Changes in Apparent Diffusion Coefficient (ADC) in Serial Weekly MRI during Radiotherapy in Patients with Head and Neck Cancer: Results from the PREDICT-HN Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Radiotherapy Treatment
2.3. MR Imaging
2.4. Target Delineation
2.5. Apparent Diffusion Coefficient Values
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
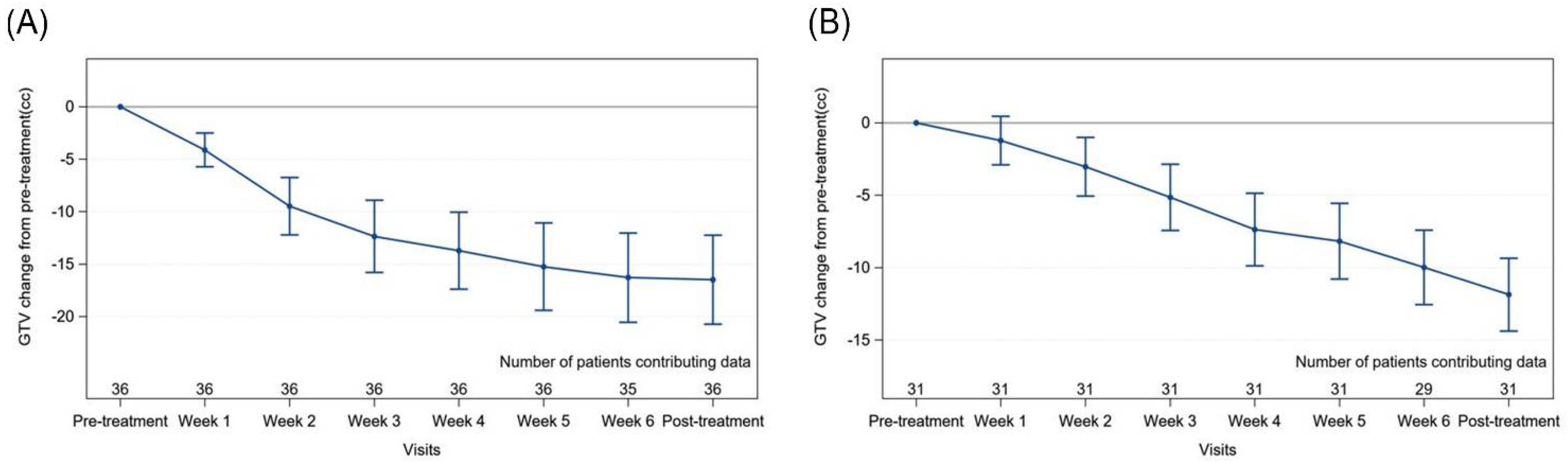
3.2. Primary Gross Tumour Volume (GTV) Changes
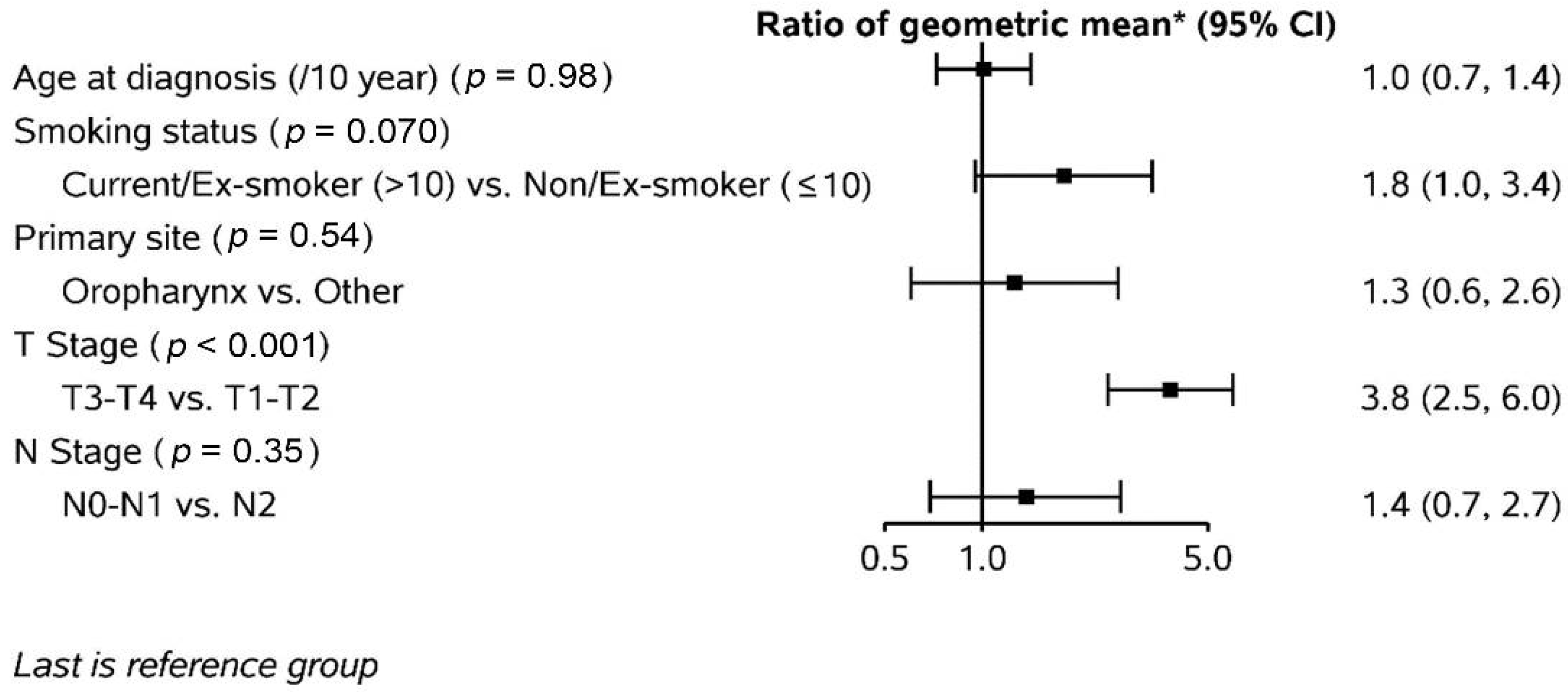
3.3. Nodal Gross Tumour Volume (GTV) Changes
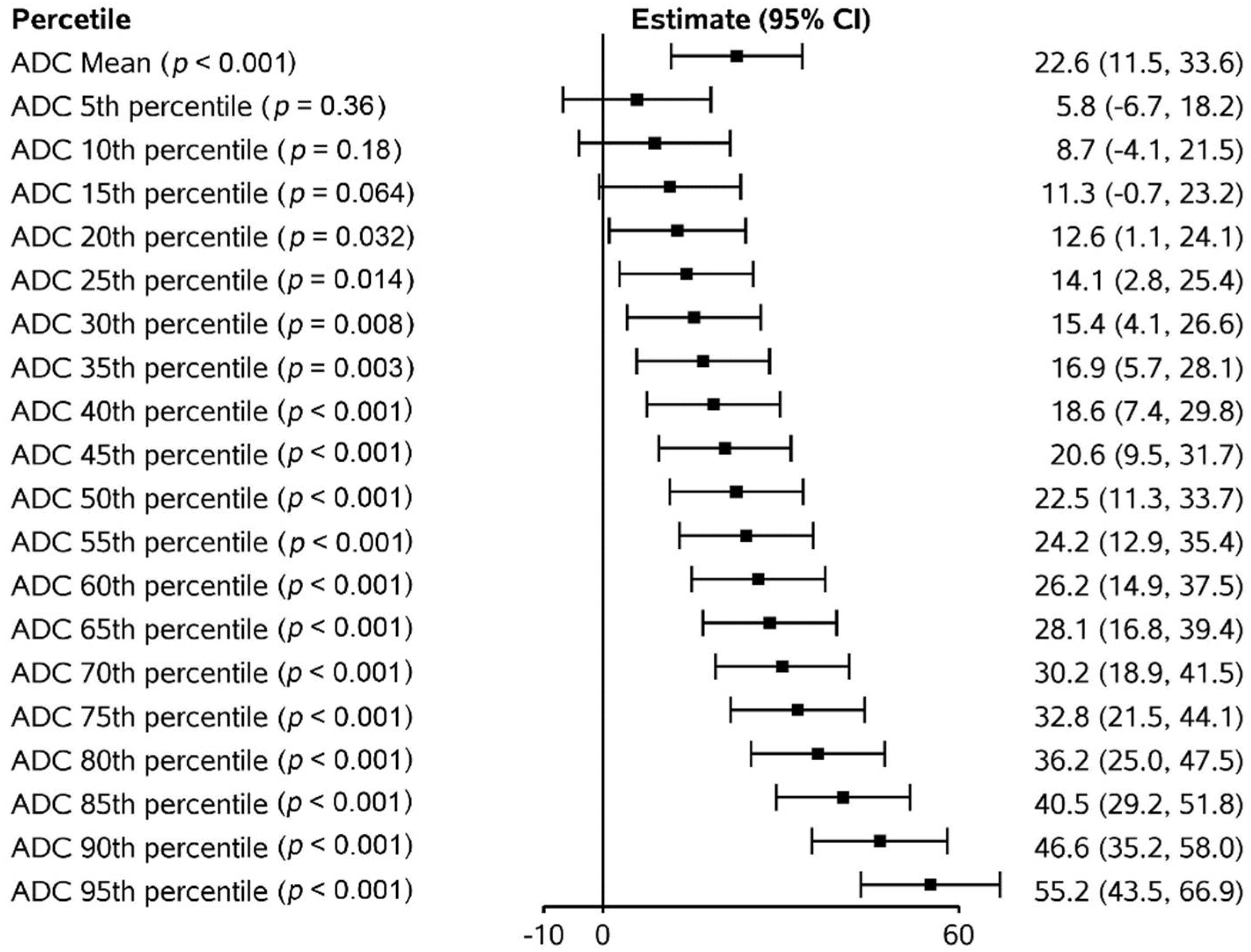
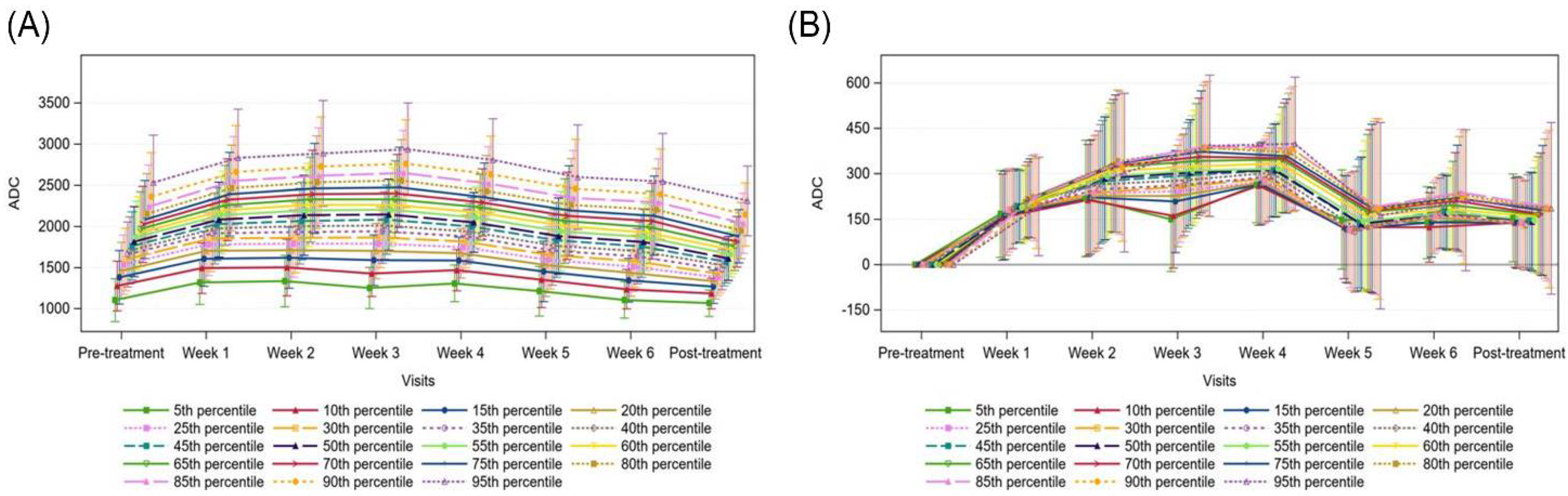
3.4. Apparent Diffusion Coefficient (ADC) Changes
3.5. Patient Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gillison, M.L.; D’Souza, G.; Westra, W.; Sugar, E.; Xiao, W.; Begum, S.; Viscidi, R. Distinct Risk Factor Profiles for Human Papillomavirus Type 16–Positive and Human Papillomavirus Type 16–Negative Head and Neck Cancers. J. Natl. Cancer Inst. 2008, 100, 407–420. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Anderson, W.F.; Lortet-Tieulent, J.; Curado, M.P.; Ferlay, J.; Franceschi, S.F.S.; Rosenberg, P.; Bray, F.; Gillison, M.L. Worldwide Trends in Incidence Rates for Oral Cavity and Oropharyngeal Cancers. J. Clin. Oncol. 2013, 31, 4550–4559. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved Survival of Patients With Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma in a Prospective Clinical Trial. JNCI: J. Natl. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef]
- Fakhry, C.; Zhang, Q.; Nguyen-Tan, P.F.; Rosenthal, D.; El-Naggar, A.; Garden, A.S.; Soulieres, D.; Trotti, A.; Avizonis, V.; Ridge, J.A.; et al. Human papillomavirus and overall survival after progression of oropharyngeal squa-mous cell carcinoma. J. Clin. Oncol. 2014, 32, 3365–3373. [Google Scholar] [CrossRef]
- Ng, S.P.; Bahig, H.; Wang, J.; Cardenas, C.E.; Lucci, A.; Hall, C.S.; Meas, S.; Sarli, V.N.; Yuan, Y.; Urbauer, D.L.; et al. Predicting treatment Response based on Dual assessment of magnetic resonance Imaging kinetics and Cir-culating Tumor cells in patients with Head and Neck cancer (PREDICT-HN): Matching ‘liquid biopsy’ and quantitative tumor modeling. BMC Cancer. 2018, 18, 903. [Google Scholar] [CrossRef]
- Musio, D.; De Felice, F.; Magnante, A.L.; Ciolina, M.; De Cecco, C.N.; Rengo, M.; Redler, A.; Laghi, A.; Raffetto, N.; Tombolini, V. Diffusion-Weighted Magnetic Resonance Application in Response Prediction before, during, and after Neoadjuvant Radiochemotherapy in Primary Rectal Cancer Carcinoma. BioMed. Res. Int. 2013, 2013, 740195. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, L.; Yang, H.; Duan, Y.; Li, G. Apparent diffusion coefficient for the prediction of tumor response to neoadjuvant chemo-radiotherapy in locally advanced rectal cancer. Radiat. Oncol. 2021, 16, 17. [Google Scholar] [CrossRef]
- Lo, C.-H.; Huang, W.-Y.; Hsiang, C.-W.; Lee, M.-S.; Lin, C.-S.; Yang, J.-F.; Hsu, H.-H.; Chang, W.-C. Prognostic Significance of Apparent Diffusion Coefficient in Hepatocellular Carcinoma Patients treated with Stereotactic Ablative Radiotherapy. Sci. Rep. 2019, 9, 14157. [Google Scholar] [CrossRef]
- Hamstra, D.A.; Rehemtulla, A.; Ross, B.D. Diffusion Magnetic Resonance Imaging: A Biomarker for Treatment Response in Oncology. J. Clin. Oncol. 2007, 25, 4104–4109. [Google Scholar] [CrossRef]
- Kim, S.; Loevner, l.; Quon, H.; Sherman, E.; Weinstein, G.; Kilger, A.; Poptani, H. Diffusion-weighted magnetic resonance imaging for predicting and detecting early response to chemoradiation therapy of squamous cell carcinomas of the head and neck. Clin. Cancer Res. 2009, 15, 986–994. [Google Scholar] [CrossRef]
- Vandecaveye, V.; Dirix, P.; De Keyzer, F.; de Beeck, K.O.; Poorten, V.V.; Roebben, I.; Nuyts, S.; Hermans, R. Predictive value of diffusion-weighted magnetic resonance imaging during chemoradiotherapy for head and neck squamous cell carcinoma. Eur. Radiol. 2010, 20, 1703–1714. [Google Scholar] [CrossRef]
- Cardenas, C.E.; Mohamed, A.S.R.; Tao, R.; Wong, A.J.R.; Awan, M.J.; Kuruvila, S.; Aristophanous, M.; Gunn, G.B.; Phan, J.; Beadle, B.M.; et al. Prospective Qualitative and Quantitative Analysis of Real-Time Peer Review Quality As-surance Rounds Incorporating Direct Physical Examination for Head and Neck Cancer Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 532–540. [Google Scholar] [CrossRef]
- Ding, Y.; Mohamed, A.S.; Yang, J.; Colen, R.R.; Frank, S.J.; Wang, J.; Wassal, E.Y.; Wang, W.; Kantor, M.E.; Balter, P.A.; et al. Prospective observer and software-based assessment of magnetic resonance imaging quality in head and neck cancer: Should standard positioning and immobilization be required for radiation therapy applications? Pract. Radiat. Oncol. 2015, 5, e299–e308. [Google Scholar] [CrossRef]
- Maeda, M.; Kato, H.; Sakuma, H.; Maier, S.E.; Takeda, K. Usefulness of the apparent diffusion coefficient in line scan diffusion-weighted imaging for distinguishing between squamous cell carcinomas and malignant lymphomas of the head and neck. AJNR Am. J. Neuroradiol. 2005, 26, 1186–1192. [Google Scholar]
- Sumi, M.; Sakihama, N.; Sumi, T.; Morikawa, M.; Uetani, M.; Kabasawa, H.; Shigeno, K.; Hayashi, K.; Takahashi, H.; Nakamura, T. Discrimination of metastatic cervical lymph nodes with diffusion-weighted MR imaging in patients with head and neck cancer. AJNR Am. J. Neuroradiol. 2003, 24, 1627–1634. [Google Scholar]
- King, A.D.; Ahuja, A.T.; Yeung, D.K.W.; Fong, D.K.Y.; Lee, Y.Y.P.; Lei, K.I.K.; Tse, G.M.K. Malignant Cervical Lymphadenopathy: Diagnostic Accuracy of Diffusion-weighted MR Imaging. Radiology 2007, 245, 806–813. [Google Scholar] [CrossRef]
- Vandecaveye, V.; Dirix, P.; De Keyzer, F.; de Beeck, K.O.; Poorten, V.V.; Hauben, E.; Lambrecht, M.; Nuyts, S.; Hermans, R. Diffusion-Weighted Magnetic Resonance Imaging Early After Chemoradiotherapy to Monitor Treatment Response in Head-and-Neck Squamous Cell Carcinoma. Int. J. Radiat. Oncol. 2012, 82, 1098–1107. [Google Scholar] [CrossRef]
- Fukui, M.B.; Blodgett, T.M.; Snyderman, C.H.; Johnson, J.J.; Myers, E.N.; Townsend, D.W.; Meltzer, C.C. Combined PET-CT in the head and neck: Part 2. Diagnostic uses and pitfalls of on-cologic imaging. Radiographics 2005, 25, 913–930. [Google Scholar] [CrossRef]
- Kapoor, V.; Fukui, M.B.; McCook, B.M. Role of 18FFDG PET/CT in the Treatment of Head and Neck Cancers: Posttherapy Evaluation and Pitfalls. Am. J. Roentgenol. 2005, 184, 589–597. [Google Scholar] [CrossRef]
- Subramaniam, R.M.; Truong, M.; Peller, P.; Sakai, O.; Mercier, G. Fluorodeoxyglu-cose-positron-emission tomography imaging of head and neck squamous cell cancer. AJNR Am. J. Neuroradiol. 2010, 31, 598–604. [Google Scholar] [CrossRef]
- Purohit, B.S.; Ailianou, A.; Dulguerov, P.; Becker, C.D.; Ratib, O.; Becker, M. FDG-PET/CT pitfalls in oncological head and neck imaging. Insights into Imaging 2014, 5, 585–602. [Google Scholar] [CrossRef]
- King, A.D.; Mo, F.K.; Yu, K.H.; Yeung, D.K.; Zhou, H.; Bhatia, K.S.; Tse, G.M.; Vlantis, A.C.; Wong, J.K.; Ahuja, A.T. Squamous cell car-cinoma of the head and neck: Diffusion-weighted MR imaging for prediction and mon-itoring of treatment response. Eur. Radiol. 2010, 20, 2213–2220. [Google Scholar] [CrossRef]

| Parameter | T1 | T2 | DWI (BLADE) |
|---|---|---|---|
| Slice Orientation | Axial | Axial | Axial |
| Field of View (mm) | 256 | 256 | 256 |
| Voxel Size (mm) | 1 × 1 × 2 | 1 × 1 × 2 | 2 × 2 × 4 |
| Recon Voxel Size (mm) | 0.5 × 0.5 × 1 | 0.5 × 0.5 × 2 | 2 × 2 × 4 |
| Parallel Imaging | No | Yes; Factor 2 | No |
| Slice Number | 240 | 120 | 25 |
| Fold-over Direction | AP | AP | N/A |
| Slice Oversampling | 100% | No | No |
| Shim | Auto | Auto | Auto |
| Scan Mode | 3D | M2D | M2D |
| Technique | GRE | SE | BLADE |
| Fast Imaging Mode | No | TSE | TSE and EPI |
| Echoes | 1 | 1 | 1 |
| Flip Angle(deg) | 20 | 90 | 90 |
| TR (ms) | 7.38 | 4800 | 5400 |
| Echo Time (ms) | 4.77 | 80 | 50 |
| Fat Suppression | No | No | Yes |
| b-values (s/mm2) | N/A | N/A | 0.800 |
| NEX | 1 | 1 | 8 |
| Geometry Correction | 3D | 2D | No |
| Echo Train Length | 1 | 15 | 15 |
| Percent Sampling (%) | 80 | 90 | 100 |
| Pixel Bandwidth (Hz) | 400 | 300 | 1220 |
| Scan Duration (min) | 6:05 | 4:48 | 7:08 |
| Parameters | N = 41 | % | |
|---|---|---|---|
| Age (median, range) | 59 (41–81) | ||
| Sex | Male | 37 | 90 |
| Female | 4 | 10 | |
| Smoking status | Never | 17 | 41 |
| Ex < 10 | 6 | 15 | |
| Ex ≥ 10 | 10 | 24 | |
| Current | 8 | 20 | |
| ECOG performance status | 0 | 20 | 49 |
| 1 | 21 | 51 | |
| Primary tumour site | Oropharynx | 28 | 76 |
| Larynx | 4 | 11 | |
| Nasopharynx | 4 | 11 | |
| Nasal cavity | 1 | 3 | |
| Primary tumour (T) stage (AJCC 7th edition) | T0 | 3 | 7 |
| T1 | 5 | 12 | |
| T2 | 15 | 37 | |
| T3 | 7 | 17 | |
| T4 | 11 | 27 | |
| Nodal (N) stage (AJCC 7th edition) | N0 | 5 | 12 |
| N1 | 7 | 17 | |
| N2 (nasopharynx) | 2 | 5 | |
| N2a | 3 | 7 | |
| N2b | 22 | 54 | |
| N2c | 2 | 5 | |
| Radiation modality | Photon | 27 | 66 |
| Proton | 14 | 34 | |
| Concurrent chemotherapy | Yes | 33 | 80 |
| No | 8 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, S.P.; Cardenas, C.E.; Bahig, H.; Elgohari, B.; Wang, J.; Johnson, J.M.; Moreno, A.C.; Shah, S.J.; Garden, A.S.; Phan, J.; et al. Changes in Apparent Diffusion Coefficient (ADC) in Serial Weekly MRI during Radiotherapy in Patients with Head and Neck Cancer: Results from the PREDICT-HN Study. Curr. Oncol. 2022, 29, 6303-6313. https://doi.org/10.3390/curroncol29090495
Ng SP, Cardenas CE, Bahig H, Elgohari B, Wang J, Johnson JM, Moreno AC, Shah SJ, Garden AS, Phan J, et al. Changes in Apparent Diffusion Coefficient (ADC) in Serial Weekly MRI during Radiotherapy in Patients with Head and Neck Cancer: Results from the PREDICT-HN Study. Current Oncology. 2022; 29(9):6303-6313. https://doi.org/10.3390/curroncol29090495
Chicago/Turabian StyleNg, Sweet Ping, Carlos E. Cardenas, Houda Bahig, Baher Elgohari, Jihong Wang, Jason M. Johnson, Amy C. Moreno, Shalin J. Shah, Adam S. Garden, Jack Phan, and et al. 2022. "Changes in Apparent Diffusion Coefficient (ADC) in Serial Weekly MRI during Radiotherapy in Patients with Head and Neck Cancer: Results from the PREDICT-HN Study" Current Oncology 29, no. 9: 6303-6313. https://doi.org/10.3390/curroncol29090495
APA StyleNg, S. P., Cardenas, C. E., Bahig, H., Elgohari, B., Wang, J., Johnson, J. M., Moreno, A. C., Shah, S. J., Garden, A. S., Phan, J., Gunn, G. B., Frank, S. J., Ding, Y., Na, L., Yuan, Y., Urbauer, D., Mohamed, A. S. R., Rosenthal, D. I., Morrison, W. H., ... Fuller, C. D. (2022). Changes in Apparent Diffusion Coefficient (ADC) in Serial Weekly MRI during Radiotherapy in Patients with Head and Neck Cancer: Results from the PREDICT-HN Study. Current Oncology, 29(9), 6303-6313. https://doi.org/10.3390/curroncol29090495







