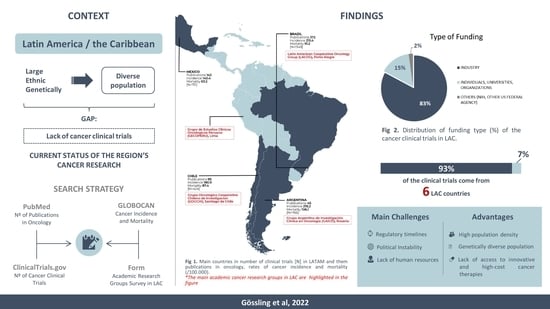
Current Scenario of Clinical Cancer Research in Latin America and the Caribbean
Abstract
1. Introduction
2. Current Status of Cancer Clinical Trials in Latin America and the Caribbean
3. Search Strategy and Selection Criteria
4. Academic Cancer Research Groups and Investigator-Initiated Research in Latin America
5. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Agency for Research on Cancer—GLOBOCAN 2020. Available online: https://gco.iarc.fr/ (accessed on 30 March 2021).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer; World Health Organization. Cancer Tomorrow; Global Cancer Observatory: Lyon, France, 2021. [Google Scholar]
- Kocarnik, J.M.; Compton, K.; Dean, F.E.; Fu, W.; Gaw, B.L.; Harvey, J.D.; Henrikson, H.J.; Lu, D.; Pennini, A.; Xu, R.; et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disabil-ity-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022, 8, 420–444. [Google Scholar] [PubMed]
- Anampa-Guzmán, A.; Acevedo, F.; Partridge, A.H.; Alfano, C.M.; Nekhlyudov, L. Cancer Survivorship in Latin America: Current Status and Opportunities. JCO Glob. Oncol. 2021, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.H.; Werutsky, G.; Mohar, A.; Ferrigno, A.S.; Müller, B.G.; Bychkovsky, B.L.; Uribe, C.J.; Villarreal-Garza, C.; Soto-Perez-de-Celis, E.; Gutiérrez-Delgado, F.; et al. Cancer control in Latin America and the Caribbean: Recent advances and opportunities to move forward. Lancet Oncol. 2021, 22, e474–e487. [Google Scholar] [CrossRef]
- Pramesh, C.S.; Badwe, R.A.; Bhoo-Pathy, N.; Booth, C.M.; Chinnaswamy, G.; Dare, A.J.; de Andrade, V.P.; Hunter, D.J.; Gopal, S.; Gospodarowicz, M.; et al. Priorities for cancer research in low- and middle-income countries: A global perspective. Nat. Med. 2022, 28, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Werutsky, G.; Barrios, C.H.; Cardona, A.F.; Albergaria, A.; Valencia, A.; Ferreira, C.G.; Rolfo, C.; de Azambuja, E.; A Rabinovich, G.; Sposetti, G.; et al. Perspectives on emerging technologies, personalised medicine, and clinical research for cancer control in Latin America and the Caribbean. Lancet Oncol. 2021, 22, e488–e500. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, C.; Blacklock, C.; Perera, R.; Davis, R.; Banerjee, A.; Gill, P.; Liew, S.M.; Chamas, L.; Hernandez, J.; Mahtani, K.; et al. Evidence for non-communicable diseases: Analysis of Cochrane reviews and randomised trials by World Bank classification. BMJ Open 2013, 3, e003298. [Google Scholar] [CrossRef]
- Wells, J.C.; Sharma, S.; Del Paggio, J.C.; Hopman, W.M.; Gyawali, B.; Mukherji, D.; Hammad, N.; Pramesh, C.S.; Aggarwal, A.; Sullivan, R.; et al. An Analysis of Contemporary Oncology Randomized Clinical Trials From Low/Middle-Income vs High-Income Countries. JAMA Oncol. 2021, 7, 379–385. [Google Scholar] [CrossRef]
- Rolfo, C.; Caglevic, C.; Bretel, D.; Hong, D.; Raez, L.E.; Cardona, A.F.; Oton, A.B.; Gomez, H.; Dafni, U.; Vallejos, C.; et al. Cancer clinical research in Latin America: Current situation and opportunities. Expert opinion from the first ESMO workshop on clinical trials, Lima, 2015. ESMO Open 2016, 1, e000055. [Google Scholar] [CrossRef]
- Van Hemelrijck, M.; Lewison, G.; Fox, L.; Vanderpuye, V.D.; Murillo, R.; Booth, C.M.; Canfell, K.; Pramesh, C.; Sullivan, R.; Mukherij, D. Global cancer research in the era of COVID-19: A bibliometric analysis. Ecancermedicalscience 2021, 15. [Google Scholar] [CrossRef]
- Booth, C.M.; Cescon, D.W.; Wang, L.; Tannock, I.F.; Krzyzanowska, M.K. Evolution of the Randomized Controlled Trial in Oncology Over Three Decades. J. Clin. Oncol. 2008, 26, 5458–5464. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.; Higgins, J.; Day, A.G.; Meyer, R.M.; Booth, C.M. Randomized controlled trials in the era of molecular oncology: Methodology, biomarkers, and end points. Ann. Oncol. 2011, 23, 1646–1651. [Google Scholar] [CrossRef] [PubMed]
- Seruga, B.; Hertz, P.C.; Wang, L.; Booth, C.M.; Cescon, D.W.; Krzyzanowska, M.; Tannock, I.F. Absolute benefits of medical therapies in phase III clinical trials for breast and colorectal cancer. Ann. Oncol. 2009, 21, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Fundytus, A.; Sengar, M.; Lombe, D.; Hopman, W.; Jalink, M.; Gyawali, B.; Trapani, D.; Roitberg, F.; De Vries, E.G.E.; Moja, L.; et al. Access to cancer medicine in low-resource settings. Lancet Oncol. 2013, 14, 1. [Google Scholar]
- UNESCO Institute for Statistics. Research and Development Expenditure (% of GDP)—Latin America & Caribbean; UNESCO Institute for Statistics: Montreal, QC, Canada, 2021. [Google Scholar]
- Mathew, A. Global Survey of Clinical Oncology Workforce. J. Glob. Oncol. 2018, 4, 1–12. [Google Scholar] [CrossRef]
- Portich, J.P.; Giacomazzi, J.; Morelle, A.M.; Rosa, D.D.; Rossari, J.R.; Azambuja, E.D. Brazilian medical oncologists: Current and future perspectives for 2020. Braz. J. Oncol. 2017, 13, 1–8. [Google Scholar]
- Moloney, A. Latin America faces hurdles in health research. Lancet 2009, 374, 1053–1054. [Google Scholar] [CrossRef]
- Ciocca, D.R.; Delgado, G. The reality of scientific research in Latin America; an insider’s perspective. Cell Stress Chaperons 2017, 22, 847–852. [Google Scholar] [CrossRef]
- CONACYT. Datos Abiertos de México—Sistema Nacional de Investigadores; CONACYT: Mexico City, Mexico, 2018.
- Shamash, K. Article Processing Charges (APCs) and Subscriptions; JISC: Bristol, UK, 2016. [Google Scholar]
- Gómez, H.L.; Pinto, J.A.; Castañeda, C.; Vallejos, C.S. Current barriers for developing clinical research in Latin America: A cross-sectional survey of medical oncologists. Clin. Res. Trials 2015, 1, 22–28. [Google Scholar] [CrossRef]
- Medicine, U.N.L.o. Clinical Trials Platform. 2022. Available online: https://clinicaltrials.gov/ (accessed on 28 October 2022).
- World Bank. Countries in Latin America & Caribbean. Available online: https://wits.worldbank.org/chatbot/SearchItem.aspx?RegionId=LCN (accessed on 28 October 2022).
- The U.S. National Institutes of Health and the National Library of Medicine, Clinicaltrials. Gov identifier: Nct01291329. Available online: http://clinicaltrials.gov/ct2/home (accessed on 22 August 2012).
- National Library of Medicine. Available online: https://pubmed.ncbi.nlm.nih.gov (accessed on 1 December 2021).
- Silva, R.E.; Amato, A.A.; Guilhem, D.B.; Carvalho, M.R.; Novaes, M.R. International Clinical Trials in Latin American and Caribbean Countries: Research and Development to Meet Local Health Needs. Front. Pharmacol. 2017, 8, 961. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vello, P.; Smith, E.; Elias, V.; Florez-Pinzon, C.; Reveiz, L. Adherence to clinical trial registration in countries of Latin America and the Caribbean, 2015. Rev. Panam. Salud Pública 2018, 42, e44. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Clinical Trials Registry Platform; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Calderon-Aparicio, A.; Orue, A. Precision oncology in Latin America: Current situation, challenges and perspectives. Ecancermedicalscience 2019, 13, 920. [Google Scholar] [CrossRef] [PubMed]
- Lab, S. SJR—International Science Ranking. 2021. Available online: https://www.scimagojr.com/countryrank.php (accessed on 28 October 2022).
- Konwar, M.; Bose, D.; Gogtay, N.J.; Thatte, U.M. Investigator-initiated studies: Challenges and solutions. Perspect. Clin. Res. 2018, 9, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Saúde, M.d. Programa Nacional de Apoio à Atenção Oncológica (PRONON). 2022. Available online: https://www.gov.br/saude/pt-br/acesso-a-informacao/acoes-e-programas/pronon-e-pronas-pcd (accessed on 28 October 2022).
- Piñeros, M.; Laversanne, M.; Barrios, E.; de Camargo Cancela, M.; de Vries, E.; Pardo, C.; Bray, F. An updated profile of the cancer burden, patterns and trends in Latin America and the Caribbean. Lancet Reg. Health-Am. 2022, 13, 100294. [Google Scholar] [CrossRef]
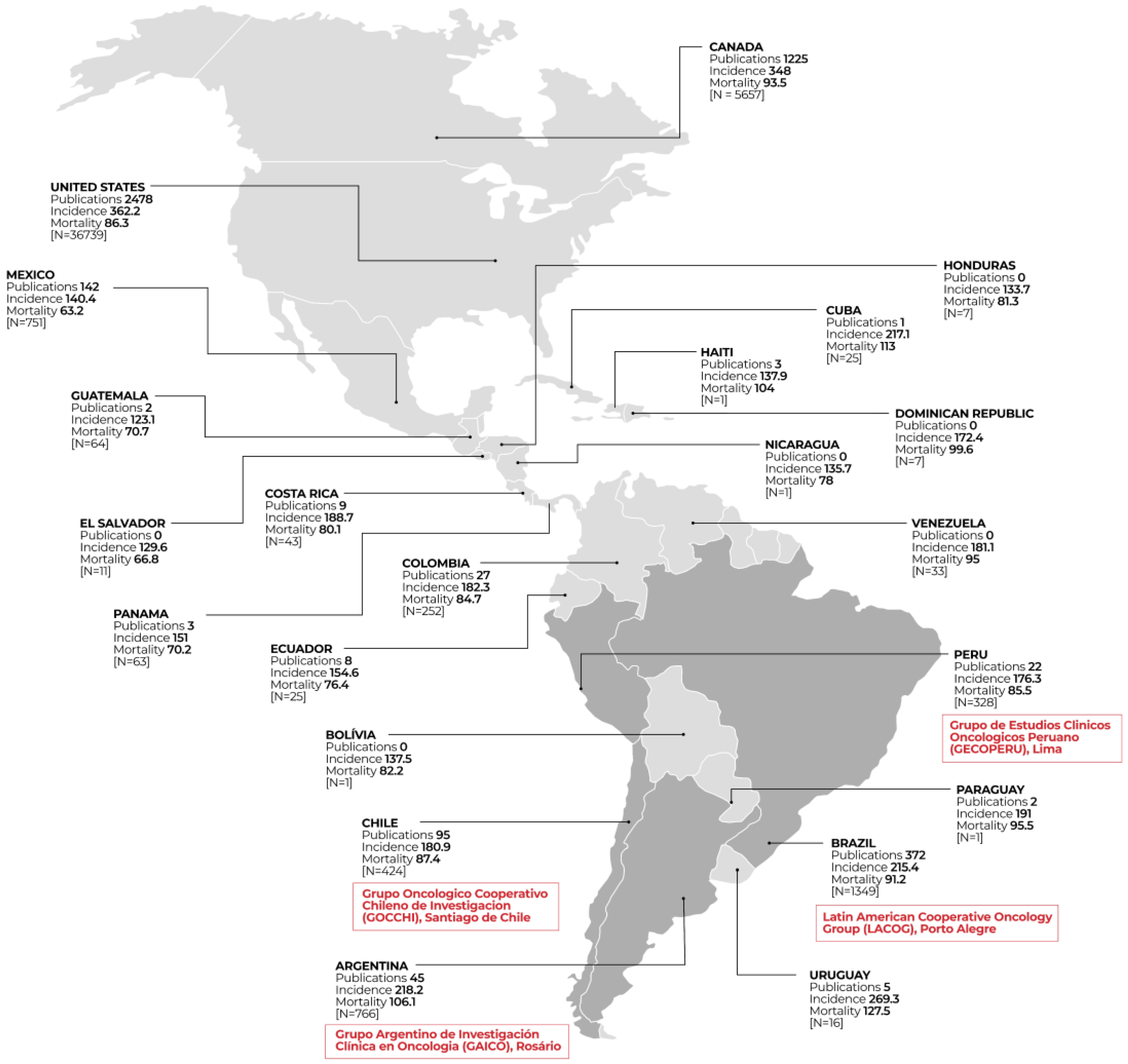
| Country | Clinical Trials | Study Phase | Status | Funder Type | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early Phase 1/Phase I | II | III | IV | Completed | Not Yet Recruiting/Recruiting /by Invitation/Active Not Recruiting | Industry | All Others (Individuals, Universities, Organizations) | Others (Nih, Other US Federal Agencies) | |||||||||
| Brazil | 1349 | 63 | 5.2% | 350 | 28.7% | 759 | 62.3% | 47 | 3.9% | 605 | 529 | 964 | 71.2% | 371 | 27.4% | 18 | 1.3% |
| Argentina | 766 | 42 | 5.3% | 190 | 24.1% | 536 | 68.1% | 19 | 2.4% | 368 | 319 | 719 | 93.7% | 42 | 5.5% | 6 | 0.8% |
| Mexico | 751 | 31 | 4.2% | 176 | 23.8% | 505 | 68.4% | 26 | 3.5% | 329 | 307 | 627 | 83.3% | 117 | 15.5% | 9 | 1.2% |
| Chile | 424 | 27 | 6.3% | 89 | 20.6% | 302 | 70.1% | 13 | 3.0% | 180 | 211 | 386 | 90.6% | 35 | 8.2% | 5 | 1.2% |
| Peru | 328 | 9 | 2.8% | 79 | 24.5% | 227 | 70.3% | 8 | 2.5% | 188 | 104 | 277 | 84.2% | 18 | 5.5% | 34 | 10.3% |
| Colombia | 252 | 7 | 2.9% | 36 | 15.0% | 189 | 78.8% | 8 | 3.3% | 124 | 111 | 224 | 88.9% | 22 | 8.7% | 6 | 2.4% |
| Guatemala | 64 | 0 | 0.0% | 10 | 16.1% | 49 | 79.0% | 3 | 4.8% | 28 | 29 | 61 | 95.3% | 2 | 3.1% | 1 | 1.6% |
| Panama | 63 | 2 | 3.1% | 11 | 17.2% | 49 | 76.6% | 2 | 3.1% | 40 | 13 | 60 | 95.2% | 3 | 4.8% | 0 | 0.0% |
| Costa rica | 43 | 0 | 0.0% | 7 | 15.9% | 34 | 77.3% | 3 | 6.8% | 13 | 29 | 40 | 93.0% | 0 | 0.0% | 3 | 7.0% |
| Venezuela | 33 | 0 | 0.0% | 3 | 9.7% | 22 | 71.0% | 6 | 19.4% | 24 | 2 | 30 | 90.9% | 2 | 6.1% | 1 | 3.0% |
| Cuba | 25 | 0 | 0.0% | 17 | 63.0% | 10 | 37.0% | 0 | 0.0% | 12 | 7 | 19 | 76.0% | 6 | 24.0% | 0 | 0.0% |
| Ecuador | 25 | 1 | 5.6% | 1 | 5.6% | 13 | 72.2% | 3 | 16.7% | 18 | 5 | 18 | 72.0% | 7 | 28.0% | 0 | 0.0% |
| Uruguay | 16 | 0 | 0.0% | 2 | 12.5% | 12 | 75.0% | 2 | 12.5% | 14 | 0 | 14 | 87.5% | 2 | 12.5% | 0 | 0.0% |
| El salvador | 11 | 0 | 0.0% | 2 | 25.0% | 6 | 75.0% | 0 | 0.0% | 7 | 4 | 8 | 72.7% | 0 | 0.0% | 3 | 27.3% |
| Honduras | 7 | 0 | 0.0% | 2 | 33.3% | 4 | 66.7% | 0 | 0.0% | 5 | 2 | 5 | 62.5% | 1 | 12.5% | 2 | 25.0% |
| Dominican Republic | 7 | 0 | 0.0% | 0 | 0.0% | 4 | 100.0% | 0 | 0.0% | 6 | 1 | 6 | 85.7% | 0 | 0.0% | 1 | 14.3% |
| Bolívia | 1 | 1 | 50.0% | 1 | 50.0% | 0 | 0.0% | 0 | 0.0% | 1 | 0 | 1 | 100.0% | 0 | 0.0% | 0 | 0.0% |
| Nicaragua | 1 | 0 | 0.0% | 0 | 0.0% | 1 | 100.0% | 0 | 0.0% | 0 | 0 | 0 | 0.0% | 1 | 100.0% | 0 | 0.0% |
| Paraguay | 1 | 0 | 0.0% | 0 | 0.0% | 1 | 100.0% | 0 | 0.0% | 1 | 0 | 1 | 100.0% | 0 | 0.0% | 0 | 0.0% |
| Haiti | 1 | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0 | 0 | 0.0% | 1 | 100.0% | 0 | 0.0% |
| TOTAL | 4168 | ||||||||||||||||
| Variable | Group (n) |
|---|---|
| Epidemiological Studies | |
| Ongoing | GAICO (4); GOCCHI (1); Gecoperu (6); LACOG (14) |
| Completed | GAICO (0); GOCCHI (0); Gecoperu (4); LACOG (9) |
| Investigator-initiated | GAICO (4); GOCCHI (0); Gecoperu (4); LACOG (21) |
| Sponsored by industries | GAICO (4); GOCCHI (1); Gecoperu (6); LACOG (21) |
| Intergroup contributions | GAICO (4); GOCCHI (1); Gecoperu (2); LACOG (3) |
| Clinical Trials | Group (n) |
| Ongoing/Completed | GAICO (11); GOCCHI (20); Gecoperu (4); LACOG (20) |
| Investigator-initiated | GAICO (0); GOCCHI (5); Gecoperu (0); LACOG (13) |
| Sponsored by industries | GAICO (11); GOCCHI (3); Gecoperu (3); LACOG (4) |
| Intergroup contributions | GAICO (7); GOCCHI (12); Gecoperu (1); LACOG (1) |
| Infrastructure of Clinical Research | Description (%) |
| Areas in oncology | Breast (100%), Radiation (50%), Kidney (50%), Lung (75%), Colon (25%), Pancreatobiliary (25%), Upper GI (Esophageal or Gastric) (25%), Cervical (50%), Prostate (25%), Ovarian (25%), Lymphoma (25%), Head and Neck (25%) |
| Process executed (without subcontracting third parties) | Ethical Process (100%), Regulatory Process (100%, Pharmacovigilance (75%), Monitoring activities and Data Management (100%), Site contractualization (100%), Site Payments (100%), CRF development (75%), Statistics (25%) |
| Positions applied to employees | Project Manager (100%), DM and Monitor (100%), CRA (100%), Medical Team (100%), Legal Assistant (50%), IT Support (75%), Statistician (25%) |
| Challenges, Advantages, and Opportunities for Clinical Research | Description |
| Main challenges | Regulatory timelines, political instability, population trust in the pharmaceutical industry, disparity in access to standard therapies, costs, lack of human resources, cultural diversity, lack of protected time for clinical investigators, competing for industry-driven trials |
| Competitive advantages/opportunities | Availability of trained health professionals, high level of trust from patients (high willingness to participate in clinical trials), relative lack of language barriers (continent with only two dominant languages), genetically diverse population, lack of access to innovative or high-cost therapies, high population density (and increased ease of access to investigator and patients), availability of health professionals, large market, presence of rare diseases |
| Actions that could further reinforce | Aim to develop academic trials including prevalent tumors of underdeveloped countries such as cervix cancer, support for independent academic research to address questions that are meaningful for the local population (i.e., de-escalating strategies, cost-effective treatments) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gössling, G.; Rebelatto, T.F.; Villarreal-Garza, C.; Ferrigno, A.S.; Bretel, D.; Sala, R.; Giacomazzi, J.; William, W.N., Jr.; Werutsky, G. Current Scenario of Clinical Cancer Research in Latin America and the Caribbean. Curr. Oncol. 2023, 30, 653-662. https://doi.org/10.3390/curroncol30010050
Gössling G, Rebelatto TF, Villarreal-Garza C, Ferrigno AS, Bretel D, Sala R, Giacomazzi J, William WN Jr., Werutsky G. Current Scenario of Clinical Cancer Research in Latin America and the Caribbean. Current Oncology. 2023; 30(1):653-662. https://doi.org/10.3390/curroncol30010050
Chicago/Turabian StyleGössling, Gustavo, Taiane F. Rebelatto, Cynthia Villarreal-Garza, Ana S. Ferrigno, Denisse Bretel, Raul Sala, Juliana Giacomazzi, William N. William, Jr., and Gustavo Werutsky. 2023. "Current Scenario of Clinical Cancer Research in Latin America and the Caribbean" Current Oncology 30, no. 1: 653-662. https://doi.org/10.3390/curroncol30010050
APA StyleGössling, G., Rebelatto, T. F., Villarreal-Garza, C., Ferrigno, A. S., Bretel, D., Sala, R., Giacomazzi, J., William, W. N., Jr., & Werutsky, G. (2023). Current Scenario of Clinical Cancer Research in Latin America and the Caribbean. Current Oncology, 30(1), 653-662. https://doi.org/10.3390/curroncol30010050