. J. Pathol. Transl. Med. 2021, 55, 94–101. [Google Scholar] [CrossRef]
- Tufano, A.; Perdonà, S.; Viscuso, P.; Frisenda, M.; Canale, V.; Rossi, A.; Del Prete, P.; Passaro, F.; Calarco, A. The Impact of Ethnicity and Age on Distribution of Metastases in Patients with Upper Tract Urothelial Carcinoma: Analysis of SEER Data. Biomedicines 2023, 11, 1943. [Google Scholar] [CrossRef] [PubMed]
- Hammouz, R.Y.; Kołat, D.; Kałuzińska, Ż.; Płuciennik, E.; Bednarek, A.K. MicroRNAs: Their Role in Metastasis, Angiogenesis, and the Potential for Biomarker Utility in Bladder Carcinomas. Cancers 2021, 13, 891. [Google Scholar] [CrossRef]
- Nikic, P.; Dragicevic, D.; Jerotic, D.; Savic, S.; Djukic, T.; Stankovic, B.; Kovacevic, L.; Simic, T.; Matic, M. Polymorphisms of Antioxidant Enzymes SOD2 (Rs4880) and GPX1 (Rs1050450) Are Associated with Bladder Cancer Risk or Its Aggressiveness. Medicina 2023, 59, 131. [Google Scholar] [CrossRef]
- Park, H.K.; Han, J.; Kwon, G.Y.; Yeo, M.-K.; Bae, G.E. Patterns of Extrathoracic Metastasis in Lung Cancer Patients. Curr. Oncol. 2022, 29, 8794–8801. [Google Scholar] [CrossRef]
- Park, H.K.; Kwon, G.Y. Comparison of Metastatic Patterns Among Neuroendocrine Tumors, Neuroendocrine Carcinomas, and Nonneuroendocrine Carcinomas of Various Primary Organs. J. Korean Med. Sci. 2023, 38, e85. [Google Scholar] [CrossRef]
- Barletta, F.; Tappero, S.; Panunzio, A.; Incesu, R.-B.; Cano Garcia, C.; Piccinelli, M.L.; Tian, Z.; Gandaglia, G.; Moschini, M.; Terrone, C.; et al. Differences in Cancer-Specific Mortality after Trimodal Therapy for T2N0M0 Bladder Cancer According to Histological Subtype. Cancers 2022, 14, 5766. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, A.; Ferrara, F.; Lasala, R.; Zovi, A. Precision Medicine in the Treatment of Locally Advanced or Metastatic Urothelial Cancer: New Molecular Targets and Pharmacological Therapies. Cancers 2022, 14, 5167. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Mohammed, Y.; Basiony, M.; Hanbazazh, M.; Samman, A.; Abdelaleem, M.F.; Nasr, M.; Abozeid, H.; Mohamed, H.I.; Faisal, M.; et al. Clinico-Pathological Features and Immunohistochemical Comparison of P16, P53, and Ki-67 Expression in Muscle-Invasive and Non-Muscle-Invasive Conventional Urothelial Bladder Carcinoma. Clin. Pract. 2023, 13, 806–819. [Google Scholar] [CrossRef] [PubMed]
- Blanca, A.; Lopez-Beltran, A.; Lopez-Porcheron, K.; Gomez-Gomez, E.; Cimadamore, A.; Bilé-Silva, A.; Gogna, R.; Montironi, R.; Cheng, L. Risk Classification of Bladder Cancer by Gene Expression and Molecular Subtype. Cancers 2023, 15, 2149. [Google Scholar] [CrossRef] [PubMed]
- Green, D.A.; Rink, M.; Xylinas, E.; Matin, S.F.; Stenzl, A.; Roupret, M.; Karakiewicz, P.I.; Scherr, D.S.; Shariat, S.F. Urothelial Carcinoma of the Bladder and the Upper Tract: Disparate Twins. J. Urol. 2013, 189, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Bilim, V.; Kuroki, H.; Shirono, Y.; Murata, M.; Hiruma, K.; Tomita, Y. Advanced Bladder Cancer: Changing the Treatment Landscape. J. Pers. Med. 2022, 12, 1745. [Google Scholar] [CrossRef]
- Evmorfopoulos, K.; Mitrakas, L.; Karathanasis, A.; Zachos, I.; Tzortzis, V.; Vlachostergios, P.J. Upper Tract Urothelial Carcinoma: A Rare Malignancy with Distinct Immuno-Genomic Features in the Era of Precision-Based Therapies. Biomedicines 2023, 11, 1775. [Google Scholar] [CrossRef]
- Aveta, A.; Cilio, S.; Contieri, R.; Spena, G.; Napolitano, L.; Manfredi, C.; Franco, A.; Crocerossa, F.; Cerrato, C.; Ferro, M.; et al. Urinary MicroRNAs as Biomarkers of Urological Cancers: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 10846. [Google Scholar] [CrossRef]
- Surveillance Research Program, National Cancer Institute. SEER*Stat Software, Version 8.4.2; National Cancer Institute: Bethesda, MD, USA, 2023. [Google Scholar]
- Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence—SEER Research Data, 17 Registries, Nov 2022 Sub (2000–2020)—Linked To County Attributes—Time Dependent (1990–2021) Income/Rurality, 1969–2021 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Released April 2023, Based on the November 2022 Submission. Available online: www.seer.cancer.gov (accessed on 23 July 2023).
- Dickie, L.; Johnson, C.H.; Adams, S.; Negoita, S. 2023 Solid Tumor Rules. Available online: https://seer.cancer.gov/tools/solidtumor/ (accessed on 27 August 2023).
- Park, H.K. Neuroendocrine Carcinomas of the Uterine Cervix, Endometrium, and Ovary Show Higher Tendencies for Bone, Brain, and Liver Organotrophic Metastases. Curr. Oncol. 2022, 29, 7461–7469. [Google Scholar] [CrossRef]
- Kong, J.; Diao, X.; Diao, F.; Fan, X.; Zheng, J.; Yan, D.; Huang, J.; Qin, H.; Lin, T. Causes of Death in Long-Term Bladder Cancer Survivors: A Population-Based Study. Asia Pac. J. Clin. Oncol. 2019, 15, e167–e174. [Google Scholar] [CrossRef]
- Guan, T.; Su, M.; Luo, Z.; Peng, W.; Zhou, R.; Lu, Z.; Feng, M.; Li, W.; Teng, Y.; Jiang, Y.; et al. Long-Term Cardiovascular Mortality among 80,042 Older Patients with Bladder Cancer. Cancers 2022, 14, 4572. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Nishimura, N.; Iida, K.; Fujii, T.; Nishikawa, R.; Teraoka, S.; Takenaka, A.; Kikuchi, H.; Abe, T.; Shinohara, N.; et al. Intravesical Bacillus Calmette–Guérin Treatment for T1 High-Grade Non-Muscle Invasive Bladder Cancer with Divergent Differentiation or Variant Morphologies. Cancers 2021, 13, 2615. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, F.W.; LaGrange, C.A.; Hemstreet III, G.P.; Kessinger, A. Clinical Features of Sarcomatoid Carcinoma (Carcinosarcoma) of the Urinary Bladder: Analysis of 221 Cases. Sarcoma 2010, 2010, 454792. [Google Scholar] [CrossRef] [PubMed]
- McQuitty, E.; Ro, J.Y.; Truong, L.D.; Shen, S.S.; Zhai, Q.; Ayala, A.G. Lymphovascular Invasion in Micropapillary Urothelial Carcinoma: A Study of 22 Cases. Arch. Pathol. Lab. Med. 2012, 136, 635–639. [Google Scholar] [CrossRef] [PubMed]
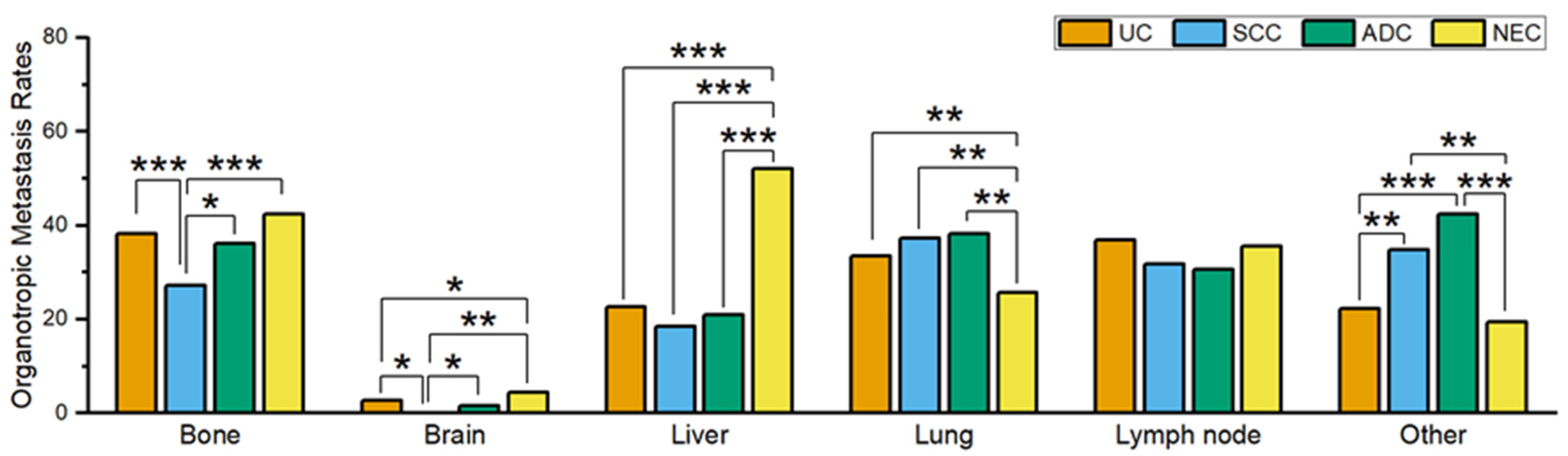


| UC | SCC | ADC | NEC | |
|---|---|---|---|---|
| Overall Metastasis Rates (Pts with metastasis/total number of Pts) | ||||
| 8.8% (4317/48,789) | 15.7% (262/1667) | 18.4% (185/1003) | 26.0% (438/1683) | |
| Organotropic Metastasis Rates (Pts with metastasis to the indicated organ/Pts with metastasis) | ||||
| Bone | 38.3% (1608/4194) | 27.2% (68/250) | 36.1% (65/180) | 42.3% (180/426) |
| Brain | 2.6% (109/4175) | 0% (0/251) | 1.7% (3/174) | 4.5% (19/422) |
| Liver | 22.6% (948/4190) | 18.4% (46/250) | 20.9% (37/177) | 52.1% (223/428) |
| Lung | 33.5% (1399/4181) | 37.2% (93/250) | 38.3% (67/175) | 25.7% (109/424) |
| LN | 36.8% (792/2153) | 31.8% (42/132) | 30.6% (30/98) | 35.5% (78/220) |
| Other | 22.2% (481/2166) | 34.8% (46/132) | 42.3% (41/97) | 19.5% (43/220) |
| Conventional UC | Sarcomatoid UC | Micropapillary UC | |
|---|---|---|---|
| Overall Metastasis Rates (Pts with metastasis/total number of Pts) | |||
| 8.8% (4317/48,789) | 14.4% (97/675) | 10.5% (66/627) | |
| Organotropic Metastasis Rates (Pts with metastasis to the indicated organ/Pts with metastasis) | |||
| Bone | 38.3% (1608/4194) | 25.3% (24/95) | 27.3% (18/66) |
| Brain | 2.6% (109/4175) | 6.3% (6/95) | 3.0% (2/66) |
| Liver | 22.6% (948/4190) | 12.9% (12/93) | 13.6% (9/66) |
| Lung | 33.5% (1399/4181) | 51.6% (47/91) | 12.1% (8/66) |
| LN | 36.8% (792/2153) | 22.6% (12/53) | 54.1% (20/37) |
| Other | 22.2% (481/2166) | 20.8% (11/53) | 24.3% (9/37) |
| Renal Pelvis | Ureter | Bladder | |
|---|---|---|---|
| Overall Metastasis Rates (Patients with metastasis/total number of patients) | |||
| 28.8% (1438/4993) | 15.6% (414/2659) | 8.8% (4317/48,789) | |
| Organotropic Metastasis Rates (Patients with metastasis to the indicated organ/Patients with metastasis) | |||
| Bone | 37.3% (522/1401) | 34.4% (139/404) | 38.3% (1608/4194) |
| Brain | 3.1% (43/1383) | 1.8% (7/398) | 2.6% (109/4175) |
| Liver | 35.0% (491/1404) | 35.8% (144/402) | 22.6% (948/4190) |
| Lung | 50.5% (705/1396) | 36.7% (148/403) | 33.5% (1399/4181) |
| Lymph node | 30.0% (207/689) | 30.6% (63/206) | 36.8% (792/2153) |
| Other | 23.6% (164/696) | 33.0% (69/209) | 22.2% (481/2166) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.K. Do Histology and Primary Tumor Location Influence Metastatic Patterns in Bladder Cancer? Curr. Oncol. 2023, 30, 9078-9089. https://doi.org/10.3390/curroncol30100656
Park HK. Do Histology and Primary Tumor Location Influence Metastatic Patterns in Bladder Cancer? Current Oncology. 2023; 30(10):9078-9089. https://doi.org/10.3390/curroncol30100656
Chicago/Turabian StylePark, Hyung Kyu. 2023. "Do Histology and Primary Tumor Location Influence Metastatic Patterns in Bladder Cancer?" Current Oncology 30, no. 10: 9078-9089. https://doi.org/10.3390/curroncol30100656
APA StylePark, H. K. (2023). Do Histology and Primary Tumor Location Influence Metastatic Patterns in Bladder Cancer? Current Oncology, 30(10), 9078-9089. https://doi.org/10.3390/curroncol30100656





