CD5-Negative Primary Mantle Cell Lymphoma Presenting with a Bilateral Conjunctival Mass: A Potential Diagnostic Pitfall
Abstract
1. Introduction
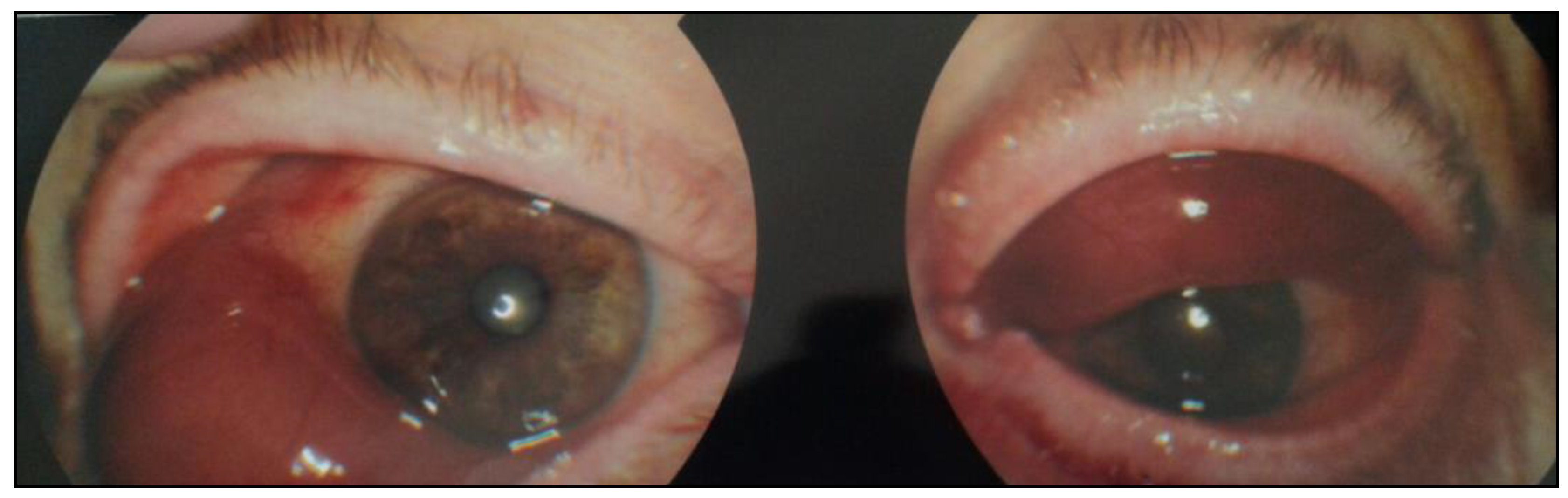
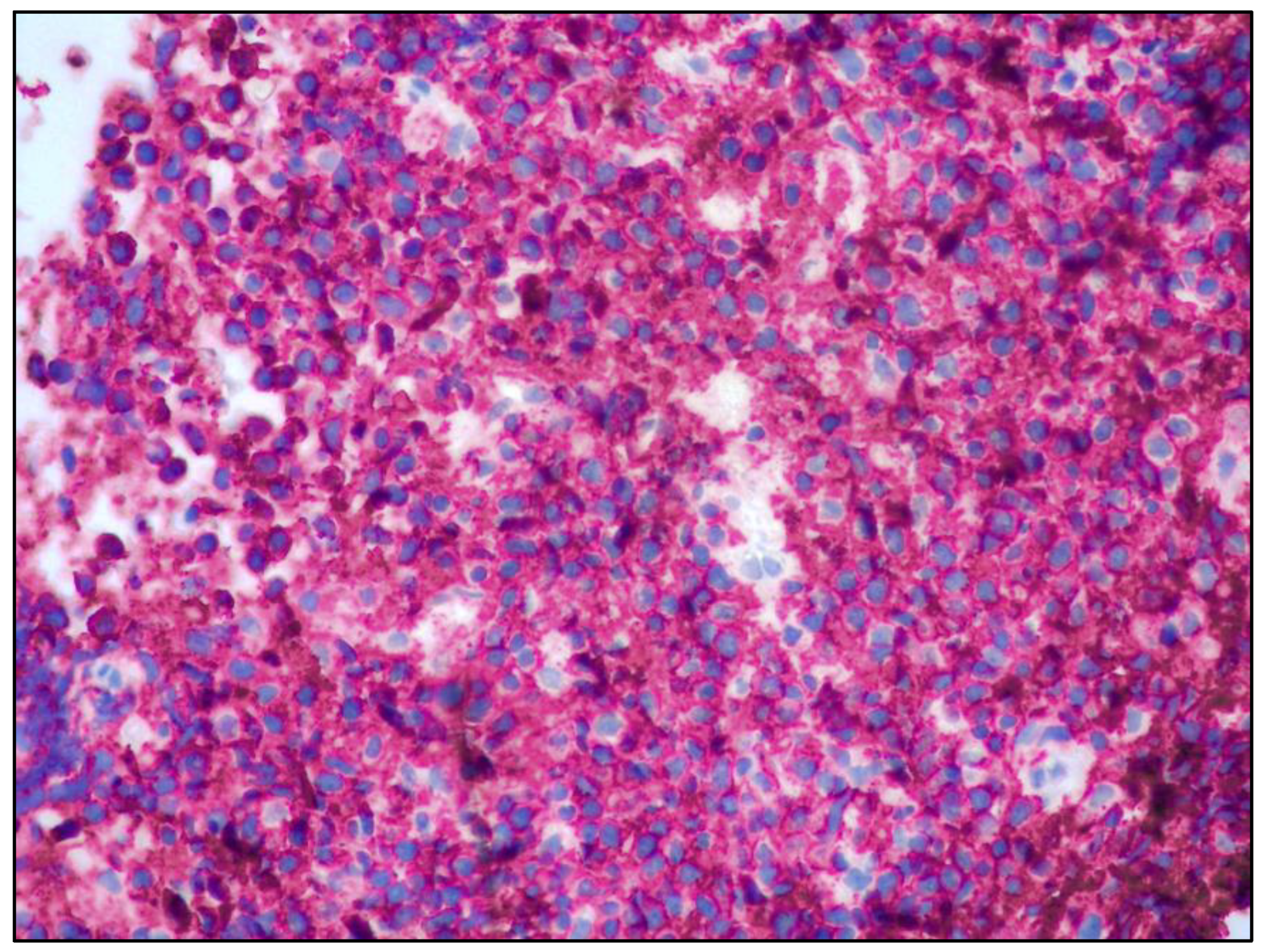
2. Case History
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Otter, R.; Gerrits, W.B.; vd Sandt, M.M.; Hermans, J.; Willemze, R. Primary extranodal and nodal non-Hodgkin’s lymphoma. A survey of a population-based registry. Eur. J. Cancer Clin. Oncol. 1989, 25, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Knowles, D.M.; Jakobiec, F.A.; McNally, L.; Burke, J.S. Lymphoid hyperplasia and malignant lymphoma occurring in the ocular adnexa (orbit, conjunctiva, and eyelids): A prospective multiparametric analysis of 108 cases during 1977–1987. Hum. Pathol. 1990, 21, 959–973. [Google Scholar] [CrossRef] [PubMed]
- Bairey, O.; Kremer, I.; Rakowsky, E.; Hadar, H.; Shaklai, M. Orbital and adnexal involvement in systemic non-Hodgkin’s lymphoma. Cancer 1994, 73, 2395–2399. [Google Scholar] [CrossRef]
- Wotherspoon, A.C.; Diss, T.C.; Pan, L.X.; Schmid, C.; Kerr-Muir, M.G.; Lea, S.H.; Isaacson, P.G. Primary low-grade B-cell lymphoma of the conjunctiva: A mucosa-associated lymphoid tissue type lymphoma. Histopathology 1993, 23, 417–424. [Google Scholar] [CrossRef]
- Smedby, K.E.; Ponzoni, M. The aetiology of B-cell lymphoid malignancies with a focus on chronic inflammation and infections. J. Intern. Med. 2017, 282, 360–370. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Haematopoietic and Lymphoid Tissues, Revised 4th ed.; WHO Classification of Tumours Editorial Board, Ed.; IARC: Lyon, France, 2017. [Google Scholar]
- Maddocks, K. Update on mantle cell lymphoma. Blood 2018, 132, 1647–1656. [Google Scholar] [CrossRef]
- Morello, L.; Rattotti, S.; Giordano, L.; Jerkeman, M.; van Meerten, T.; Krawczyk, K.; Moita, F.; Marino, D.; Ferrero, S.; Szymczyk, M.; et al. Mantle cell lymphoma of mucosa-associated lymphoid tissue: A European mantle cell lymphoma network study. Hemasphere 2020, 4, 1. [Google Scholar] [CrossRef]
- Looi, A.; Gascoyne, R.D.; Chhanabhai, M.; Connors, J.M.; Rootman, J.; White, V.A. Mantle cell lymphoma in the ocular adnexal region. Ophthalmology 2005, 112, 114–119. [Google Scholar] [CrossRef]
- Aspiotis, M.; Gorezis, S.; Asproudis, I.; Tsanou, E.; Papadiotis, E.; Kamina, S.; Agnantis, N.J.; Bai, M. Primary mantle cell lymphoma of the conjunctiva: A case report. Virchows Arch. 2006, 449, 472–475. [Google Scholar] [CrossRef]
- Yoo, S.B.; Kim, Y.A.; Jeon, Y.K.; Kim, C.W. CD5-undetected by immunohistochemistry, t(11;14)(q13;q32)-positive conjunctival mantle cell lymphoma: A case report. Pathol. Res. Pract. 2008, 204, 779–783. [Google Scholar] [CrossRef]
- Rasmussen, P.; Sjo, L.D.; Prause, J.U.; Ralfklaer, E.; Heegard, S. Mantle cell lymphoma in the orbital and adnexal region. Br. J. Ophthalmol. 2009, 93, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Khanlari, M.; Bagheri, B.; Vojdani, R.; Mohammadianpanath, M.; Paydar, S.; Daneshbod, Y. Conjunctival mass as an initial presentation of mantle cell lymphoma: A case report. BMC Res. Notes 2012, 5, 671. [Google Scholar] [CrossRef] [PubMed]
- Sahu, K.K.; Uthamalingam, P.; Sampath, S.; Jinagal, J.; Das, A.; Prakash, G.; Malhotra, P.; Varma, S.C. Ocular adnexal lymphomas: Report of 2 cases of mantle cell lymphomas and short review of the literature. Indian J. Hematol. Blood Transf. 2014, 30, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Rasic, D.M.; Knezevic, M.; Terzic, T.; Vlajkovic, G. Bilateral ocular paraadnexal mass as initial presentation of systemic blastoid variant of mantle-cell lymphoma. Surv. Ophthalmol. 2017, 62, 83–88. [Google Scholar] [CrossRef]
- Zhang, X.; Gordon, K.B.; Zaldivar, R.A.; Rajjoub, L.Z. mantle cell lymphoma: Conjunctival mass in a female patient. Can. J. Ophthalmol. 2018, 53, 109–110. [Google Scholar] [CrossRef]
- Kirkegaard, M.K. Ocular adnexal lymphoma: Subtype-specific clinical and genetic features. Acta Ophthalmol. 2022, 100, 3–37. [Google Scholar] [CrossRef]
- Melli, B.; Gentile, P.; Nicoli, D.; Farnetti, E.; Croci, S.; Gozzi, F.; Bolletta, E.; De Simone, L.; Sanguedolce, F.; Palicelli, A.; et al. Primary vitreoretinal lymphoma: Current diagnostic laboratory tests and new emerging molecular tools. Curr. Oncol. 2022, 29, 6908–6921. [Google Scholar] [CrossRef]
- Salaverria, I.; Royo, C.; Carvajal-Cuenca, A.; Clot, G.; Navarro, A.; Valera, A.; Song, J.Y.; Woroniecka, R.; Rymkiewicz, G.; Klapper, W.; et al. CCND2 rearrangements are the most frequent genetic events in cyclin D1(-) mantle cell lymphoma. Blood 2013, 121, 1394–1402. [Google Scholar] [CrossRef]
- Olsen, T.G.; Holm, F.; Mikkelsen, L.H.; Rasmussen, P.K.; Coupland, S.E.; Esmaeli, B.; Finger, P.T.; Graue, G.F.; Grossniklaus, H.E.; Honavar, S.G.; et al. Orbital lymphoma. An international multicenter retrospective study. Am. J. Ophthalmol. 2019, 199, 44–57. [Google Scholar] [CrossRef]
- Vest, S.D.; Mikkelsen, L.H.; Holm, F.; Rasmussen, P.K.; Hindso, T.G.; Knudsen, M.K.H.; Coupland, S.E.; Esmaeli, B.; Dito, P.T.; Graue, G.F.; et al. Lymphoma of the lacrimal gland. An international multicenter retrospective study. Am. J. Ophthalmol. 2020, 219, 107–120. [Google Scholar] [CrossRef]
- Jenkins, C.; Rose, G.E.; Bunce, C.; Wright, J.E.; Cree, I.A.; Plowman, N.; Lightman, S.; Moseley, I.; Norton, A. Histological features of ocular adnexal lymphoma (REAL classification) and their association with patient morbidity and survival. Br. J. Ophthalmol. 2000, 84, 907–913. [Google Scholar] [CrossRef]
- Mansoor, A.; Akbari, M.; Auer, I.; Lai, R. Cyclin D1 and t(11;14)-positive B-cell neoplasms resembling marginal zone B-cell lymphoma: A morphological variant of mantle cell lymphoma. Hum. Pathol. 2007, 38, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Ponzoni, M.; Govi, S.; Licata, G.; Mappa, S.; Resti, A.G.; Politi, L.S.; Spagnuolo, L.; Di Cairano, E.; Doglioni, C.; Ferreri, A.J.M. A reapparisal of the diagnostic and therapeutic management of uncommon histologies of primary ocular adnexal lymphoma. Oncologist 2013, 18, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Ge, J.; Sun, Z.; Nan, F.; Wan, W.; Xu, D.; Zhang, M.; Fu, X. CD5-positive marginal zone lymphoma: Clinicopathological features and survival outcomes. Leuk. Res. 2022, 117, 106840. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Lin, P.; Saksena, A.; Xu, J.; Wang, M.; Romaguera, J.; Yin, C.C.; Medeiros, L.J.; Li, S. CD5-negative mantle cell lymphoma: Clinicopathologic correlations and outcome in 58 patients. Am. J. Surg. Pathol. 2019, 43, 1052–1060. [Google Scholar] [CrossRef]
- Ferry, J.A.; Fung, C.Y.; Zukenberg, L.; Lucarelli, M.J.; Hasserjian, R.P.; Preffer, F.I.; Harris, N.L. Lymphoma of the ocular adnexa: A study of 353 cases. Am. J. Surg. Pathol. 2007, 31, 170–184. [Google Scholar] [CrossRef]
- Jain, P.; Wang, M.L. Mantle cell lymphoma in 2022-A comprehensive update on molecular pathogenesis, risk stratification, clinical approach and current and novel treatments. Am. J. Hematol. 2022, 97, 638–656. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanelli, M.; Lugli, A.; Palicelli, A.; Sanguedolce, F.; Zizzo, M.; Cresta, C.; Biancafarina, S.; Martino, G.; Crescenzi, B.; Pancetti, S.; et al. CD5-Negative Primary Mantle Cell Lymphoma Presenting with a Bilateral Conjunctival Mass: A Potential Diagnostic Pitfall. Curr. Oncol. 2023, 30, 824-831. https://doi.org/10.3390/curroncol30010062
Zanelli M, Lugli A, Palicelli A, Sanguedolce F, Zizzo M, Cresta C, Biancafarina S, Martino G, Crescenzi B, Pancetti S, et al. CD5-Negative Primary Mantle Cell Lymphoma Presenting with a Bilateral Conjunctival Mass: A Potential Diagnostic Pitfall. Current Oncology. 2023; 30(1):824-831. https://doi.org/10.3390/curroncol30010062
Chicago/Turabian StyleZanelli, Magda, Alberto Lugli, Andrea Palicelli, Francesca Sanguedolce, Maurizio Zizzo, Camilla Cresta, Samuele Biancafarina, Giovanni Martino, Barbara Crescenzi, Saverio Pancetti, and et al. 2023. "CD5-Negative Primary Mantle Cell Lymphoma Presenting with a Bilateral Conjunctival Mass: A Potential Diagnostic Pitfall" Current Oncology 30, no. 1: 824-831. https://doi.org/10.3390/curroncol30010062
APA StyleZanelli, M., Lugli, A., Palicelli, A., Sanguedolce, F., Zizzo, M., Cresta, C., Biancafarina, S., Martino, G., Crescenzi, B., Pancetti, S., Broggi, G., Caltabiano, R., Cimino, L., Mecucci, C., & Ascani, S. (2023). CD5-Negative Primary Mantle Cell Lymphoma Presenting with a Bilateral Conjunctival Mass: A Potential Diagnostic Pitfall. Current Oncology, 30(1), 824-831. https://doi.org/10.3390/curroncol30010062









