Neoadjuvant Chemo-Radiation Using IGRT in Patients with Locally Advanced Gastric Cancer
Abstract
1. Introduction
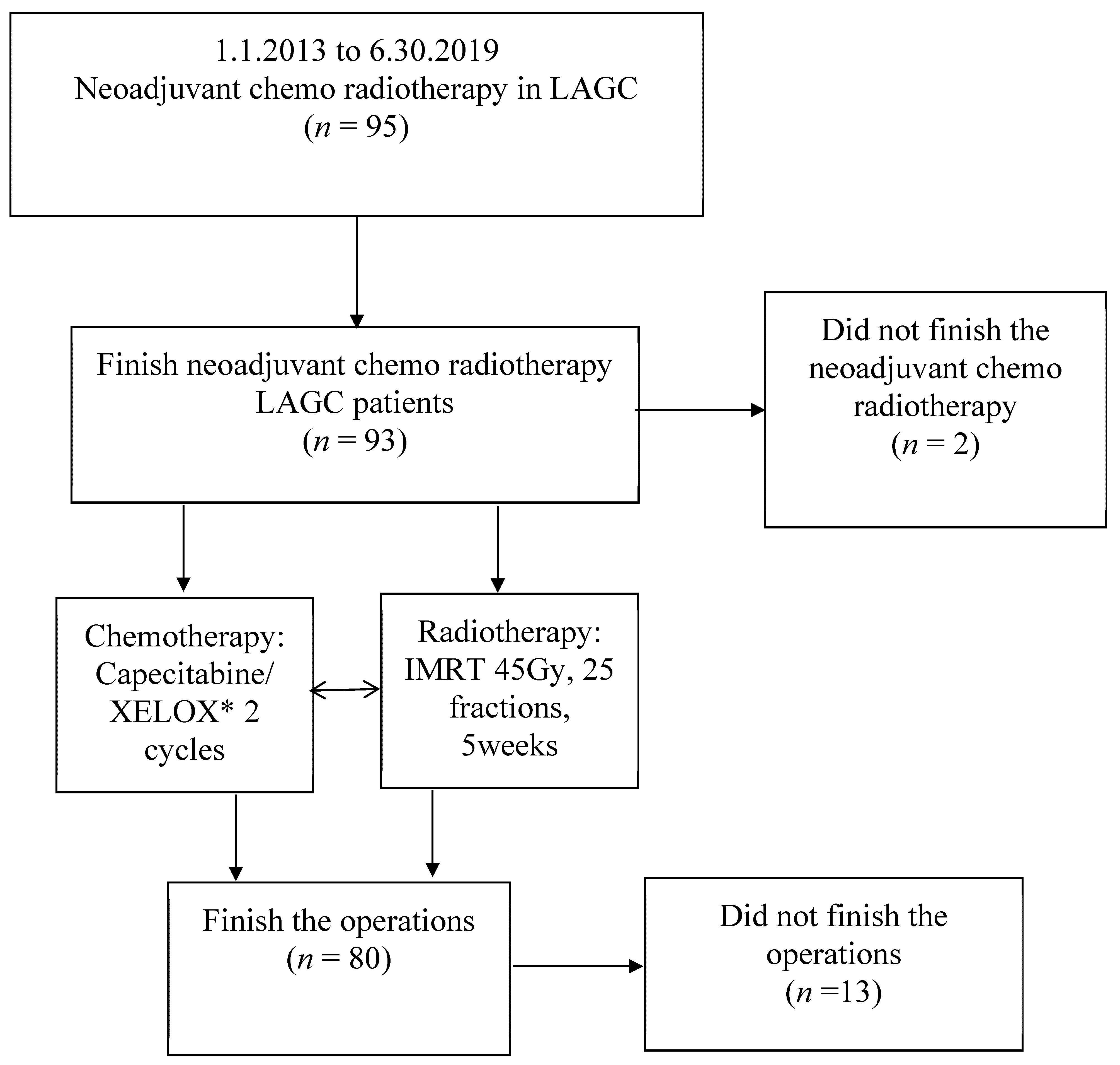
2. Patients and Methods
2.1. Patients
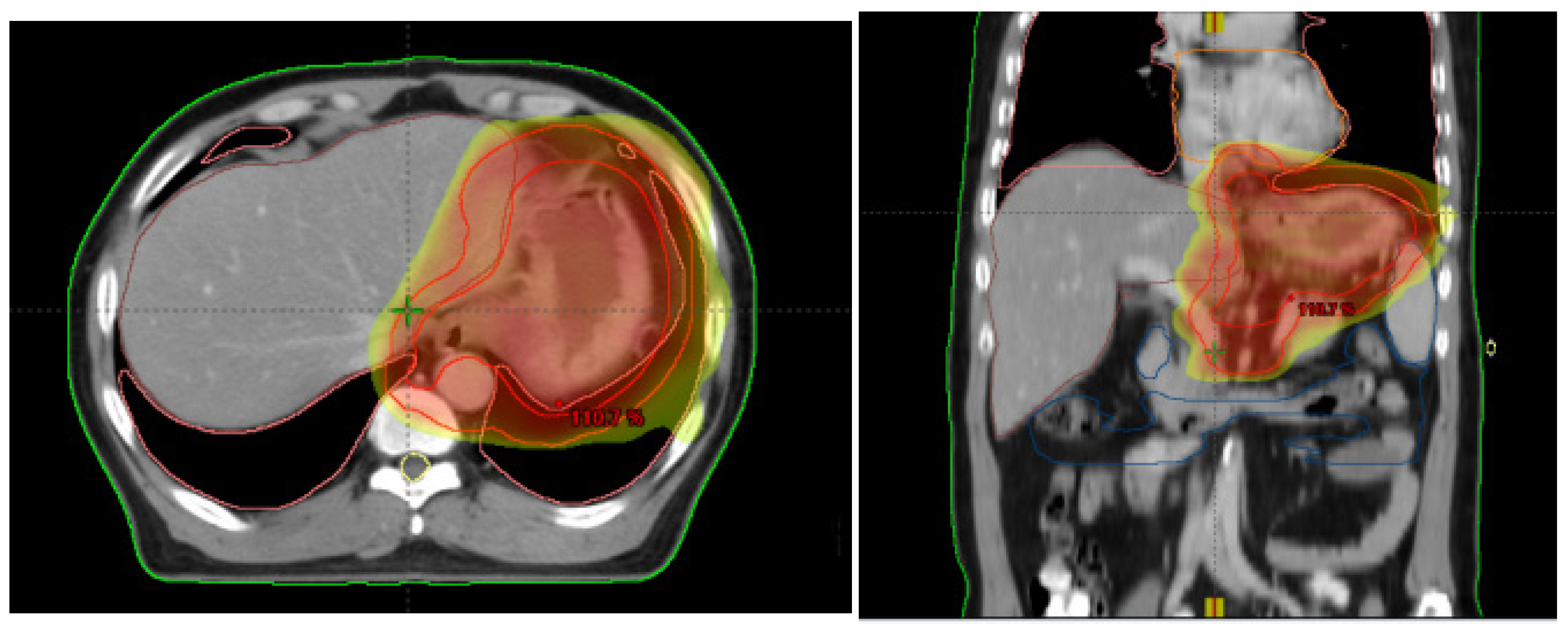
2.2. Radiotherapy
2.3. Chemotherapy
2.4. Surgery
2.5. Tumor Response and Toxicity Criteria
2.6. Follow-Up and Evaluation of Toxicities
2.7. Statistics
3. Results
3.1. Patients’ Characteristics
3.2. Treatment and Acute Toxicity
3.3. Surgery and Postoperative Complications
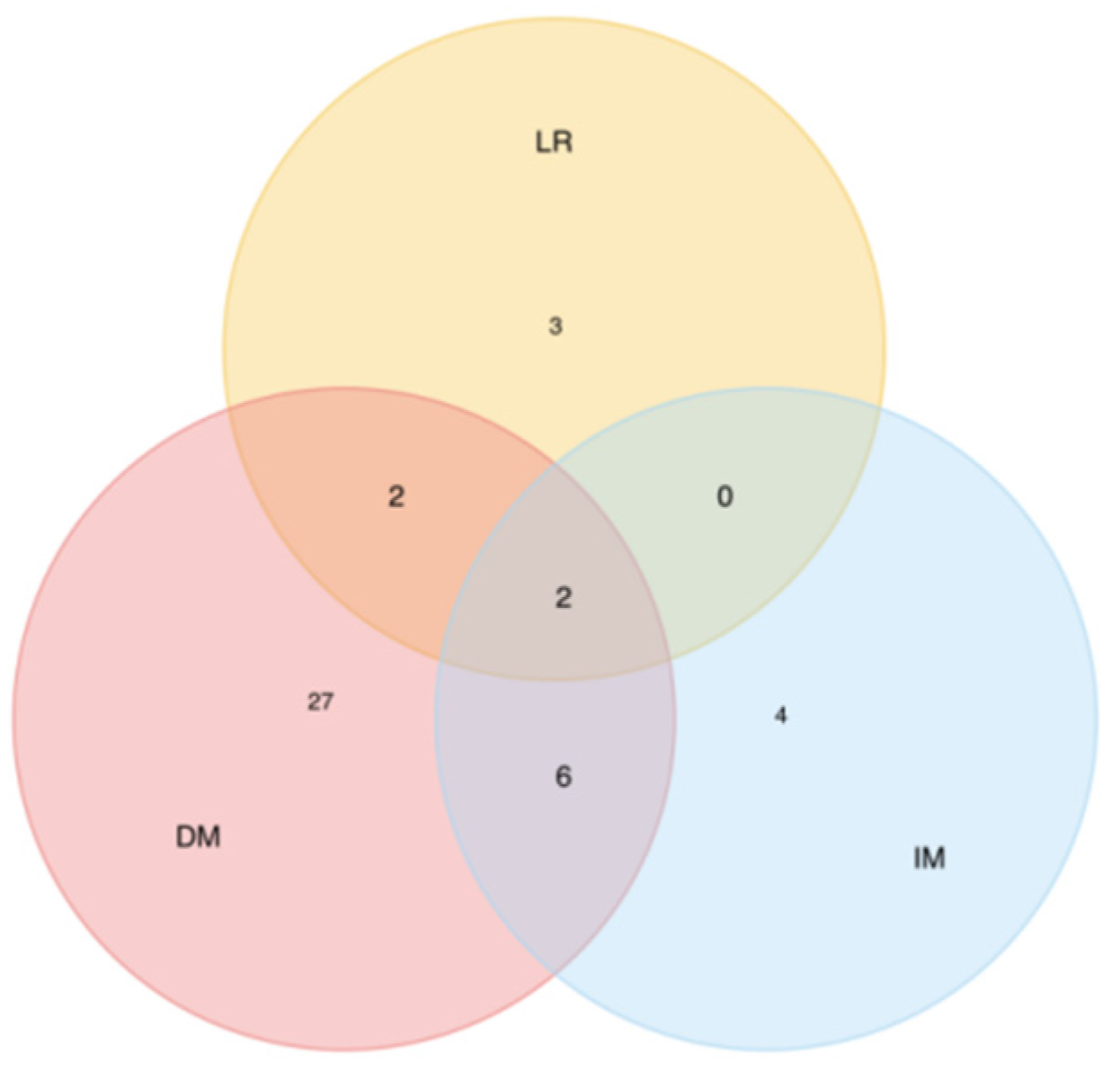
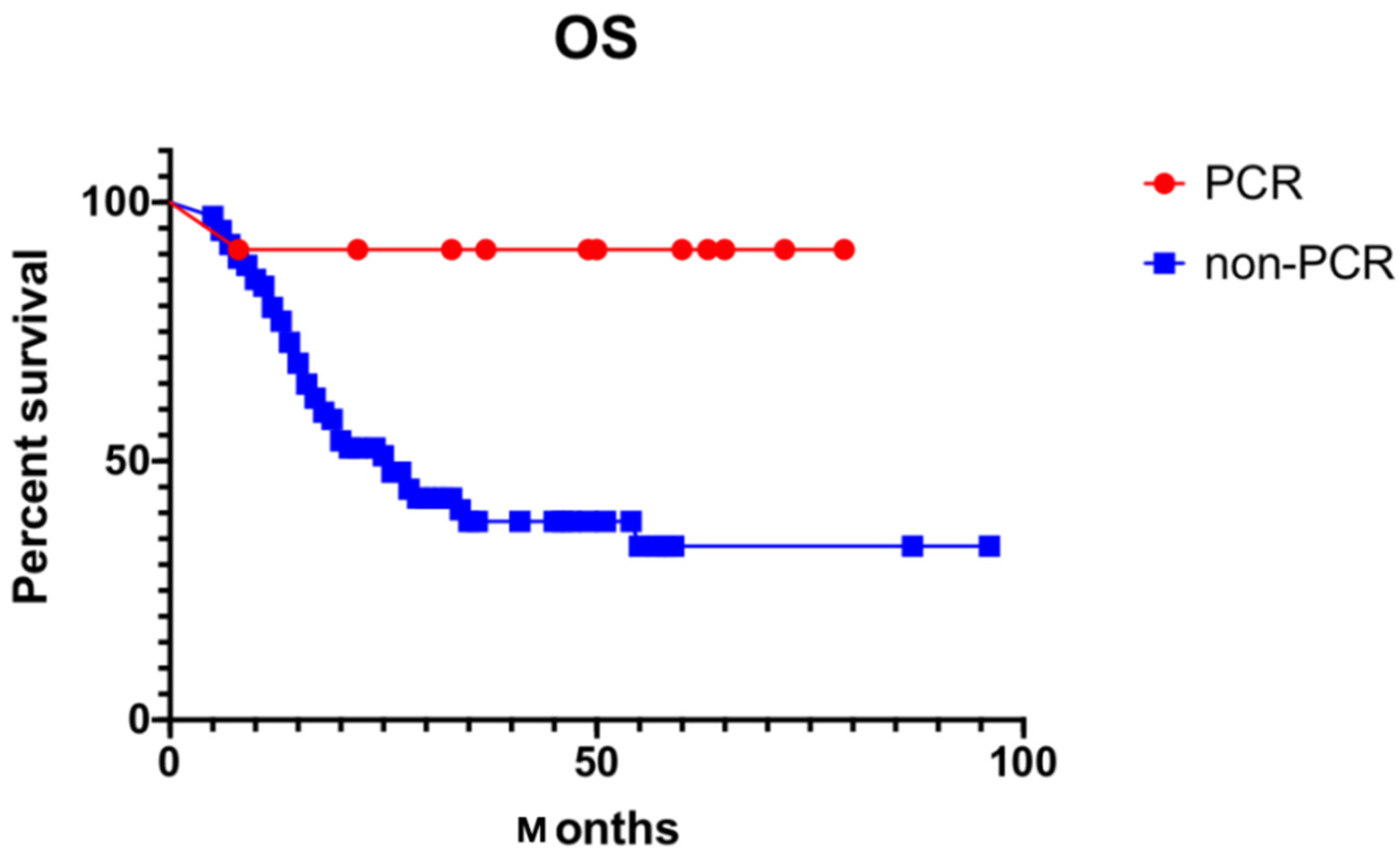
3.4. Pattern of Failure and Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2017 Stomach Cancer Collaborators. The global, regional, and national burden of stomach cancer in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 42–54. [Google Scholar] [CrossRef]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int. J. Mol. Sci. 2020, 21, 4012. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllou, T.; Wijnhoven, B. Multidisciplinary treatment of esophageal cancer: The role of active surveillance after neoadjuvant chemoradiation. Ann. Gastroenterol. Surg. 2020, 4, 352–359. [Google Scholar] [CrossRef]
- Recio-Boiles, A.; Hammad, H.; Howell, K.; Kalb, B.T.; Nfonsam, V.N.; Scott, A.J.; Babiker, H.M.; Elquza, E. Locally Advanced Rectal Cancer Evaluation by Magnetic Resonance Imaging after Neoadjuvant Therapy on Decision Making: Cancer Center Experience and Literature Review. J. Gastrointest. Cancer. 2020, 51, 254–259. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Sagaert, X.; Topal, B.; Haustermans, K.; Prenen, H. Gastric cancer. Lancet 2016, 388, 2654–2664. [Google Scholar] [CrossRef]
- Van Hagen, P.; Hulshof, M.C.; van Lanschot, J.J.; Steyerberg, E.W.; Henegouwen, M.V.; Wijnhoven, B.P.; Richel, D.J.; Nieuwenhuijzen, G.A.; Hospers, G.A.; Bonenkamp, J.J.; et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef]
- Chavaudra, J.; Bridier, A. Definition of volumes in external radiotherapy: ICRU reports 50 and 62. Cancer Radiother. 2001, 5, 472–478. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Wang, X.Z.; Zeng, Z.Y.; Ye, X.; Sun, J.; Zhang, Z.M.; Kang, W.M. Interpretation of the development of neoadjuvant therapy for gastric cancer based on the vicissitudes of the NCCN guidelines. World J. Gastrointest. Oncol. 2020, 12, 37–53. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Gu, X.Z.; Yin, W.B.; Huang, G.J.; Zhang, D.W.; Zhang, R.G. Randomized clinical trial on the combination of preoperative irradiation and surgery in the treatment of adenocarcinoma of gastric cardia (AGC)—Report on 370 patients. Int. J. Radiat. Oncol. Biol. Phys. 1998, 42, 929–934. [Google Scholar] [CrossRef]
- Stahl, M.; Walz, M.K.; Riera-Knorrenschild, J.; Stuschke, M.; Sandermann, A.; Bitzer, M.; Wilke, H.; Budach, W. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): Long-term results of a controlled randomised trial. Eur. J. Cancer 2017, 81, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Stahl, M.; Walz, M.K.; Stuschke, M.; Lehmann, N.; Meyer, H.J.; Riera-Knorrenschild, J.; Langer, P.; Engenhart-Cabillic, R.; Bitzer, M.; Königsrainer, A.; et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J. Clin. Oncol. 2009, 27, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Von Dobeln, G.A.; Klevebro, F.; Jacobsen, A.-B.; Johannessen, H.-O.; Nielsen, N.H.; Johnsen, G.; Hatlevoll, I.; Glenjen, N.I.; Friesland, S.; Lundell, L.; et al. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or gastroesophageal junction: Long-term results of a randomized clinical trial. Dis. Esophagus 2019, 32, doy078. [Google Scholar] [CrossRef]
- Leong, T.; Smithers, B.M.; Haustermans, K.; Michael, M.; Gebski, V.; Miller, D.; Zalcberg, J.; Boussioutas, A.; Findlay, M.; O’Connell, R.L.; et al. TOPGEAR: A Randomized, Phase III Trial of Perioperative ECF Chemotherapy with or Without Preoperative Chemoradiation for Resectable Gastric Cancer: Interim Results from an International, Intergroup Trial of the AGITG, TROG, EORTC and CCTG. Ann. Surg. Oncol. 2017, 24, 2252–2258. [Google Scholar] [CrossRef]
- Liu, X.; Jin, J.; Cai, H.; Huang, H.; Zhao, G.; Zhou, Y.; Wu, J.; Du, C.; Long, Z.; Fang, Y.; et al. Study protocol of a randomized phase III trial of comparing preoperative chemoradiation with preoperative chemotherapy in patients with locally advanced gastric cancer or esophagogastric junction adenocarcinoma: PREACT. BMC Cancer 2019, 19, 606. [Google Scholar] [CrossRef]
- Abbas, M.N.; Bright, T.; Price, T.; Karapetis, C.; Thompson, S.; Connell, C.; Watson, D.; Barnes, M.; Bull, J.; Singhal, N.; et al. Patterns of care and outcomes for gastric and gastro-oesophageal junction cancer in an Australian population. ANZ J. Surg. 2021, 91, 2675–2682. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, G.; Zhang, T.; Zhao, Y.; Zheng, Z. Is pathologic tumor regression grade after neo-adjuvant chemotherapy a promising prognostic indicator for patients with locally advanced gastric cancer? A cohort study evaluating tumor regression response. Cancer Chemother. Pharmacol. 2019, 84, 635–646. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Y.; Zhao, L.; Zhou, H.; Wu, C.; Zhang, X.; Zhou, A.; Jin, J.; Zhao, D. The Effect of Neoadjuvant Therapies for Patients with Locally Advanced Gastric Cancer: A Propensity Score Matching Study. J. Cancer 2021, 12, 379–386. [Google Scholar] [CrossRef]
- Martin-Romano, P.; Sola, J.J.; Diaz-Gonzalez, J.A.; Chopitea, A.; Iragorri, Y.; Martínez-Regueira, F.; Ponz-Sarvise, M.; Arbea, L.; Subtil, J.C.; Cano, D.; et al. Role of histological regression grade after two neoadjuvant approaches with or without radiotherapy in locally advanced gastric cancer. Br. J. Cancer 2016, 115, 655–663. [Google Scholar] [CrossRef]
- Cabral, F.; Cruz, A.; Casaca, R.; Monteiro, C.; Ramos, P.; Pedro, C.; Brandão, F.; Fonseca, R.; Ratão, P.; Salgado, L.; et al. Complete pathological response (PCR) in gastroesophageal cancer: Correlation with metabolic response. Cancer Radiother. 2020, 24, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Ghidini, M.; Barni, S.; Sgroi, G.; Passalacqua, R.; Tomasello, G. Neoadjuvant chemoradiotherapy or chemotherapy for gastroesophageal junction adenocarcinoma: A systematic review and meta-analysis. Gastric Cancer 2019, 22, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; D’Amico, T.A.; Almhanna, K.; Bentrem, D.J.; Chao, J.; Das, P.; Denlinger, C.S.; Fanta, P.; Farjah, F.; Fuchs, C.S.; et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2016, 14, 1286–1312. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Yamaguchi, K.; Okumura, N.; Tanahashi, T.; Kodera, Y. Is conversion therapy possible in stage IV gastric cancer: The proposal of new biological categories of classification. Gastric Cancer 2016, 19, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Yoshida, K.; Tanahashi, T.; Takahashi, T.; Matsuhashi, N.; Tanaka, Y.; Tanabe, K.; Ohdan, H. The long-term survival of stage IV gastric cancer patients with conversion therapy. Gastric Cancer 2018, 21, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
| Characteristics | n | Percentage (%) |
|---|---|---|
| Age | ||
| <60 | 41 | 43.16 |
| ≥60 | 54 | 56.84 |
| Gender | ||
| Male | 81 | 85.26 |
| Female | 14 | 14.74 |
| ECOG performance status | ||
| 0 | 77 | 81.06 |
| 1 | 18 | 18.94 |
| Tumor location | ||
| Upper 1/3 | 85 | 89.47 |
| Other | 10 | 10.53 |
| Tumor differentiation | ||
| Well differentiated | 3 | 3.16 |
| Moderately differentiated | 21 | 22.11 |
| Poorly differentiated | 64 | 67.36 |
| Others | 7 | 7.37 |
| Blood tumor markers abnormal | ||
| Carcinoembryonic antigen | 35 | 36.84 |
| CA199 | 29 | 30.53 |
| CA242 | 29 | 30.53 |
| CA724 | 19 | 20.00 |
| CA125 | 14 | 14.74 |
| CA153 | 4 | 4.21 |
| Pretreatment tumor stage | ||
| T2 | 2 | 2.1 |
| T3 | 20 | 21.06 |
| T4 | 73 | 76.84 |
| Pretreatment node status | ||
| N0 | 10 | 10.53 |
| N1 | 34 | 35.79 |
| N2 | 49 | 51.57 |
| N3 | 2 | 2.11 |
| Chemotherapy regiment | ||
| Capecitabine | 71 | 74.74 |
| XELOX | 24 | 25.26 |
| Postoperative chemotherapy | ||
| Yes | 41 | 51.25 |
| No | 39 | 48.75 |
| Failure Sites | No. of Patients | % of Recurrence Patients (n = 37) |
|---|---|---|
| Single site | ||
| Local recurrence | 3 | 6.81 |
| Implant metastasis | 4 | 9.12 |
| Distant metastasis | 27 | 61.42 |
| Two sites | ||
| LR + IM | 0 | 0 |
| LR + DM | 2 | 4.51 |
| IM + DM | 6 | 13.63 |
| Three sites | 2 | 4.51 |
| Characteristics | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| Percent | p-Value | HR | 95% CI | p-Value | |
| Age | 0.672 | ||||
| ≥60 | 43.90% | ||||
| <60 | 40.70% | ||||
| Weight loss | 0.849 | ||||
| Yes | 38.70% | ||||
| No | 43.80% | ||||
| Anemia | 0.645 | ||||
| Yes | 39.20% | ||||
| No | 43.80% | ||||
| Post-operation chemotherapy | 0.732 | ||||
| Yes | 45.60% | ||||
| No | 30.70% | ||||
| Chemotherapy | 0.002 * | 0.286 | 0.149–0.549 | 0.148 | |
| capecitabine | 20.80% | ||||
| XELOX | 49.30% | ||||
| Surgery | 0.001 * | 3.53 | 1.866–6.679 | 0.338 | |
| D1 | 13.60% | ||||
| D2 | 56.90% | ||||
| PCR | 0.003 * | 11.211 | 1.500–83.813 | 0.024 * | |
| Yes | 90.90% | ||||
| No | 35.70% | ||||
| T downstaging | 0.001 * | 2.808 | 1.352–5.832 | 0.151 | |
| Yes | 52.90% | ||||
| No | 12.00% | ||||
| N downstaging | 0.005 * | 4.505 | 1.777–11.419 | 0.25 | |
| Yes | 50.80% | ||||
| No | 26.50% | ||||
| Study | Year, Country | Phase | Sample Size | Tumor Site | Groups | Local Control | Survival |
|---|---|---|---|---|---|---|---|
| Zhang et al. [11] | 1998, China | III | 370 | EGJ | RT + S vs. S | 61.4% vs. 51.7% | 10-year OS 20.3% vs. 13.3% |
| Stahl et al. [12,13] | 2009, 2019, Germany | III | 119 | EGJ | CRT + S vs. C + S | PCR 15.6% vs. 2.0% | 3-year OS 46.7% vs. 26.1%, 5-year OS 39.5% vs. 24.4% |
| Van Hagen et al. [7] | 2012, England | III | 366 | EGJ or EC | CRT + S vs. S | LRR 14% vs. 34% | 5-year OS 47% vs. 34% |
| G A von Döbeln [14] | 2019, Sweden and Norway | II | 181 | EGJ or EC | CRT + S vs. C + S | PCR 28% vs. 9% | 5-year OS 42.2% vs. 39.6% |
| Trevor Leong et al. [15] | 2019, Australia, Europa, Canada | III | 752 | EGJ or EC | CRT + S vs. C + S | On going | On going |
| Liu, X et al. [16] | 2019, China | III | 682 | EGJ or EC | CRT + S vs. C + S | On going | On going |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.; Lian, X.; Guan, Q.; He, L.; Zhang, F.; Shen, J. Neoadjuvant Chemo-Radiation Using IGRT in Patients with Locally Advanced Gastric Cancer. Curr. Oncol. 2022, 29, 7450-7460. https://doi.org/10.3390/curroncol29100586
Shen J, Lian X, Guan Q, He L, Zhang F, Shen J. Neoadjuvant Chemo-Radiation Using IGRT in Patients with Locally Advanced Gastric Cancer. Current Oncology. 2022; 29(10):7450-7460. https://doi.org/10.3390/curroncol29100586
Chicago/Turabian StyleShen, Jing, Xin Lian, Qiu Guan, Lei He, Fuquan Zhang, and Jie Shen. 2022. "Neoadjuvant Chemo-Radiation Using IGRT in Patients with Locally Advanced Gastric Cancer" Current Oncology 29, no. 10: 7450-7460. https://doi.org/10.3390/curroncol29100586
APA StyleShen, J., Lian, X., Guan, Q., He, L., Zhang, F., & Shen, J. (2022). Neoadjuvant Chemo-Radiation Using IGRT in Patients with Locally Advanced Gastric Cancer. Current Oncology, 29(10), 7450-7460. https://doi.org/10.3390/curroncol29100586





