1. Introduction
Prostate cancer is the most common cancer in men, with an incidence of 113/100,000 men per year [
1]. Radiotherapy (RT) is one of the main curative treatment modalities besides surgery. Recently, ultra-hypofractionated radiotherapy has been proven to be noninferior to conventionally fractionated radiotherapy, and it has been increasingly employed since [
2].
The use of ultra-hypofractionated radiotherapy leads to prolonged treatment times compared with normal fractionated and moderately hypofractionated RT. Treatment duration may vary substantially from 1 min to more than 10 min and depends not only on the fractionation scheme but also on the treatment machine (linear accelerator (LINAC) vs. CyberKnife) or beam modulation (flattening filter-free (FFF) vs. flattening filter) [
3,
4].
Treatment duration plays a crucial role in the amount of intrafraction prostate motion [
3,
5]. Prolonged treatment time of 10 min compared with the 5 min one leads to increased prostate shifts by 3 mm and more [
6,
7,
8]. Consecutively, prolonged treatment times harbor the risk of geographical miss and demand either the adoption of larger planned target volume (PTV) margins at the cost of normal tissue exposure and toxicity or intrafraction tracking/monitoring. For moderately hypofractionated RT, intrafraction prostate motion is usually compensated for with the adaptation of the PTV margin [
9]. On the other hand, for stereotactic and ultra-hypofractionated RT, prostate tracking/monitoring is the preferred method. Currently, it is not clear which treatment times demand intrafraction prostate motion tracking rather than compensation with safety margins.
Various techniques have been proposed for prostate motion monitoring and compensation, including intraprostatic radiopaque fiducial markers (FMs) with in-room imaging, ultrasound-based systems, electromagnetic tracking, and MRI systems integrated into the treatment room, all of which are suitable for LINACs [
10]. For CyberKnife treatment, the use of intraprostatic radiopaque FMs is required. Radiopaque markers require repetitive intrafraction imaging. Some institutions investigated an imaging interval of 40 s [
7] or 60–180 s [
6], while others suggested an imaging frequency depending on margin size, i.e., every 15, 60, or 240 s for 1 mm, 2 mm, or 3 mm margins, respectively [
11]. Currently, there are no guidelines on what imaging frequency should be used.
This study aimed to use intraprostatic radiopaque FM tracking by repetitive in-room imaging in order to (1) evaluate the time-dependent magnitude of intrafraction translational prostate displacement, (2) determine an optimal intrafraction imaging frequency allowing minimal margins, and (3) determine a time cutoff for decision-making regarding the compensation modality, i.e., PTV margins compensation vs. intrafraction prostate tracking.
2. Materials and Methods
2.1. Study Design and Treatment
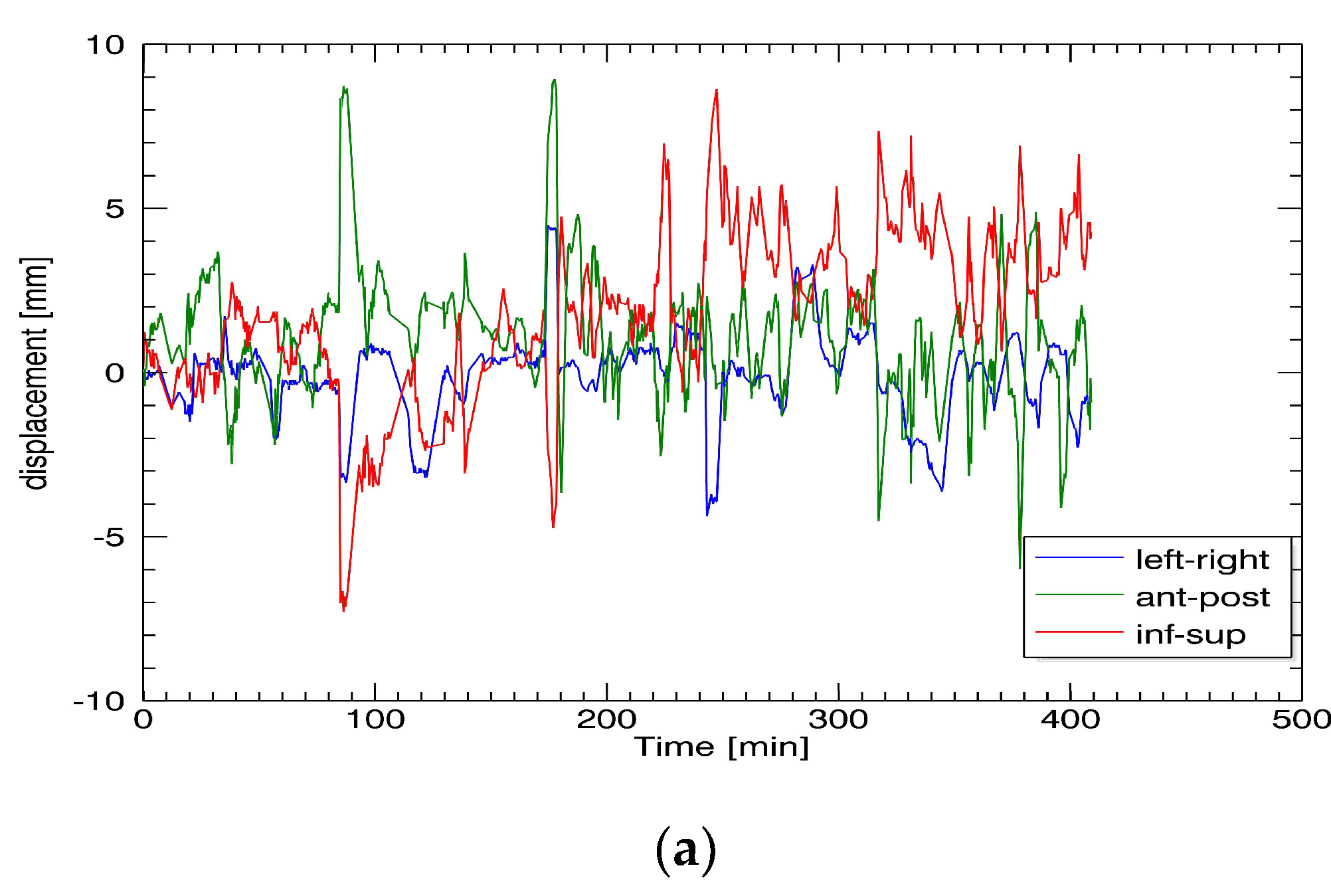
Radiotherapy treatment data of nine patients with localized prostate cancer treated with ultra-hypofractionated stereotactic RT (5 × 7.25 Gy) using a CyberKnife linear accelerator at a single institution in Switzerland between 05/2009 and 11/2012 were retrospectively analyzed. Informed consent was obtained before treatment. Patient positioning was the same for the patients treated with a LINAC: the patients were treated in supine position with comfortable bladder filling and emptied rectum using laxatives but without an endorectal balloon (ERB). For IGRT, radiopaque fiducial markers were implanted into the prostate before planning CT, and their position was tracked during treatment using a kV/kV orthogonal imaging system (integrated kV/kV system). The treatment time was between 1500 and 6000 s. Imaging was performed with an average interval of 19–92 s during treatment. FM position data were immediately analyzed, and displacement data were sent to the robotic system, allowing the robotic arm to follow the target. No table adjustments were performed.
2.2. Data Analysis
The prostate position was recorded in the x-, y-, and z-directions. Differences in the x-axis correspond to left/right movements, in the y-axis—to anterior/posterior movements, and in the z-axis—to inferior/superior movements (DICOM standard). Neither rotational nor deformational movements were analyzed. Prostate position data were recorded in a log file during treatment. For data analysis, programming language IDL (Version 8.7 interactive data language) was used. The log files created during treatment were imported into the IDL code to be evaluated statistically.
2.3. Statistics
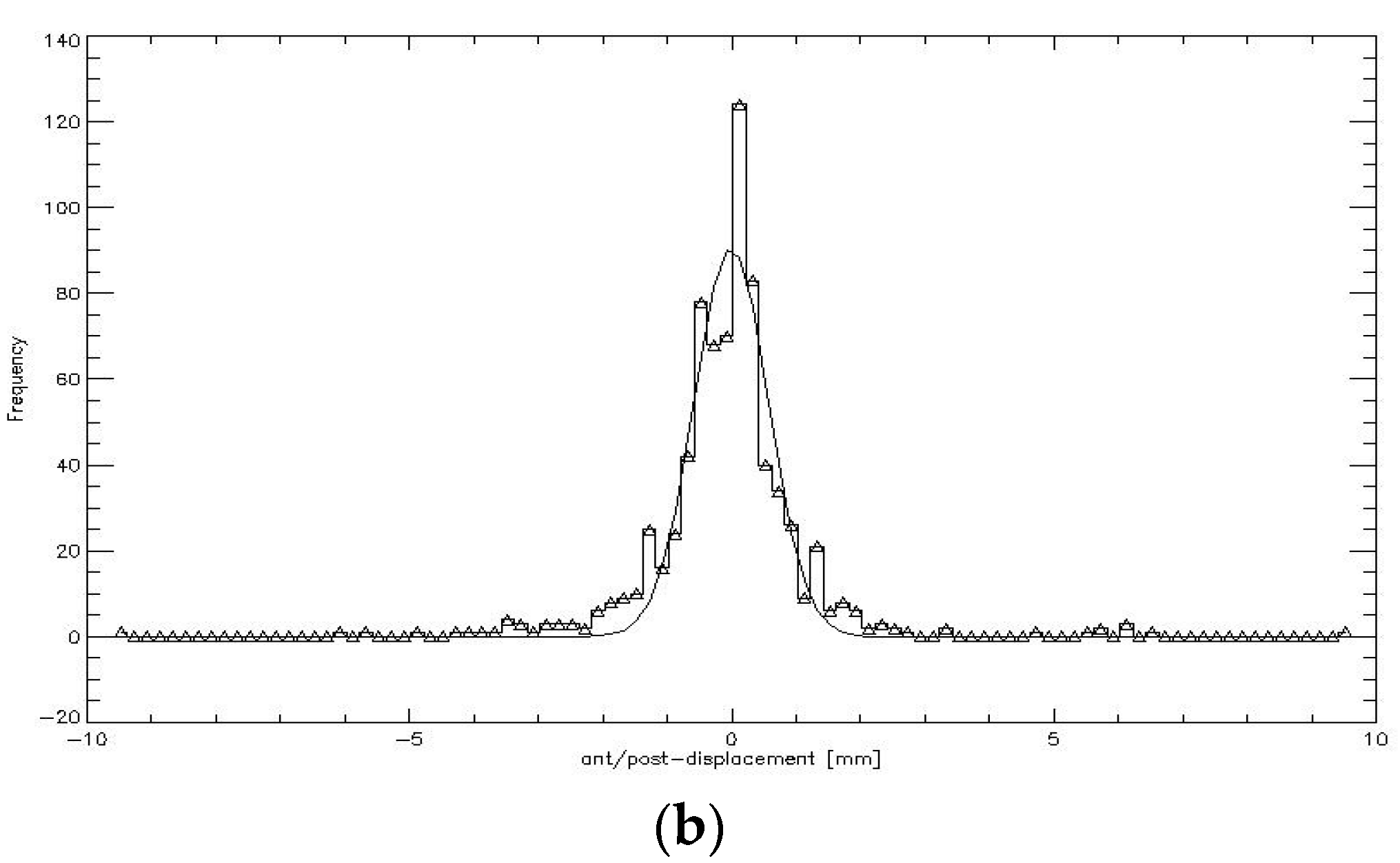
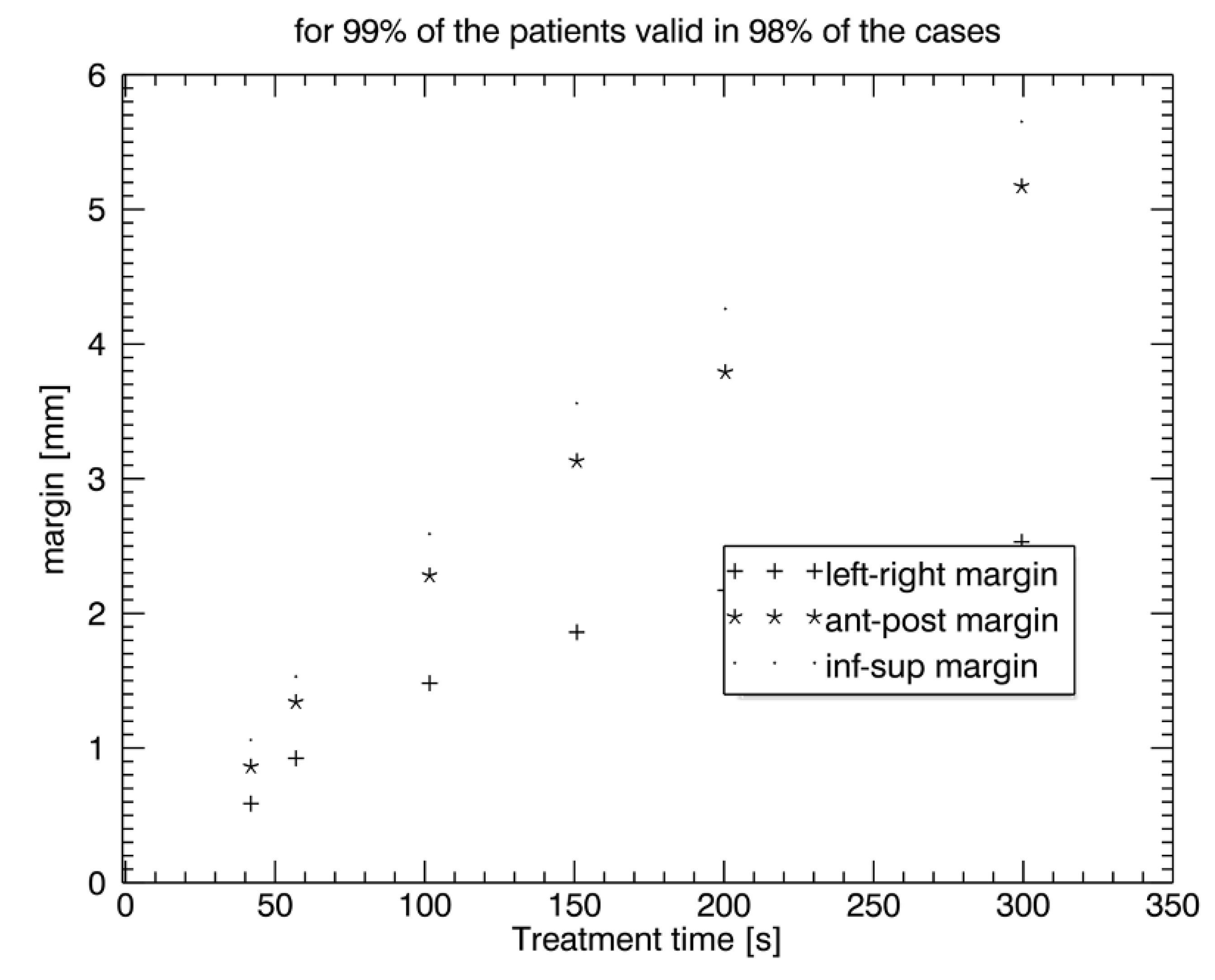
CTVprostate displacement patterns were graphically depicted as a function of timeout of the log file for each patient and all the treatment sessions in all three spatial directions by connecting data of the fractions to one total pattern. CTVprostate displacement patterns were evaluated for time intervals I of 0 s, 50 s, 100 s, 150 s, 200 s, and 300 s. Additionally, the displacement after each imaging section was assessed to calculate the necessary PTV safety margin for CyberKnife treatment. Since all the data were discrete and did not match the above time intervals, the closest datapoint was chosen. Therefore, the actual average time interval steps were I = 41.9 s (nominal, 0 s), 56.9 s (nominal, 50 s), 101 s (nominal, 100 s), 150.8 s (nominal, 150 s), 200.4 s (nominal, 200 s), and 299.4 s (nominal, 300 s). CTVprostate displacement was graphically depicted with frequency histograms with bin size 0.2 mm created for each piece of patient data and for each of the time intervals.
Assuming the prostate motion was random with a Gaussian distribution, a “propensity density function” (Equation (1)) was fitted for the
x-,
y-, and
z-directions (
σx,y,z).
This function describes a symmetric curve centered on the mean
μx with the total area under the curve equal to 1. The values
σx,y,z give the standard deviation of the distribution in all directions. CTV
prostate motion could be quantified by the variation of
σx,y,z, which is proportional to the probability that the CTV
prostate lies within the PTV. The proportionality constants that correspond to a certain coverage of the PTV are listed in
Table 1.
The variation of the CTVprostate movements (μσ) in the patient cohort was called μjΦ073 and was obtained by averaging the patients’ specific σji, and j specified the spatial direction. The standard deviation ∑j of this average corresponded to the patient variation.
The PTV safety margin into the
j direction valid for A% of all the patients for whom the CTV
prostate lies with a probability B% into the PTV was calculated as follows:
4. Discussion
Ultra-hypofractionated and stereotactic radiotherapy and adaptive radiotherapy result in prolonged treatment times. Radiation treatment time plays a crucial role in the magnitude of intrafraction prostate motion [
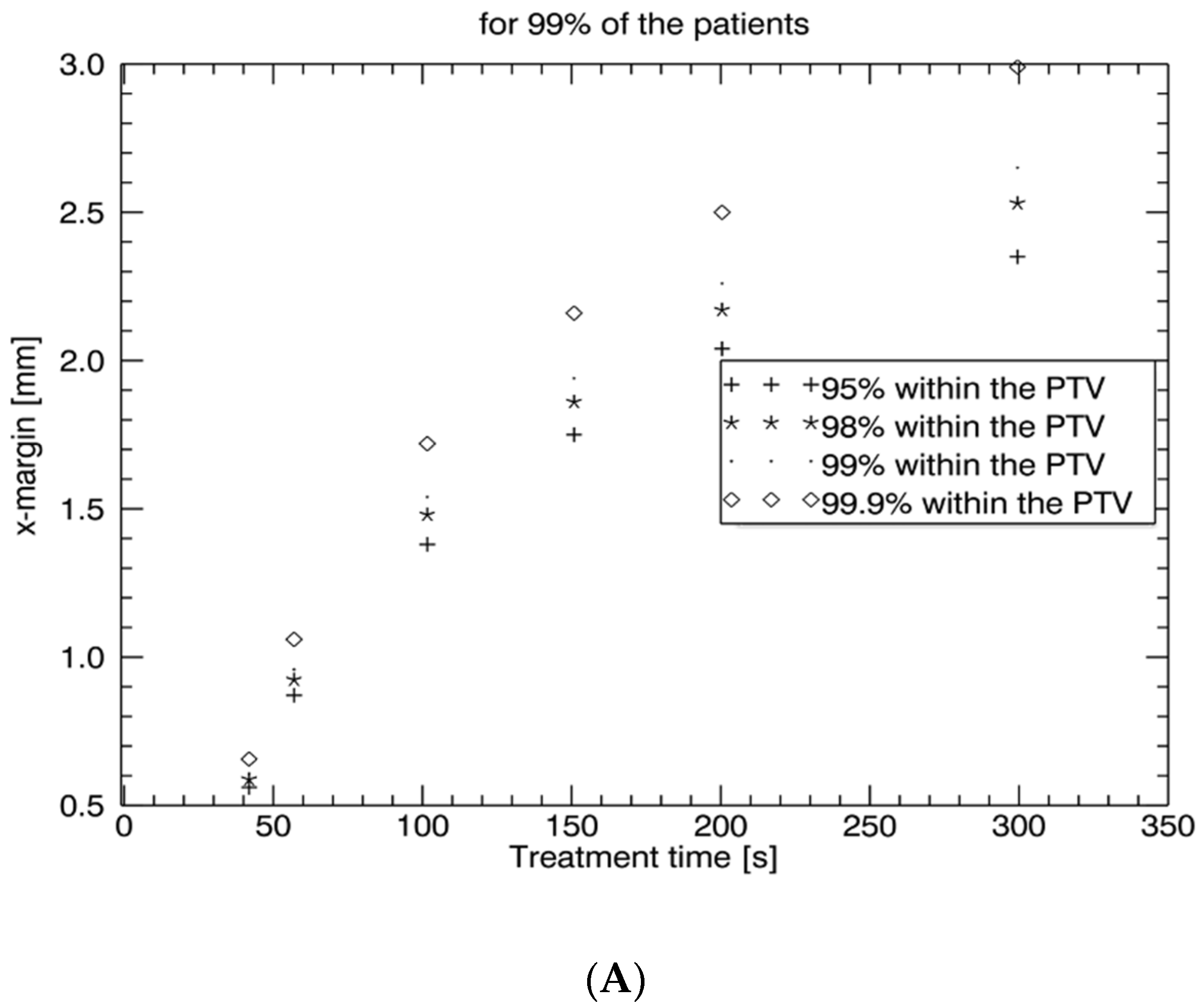
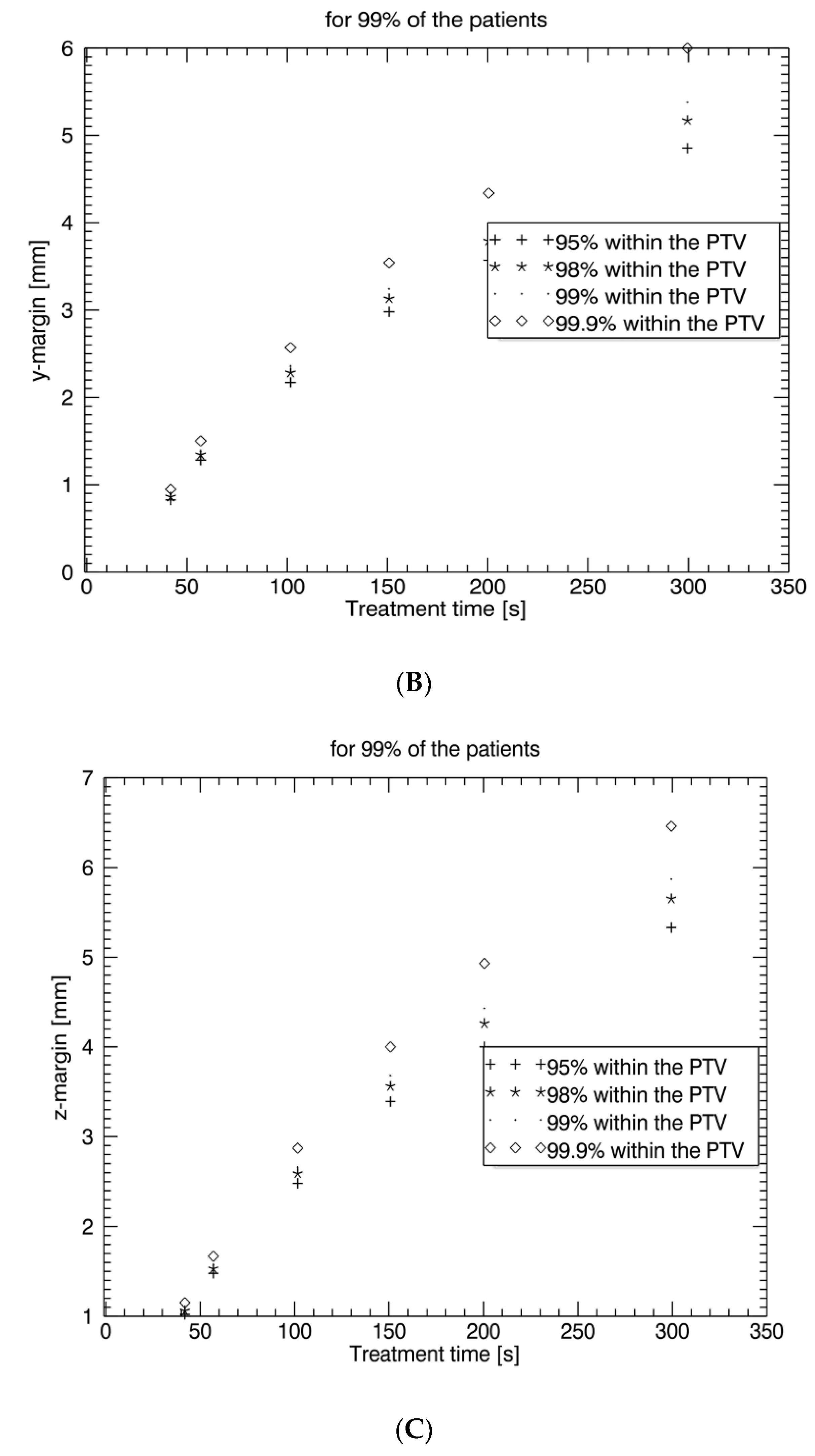
5]. Therefore, intrafraction motion management is important in these settings to avoid geographical miss and normal tissue exposure. Our study confirms that intrafraction uncertainty is time-dependent. For treatment times below 2.5 min (<150 s), which are generally needed for moderately hypofractionated radiotherapy with conventional LINACs, intrafraction prostate motion is low and may be reasonably compensated by a PTV margin of an additional 2–3 mm. However, for treatment times above 2.5 min, as generally needed for stereotactic radiotherapy and ultra-hypofractionation without an FF, intrafraction monitoring and correction are recommended since prostate dislocations are unacceptably high (5 min: > 5 mm). Our study further shows that an intrafraction imaging interval < 50 s with consecutive prostate tracking enables PTV safety margins for intrafraction uncertainty between 0.5 mm and 1 mm in all three directions,
x,
y, and
z. Due to the low number of included patients, the statistical results of this study need to be seen in consideration of other studies.
The duration of radiotherapy may vary substantially from 1 min to more than 10 min depending on the fractionation scheme, treatment machine (CyberKnife vs. LINAC), and beam modulation (FFF vs. flattening filter) [
3,
4]. Currently, with the evolution of adaptive radiotherapy systems (e.g., Ethos, Varian Medical Systems, Palo Alto, CA, USA; MR–LINAC hybrid systems), treatment times are further increased by at least 213 s [
12]. Studies have shown that prostate motion > 2 mm occurs in about 5% of the cases at 30 s, 8%—at 60 s, 11%—at 90 s, 14%—at 120 s [
7,
13,
14]. Prolonged RT times can increase intrafraction prostate movement from 3 up to 7 mm, which equals the margin from the CTV to the PTV [
15,
16]. With a median treatment time of 16 min, intrafraction shifts of the prostate > 5 mm were found in 12% of all the fractions, and a margin of 6 mm was calculated for compensation for this uncertainty [
15]. Mobility of the prostate weakly correlated with changes in the rectal volume but was independent of the bony anatomy or intrafraction bladder re-filling by 41 ccm [
15]. Other groups investigating intrafraction prostate motion with LINACs or rotational radiotherapy systems (e.g., Tomotherapy, Accuray, Sunnyvale, CA, USA) reported an overall error margin of 2–3 mm but did not indicate time dependency [
17,
18,
19]. Our study confirms that prostate shifts are time-dependent and depicts in detail rising correction margins of 0.5–1 mm at 40 s, 0.9–1.55 mm at 60 s, 1.5–2.6 mm at 100 s, 1.9–3.6 mm at 150 s, 2.2–4.2 mm at 200 s, and 2.6–5.6 mm at 300 s.
Currently, different methods for prostate motion management exist: (1) implanted radiopaque (gold) FM and kV/kV imaging for automatic three- to six-dimensional intrafraction prostate motion corrections (e.g., ExacTrac, Brainlab, Munich, Germany, as used in this study; Truebeam Auto Beam Hold
® tracking system, Varian Medical Systems, USA); (2) clarity autoscan with a monitoring system and its use of noninvasive soft tissue imaging to monitor prostate motion during the course of radiotherapy; this system uses a 4D autoscan ultrasound probe to image the prostate through the acoustic window of the perineum (Elekta Instrument AB, Stockholm, Sweden) [
20]; (3) implanted radiofrequency transponder beacons (Calypso system, Varian Medical Systems, Palo Alto, CA, USA); and more recently, (4) MRI-integrated systems (MRIdian, ViewRay, Oakwood Village, OH, USA; Electa Unity, Elekta Instrument AB, Stockholm, Sweden). Each technology is suitable for high-precision RT. Localization of the prostate using electromagnetic transponders agreed well with optical radiographic techniques [
21,
22]. However, systems using real-time adaptation were reported to significantly outperform nonadaptive delivery methods [
23]. The mean 2%/2 mm γ-fail rate was 1.4% with adaptation but 17.3% without adaptation in prostate treatment (
p < 0.001) [
23]. Some argue that real-time tracking is necessary since respiratory-induced prostate mobility within 30 s during radiotherapy is significant, especially in the superior–inferior direction, and can thus induce a geographical error [
13,
14]. Real-time couch-tracking resulted in submillimeter accuracy for prostate cancer, which transferred into high dosimetric accuracy [
24]. However, the mean displacements were minimal: 1.4 mm (–3.1 to 8.2 mm), –2.2 mm (–9.1 to 1.5 mm), and –0.3 mm (–5.0 to 1.8 mm) in the AP, SI, and LR directions, respectively [
13,
14]. A study by the MSKCC using implanted radiofrequency transponder beacons found that after the initial setup, 1.7 interventions per fraction were required to keep the prostate within 2 mm of its planned position, with a concomitant increase in time for dose delivery of approximately 65 s [
25]. The posterior PTV margin required for 95% of the dose to be delivered with the target positioned within the PTV was found to increase by 2 mm every 5 min [
25]. Our study shows that for treatment times below 2.5 min (<150 s), intrafraction prostate motion may be reasonably compensated by an additional PTV margin of 2–3 mm. However, for treatment times above 2.5 min, intrafraction monitoring and correction are recommended with an imaging interval of <50 s. This allows for PTV safety margins for intrafraction uncertainty between 0.5 mm and 1 mm. The dosimetric impact of online translation corrections based on the FM positions in kV/kV was analyzed using cine-MRI within the HypoFLAME trial, which investigated ultra-hypofractionated RT (5 × 7 Gy; average treatment time = 6 min) [
26]. They found that an overall PTV margin of 4–5 mm and online translation correction resulted in D99% coverage in 97% (CTV
prostate) and 100% (CTV
seminal vesicles) of the cases, whereas D99% for GTV
boost was only 87% [
26].
Treatment time for SBRT can be significantly reduced by using the FFF mode [
27]. Several articles reported significantly faster beam-on time (BOT) and treatment time (T × T) for FFF plans compared with flattened beam (FB) plans. The planning doses across these studies were 5–25 Gy per fraction. The mean BOT across the three studies for FB was 6.39 min, for FFF—2.42 min. The mean T × T was 11.21 min and 5.79 min for FB and FFF, respectively. Another method of reducing prostate motion for prostate SBRT is using endorectal immobilization with a rectal displacement device (RDD) [
28]. The RDD used in prostate SBRT leads to reduced intrafraction motion of the prostate and rectum, with increasing improvement with time. It also results in significant improvement in rectal wall dosimetry. Furthermore, Timmerman et al. showed that prostate immobilization with endorectal balloon (ERB) interventions reduced prostate deformation in the anterior–posterior direction, reduced errors in the sagittal rotation of the prostate, and increased the similarity in the shape of the prostate to the radiotherapy plan and resulted in improved dose–volume histogram (DVH) characteristics [
29]. Another group compared ERB interventions with a hydrogel spacer and showed comparable prostate motion between an ERB and a hydrogel spacer [
30]. The time dependencies were similar. A large majority of shifts for both ERB and hydrogel were well within a typical robust planning margin.
The evolution of adaptive radiotherapy (ART) inevitably results in further prolonged treatment preparation times in addition to beam-on times [
12,
31]. In the clinical setting, MR-based ART required an average of 45 min per fraction [
31]. Newer artificial intelligence (AI)-based ART systems might shorten the adaptation process. Nevertheless, optimized methods resulting in plans meeting the constraints required at least 213 s for the shape adaptation workflow [
12]. During the treatment–preparation time of 213 s, prostate shifts would require PTV margins of more than 4 mm, according to our results. Hence, MR or CBCT (cone-beam computed tomography) reimaging and target realignment before the start of the actual treatment is reasonable, irrespective of the intrafraction compensation method.