Risk for Arterial Thromboembolic Events (ATEs) in Patients with Advanced Urinary Tract Cancer (aUTC) Treated with First-Line Chemotherapy: Single-Center, Observational Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patient Population
2.2. Statistical Analysis
3. Results
3.1. Incidence of ATEs and Association with Clinical Characteristics
3.2. Association of ATEs with Type of Chemotherapy
3.3. Uni- and Multivariate Analysis of ATE Risk
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VTEs | Venous thromboembolic events |
| ATEs | Arterial thromboembolic events |
| RAMs | Risk Assessment Models |
| SEER | Surveillance Epidemiology and End Results |
| aUTC | Advanced urinary tract cancer |
| PAD | Peripheral arterial disease |
| LNL | Lymph-node/local |
| CIF | Cumulative incidence function |
| SHR | Sub-distribution hazard ratio |
| CVC | Central venous catheter |
| nSTEMI | Non-ST elevation myocardial infarction |
| STEMI | ST elevation myocardial infarction |
| TCC | Transitional cell carcinoma |
| CAD | Coronary artery disease |
| CAT | Cancer-associated thrombosis |
References
- Navi, B.B.; Reiner, A.S.; Kamel, H.; Iadecola, C.; Okin, P.M.; Elkind, M.S.V.; Panageas, K.S.; DeAngelis, L.M. Risk of Arterial Thromboembolism in Patients With Cancer. J. Am. Coll. Cardiol. 2017, 70, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Navi, B.B.; Reiner, A.S.; Kamel, H.; Iadecola, C.; Elkind, M.S.; Panageas, K.S.; DeAngelis, L. Association between incident cancer and subsequent stroke. Ann. Neurol. 2015, 77, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Zöller, B.; Ji, J.; Sundquist, J.; Sundquist, K. Risk of coronary heart disease in patients with cancer: A nationwide follow-up study from Sweden. Eur. J. Cancer 2012, 48, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Zöller, B.; Ji, J.; Sundquist, J.; Sundquist, K. Risk of haemorrhagic and ischaemic stroke in patients with cancer: A nationwide follow-up study from Sweden. Eur. J. Cancer 2012, 48, 1875–1883. [Google Scholar] [CrossRef]
- Yeh, E.T.H.; Chang, H.M. Cancer and clot: Between a rock and a hard place. J. Am. Coll. Cardiol. 2017, 70, 939–941. [Google Scholar] [CrossRef]
- Bang, O.Y.; Chung, J.W.; Lee, M.J.; Kim, S.J.; Cho, Y.H.; Kim, G.M.; Chung, C.-S.; Lee, K.H.; Ahn, M.-J.; Moon, G.J. Cancer cell-derived extracellular vesicles are associ- ated with coagulopathy causing ischemic stroke via tissue factor-independent way: The OASIS-CANCER Study. PLoS ONE 2016, 11, e0159170. [Google Scholar] [CrossRef]
- Demers, M.; Wagner, D.D. NETosis: A new factor in tumor progression and cancer-associated thrombosis. Semin. Thromb. Hemost. 2014, 40, 277–283. [Google Scholar] [CrossRef]
- Khorana, A.A. If Trousseau had a stroke. Blood 2019, 133, 769–770. [Google Scholar] [CrossRef]
- Barceló, R.; Muñoz, A.; López-Vivanco, G. Prospective evaluation of major vascular events in patients with non-small cell lung carcinoma treated with cisplatin and gemcitabine. Cancer 2005, 103, 994–999. [Google Scholar]
- Tully, C.M.; Apolo, A.B.; Zabor, E.C.; Regazzi, A.M.; Ostrovnaya, I.; Furberg, H.F.; Rosenberf, J.E.; Bajorin, D.F. The high incidence of vascular thromboembolic events in advanced urothelial cancer treated with platinum chemotherapy agents. Cancer 2016, 122, 712–721. [Google Scholar] [CrossRef]
- Lyman, G.H.; Bohlke, K.; Khorana, A.A.; Kuderer, N.M.; Lee, A.Y.; Arcelus, J.I.; Balaban, E.P.; Clarke, J.M.; Flowers, C.R.; Francis, C.W.; et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2016, 33, 654–656. [Google Scholar] [CrossRef]
- Agnelli, G.; George, D.J.; Kakkar, A.K.; Fisher, W.; Lassen, M.R.; Mismetti, P.; Mouret, P.; Chaudhari, U.; Lawson, F.; Turpie, A.G. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N. Engl. J. Med. 2012, 366, 601–609. [Google Scholar] [CrossRef]
- Agnelli, G.; Gussoni, G.; Bianchini, C.; Verso, M.; Mandalà, M.; Cavanna, L.; Barni, S.; Labianca, R.; Buzzi, F.; Scambia, G.; et al. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: A randomised, placebo-controlled, double-blind study. Lancet Oncol. 2009, 10, 943–949. [Google Scholar] [CrossRef]
- Carrier, M.; Abou-Nassar, K.; Mallick, R.; Tagalakis, V.; Shivakumar, S.; Schattner, A.; Kuruvilla, P.; Hill, D.; Spadafora, S.; Marquis, K.; et al. Apixaban to prevent venous thromboembolism in patients with cancer. N. Engl. J. Med. 2019, 380, 711–719. [Google Scholar] [CrossRef]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef]
- Gerotziafas, G.T.; Taher, A.; Abdel-Razeq, H.; AboElnazar, E.; Spyropoulos, A.C.; El Shemmari, S.; Larsen, A.K.; Elalamy, I.; COMPASS–CAT Working Group. A predictive score for thrombosis associated with breast, colorectal, lung, or ovarian cancer: The prospective COMPASS–Cancer Associated Thrombosis Study. Oncologist 2017, 22, 1222–1231. [Google Scholar] [CrossRef]
- Kim, A.S.; Johnston, S.C. Global variation in the relative burden of stroke and ischemic heart disease. Circulation 2011, 124, 314–323. [Google Scholar] [CrossRef]
- Velders, M.A.; Boden, H.; Hofma, S.H.; Osanto, S.; van der Hoeven, B.L.; Heestermans, A.A.; Cannegieter, S.C.; Jukema, J.W.; Umans, V.A.; Schalij, M.J.; et al. Outcome after ST elevation myocardial infarction in patients with cancer treated with primary percutaneous coronary intervention. Am. J. Cardiol. 2013, 112, 1867–1872. [Google Scholar] [CrossRef]
- Navi, B.B.; Singer, S.; Merkler, A.E.; Cheng, N.T.; Stone, J.B.; Kamel, H.; Iadecola, C.; Elkind, M.S.; DeAngelis, L.M. Recurrent thromboembolic events after ischemic stroke in patients with cancer. Neurology 2014, 83, 26–33. [Google Scholar] [CrossRef]
- Moore, R.A.; Adel, N.; Riedel, E.; Bhutani, M.; Feldman, D.R.; Tabbara, N.E.; Soff, G.; Parameswaran, R.; Hassoun, H. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: A large retrospective analysis. J. Clin. Oncol. 2011, 29, 3466–3473. [Google Scholar] [CrossRef]
- Zareba, P.; Duivenvoorden, W.C.M.; Pinthus, H.J. Thromboembolism in Patients with Bladder Cancer: Incidence, Risk Factors and Prevention. Bladder Cancer 2018, 4, 139–147. [Google Scholar] [CrossRef]
- Seng, S.; Liu, Z.; Chiu, S.K.; Proverbs-Singh, T.; Sonpavde, G.; Choueiri, T.K.; Tsao, C.-K.; Yu, M.; Hahn, N.M.; Oh, W.; et al. Risk of venous thromboembolism in patients with cancer treated with cisplatin: A systematic review and meta-analysis. J. Clin. Oncol. 2012, 30, 4416–4426. [Google Scholar] [CrossRef]
- Czaykowski, P.M.; Moore, M.J.; Tannock, I.F. High risk of vascular events in patients with urothelial transitional cell carcinoma treated with cisplatin based chemotherapy. J. Urol. 1998, 160 Pt 1, 2021–2024. [Google Scholar] [CrossRef]
- Fine, J.; Gray, R. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Bamias, A.; Dafni, U.; Karadimou, A.; Timotheadou, E.; Aravantinos, G.; Psyrri, A.; Xanthakis, I.; Tsiatas, M.; Koutoulidis, V.; Constantinidis, C.; et al. Prospective, open-label, randomized, phase III study of two dose-dense regimens MVAC versus gemcitabine/cisplatin in patients with inoperable, metastatic or relapsed urothelial cancer: A Hellenic Cooperative Oncology Group study (HE16/03). Ann. Oncol. 2013, 24, 1011–1017. [Google Scholar] [CrossRef]
- Ardavanis, A.; Tryfonopoulos, D.; Alexopoulos, A.; Kandylis, C.; Lainakis, G.; Rigatos, G. Gemcitabine and docetaxel as first-line treatment for advanced urothelial carcinoma: A phase II study. Br. J. Cancer 2005, 92, 645–650. [Google Scholar] [CrossRef][Green Version]
- Bamias, A.; Tiliakos, I.; Karali, M.-D.; Dimopoulos, M.A. Systemic chemotherapy in inoperable or metastatic bladder cancer. Ann. Oncol. 2006, 17, 553–561. [Google Scholar] [CrossRef]
- Bellmunt, J.; Albiol, S. Chemotherapy for metastatic or unresectable bladder cancer. Semin. Oncol. 2007, 34, 135–144. [Google Scholar] [CrossRef]
- Kaufman, D.S.; Carducci, M.A.; Kuzel, T.M.; Todd, M.B.; Oh, W.K.; Smith, M.R.; Ye, Z.; Nicol, S.J.; Stadler, W.M. A multi-institutional phase II trial of gemcitabine plus paclitaxel in patients with locally advanced or metastatic urothelial cancer. Urol. Oncol. 2004, 22, 393–397. [Google Scholar] [CrossRef]
- Lin, C.-C.; Hsu, C.-H.; Huang, C.-Y.; Keng, H.-Y.; Tsai, Y.-C.; Huang, K.-H.; Cheng, A.-L.; Pu, Y.-S. Gemcitabine and ifosfamide as a second-line treatment for cisplatin-refractory metastatic urothelial carcinoma: A phase II study. Anticancer. Drugs 2007, 18, 487–491. [Google Scholar] [CrossRef]
- Bamias, A.; Moulopoulos, L.A.; Koutras, A.; Aravantinos, G.; Fountzilas, G.; Pectasides, D.; Kastritis, E.; Gika, D.; Skarlos, D.; Linardou, H.; et al. The combination of gemcitabine an carboplatin as first-line treatment in patients with advanced urothelial carcinoma. A Phase II study of the Hellenic Cooperative Oncology Group. Cancer 2006, 106, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; Ribas, A.; Eres, N.; Albanell, J.; Almanza, C.; Bermejo, B.; Solé, L.-A.; Baselga, J. Carboplatin-based versus cisplatin-based chemotherapy in the treatment of surgically incurable advanced bladder carcinoma. Cancer 1997, 80, 1966–1972. [Google Scholar] [CrossRef]
- Kyriakakis, Z.; Dimopoulos, M.A.; Kostakopoulos, A.; Karayiannis, A.; Sofras, F.; Zervas, A.; Giannopoulos, A.; Dimopoulos, C. Cisplatin, ifosfamide, methotrexate and vinblastine combination chemotherapy for metastatic urothelial cancer. J. Urol. 1997, 158, 408–411. [Google Scholar] [CrossRef]
- Von der Maase, H.; Sengelov, L.; Roberts, J.T.; Ricci, S.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Zimmermann, A.; Arning, M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J. Clin. Oncol. 2005, 23, 4602–4608. [Google Scholar] [CrossRef] [PubMed]
- Dreicer, R.; Manola, J.; Roth, B.J.; See, W.A.; Kuross, S.; Edelman, M.J.; Hudes, G.R.; Wilding, G. Phase III trial of methotrexate, vinblastine, doxorubicin, and cisplatin versus carboplatin and paclitaxel in patients with advanced carcinoma of the urothelium. Cancer 2004, 100, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Pistamaltzian, N.; Tzannis, K.; Pissanidou, V.; Peroukidis, S.; Milaki, G.; Karavasilis, V.; Mitsogiannis, I.; Varkarakis, I.; Papatsoris, A.; Dellis, A.; et al. Treatment of relapsed urothelial bladder cancer with vinflunine: Real-world evidence by the Hellenic Genitourinary Cancer Group. Anticancer. Drugs 2016, 27, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Bakoyannis, C.; Georgoulias, V.; Papadimitriou, C.; Moulopoulos, L.A.; Deliveliotis, C.; Karayannis, A.; Varkarakis, I.; Aravantinos, G.; Zervas, A.; et al. Docetaxel and cisplatin combination chemotherapy in advanced carcinoma of the urothelium: A multicenter phase II study of the Hellenic Cooperative Oncology Group. Ann. Oncol. 1999, 10, 1385–1388. [Google Scholar] [CrossRef]
- McGale, P.; Darby, S.C.; Hall, P.; Adolfsson, J.; Bengtsson, N.O.; Bennet, A.M.; Fornander, T.; Gigante, B.; Jensen, M.-B.; Peto, R.; et al. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother. Oncol. 2011, 100, 167–175. [Google Scholar] [CrossRef]
- Chen, P.C.; Muo, C.H.; Lee, Y.T.; Yu, Y.H.; Sung, F.C. Lung cancer and incidence of stroke: A population-based cohort study. Stroke 2011, 42, 3034–3039. [Google Scholar] [CrossRef]
- Maduro, J.H.; den Dekker, H.A.; Pras, E.; de Vries, E.G.; van der Zee, A.G.; Klokman, W.J.; Reyners, A.K.; van Leeuwen, F.E.; Langendijk, J.A.; de Bock, G.H.; et al. Cardiovascular morbidity after radiotherapy or chemoradiation in patients with cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1337–1344. [Google Scholar] [CrossRef]
- Chu, C.N.; Chen, S.W.; Bai, L.Y.; Mou, C.H.; Hsu, C.Y.; Sung, F.C. Increase in stroke risk in patients with head and neck cancer: A retrospective cohort study. Br. J. Cancer 2011, 105, 1419–1423. [Google Scholar] [CrossRef]
- Moser, E.C.; Noordijk, E.M.; van Leeuwen, F.E.; le Cessie, S.; Baars, J.W.; Thomas, J.; Carde, P.; Meerwaldt, J.H.; Van Glabbeke, M.; Kluin-Nelemans, H.C. Long-term risk of cardiovascular disease after treatment for aggressive non-Hodgkin lymphoma. Blood 2006, 107, 2912–2919. [Google Scholar] [CrossRef]
- Kuan, A.-S.; Chen, S.-C.; Yeh, C.-M.; Hung, M.-H.; Hung, Y.-P.; Chen, T.-J.; Liu, C.-J. Risk of ischemic stroke in patients with gastric cancer: A nationwide population-based cohort study. Medicine 2015, 94, e1336. [Google Scholar] [CrossRef]
- Kuan, A.-S.; Teng, C.-J.; Wu, H.-H.; Su, V.-Y.; Chen, Y.-T.; Chien, S.-H.; Yeh, C.-M.; Hu, L.-Y.; Chen, T.-J.; Tzeng, C.-H.; et al. Risk of ischemic stroke in patients with ovarian cancer: A nationwide population-based study. BMC Med. 2014, 12, 53. [Google Scholar] [CrossRef][Green Version]
- Ramos, J.D.; Casey, M.F.; Crabb, S.J.; Bamias, A.; Harshman, L.C.; Wong, Y.N.; Bellmunt, J.; De Giorgi, U.; Ladoire, S.; Powles, T.; et al. Venous thromboembolism in metastatic urothelial carcinoma or variant histologies: Incidence, associative factors, and effect on survival. Cancer Med. 2017, 6, 186–194. [Google Scholar] [CrossRef]
- Reardon, Z.D.; Patel, S.G.; Zaid, H.B.; Stimson, C.J.; Resnick, M.J.; Keegan, K.A.; Barocas, D.A.; Chang, S.S.; Cookson, M.S. Trends in the Use of Perioperative Chemotherapy for Localized and Locally Advanced Muscle-invasive Bladder Cancer: A Sign of Changing Tides. Eur. Urol. 2015, 67, 165–170. [Google Scholar] [CrossRef]
- Goff, D.C., Jr.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J.; et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, S49–S73. [Google Scholar] [CrossRef]
- Meschia, J.F.; Bushnell, C.; Boden-Albala, B.; Braun, L.T.; Bravata, D.M.; Chaturvedi, S.; Creager, M.A.; Eckel, R.H.; Elkind, M.S.V.; Fornage, M.; et al. Guidelines for the primary prevention of stroke: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 3754–3832. [Google Scholar] [CrossRef]
- McSweeney, J.C.; Rosenfeld, A.G.; Abel, W.M.; Braun, L.T.; Burke, L.E.; Daugherty, S.L.; Fletcher, G.F.; Gulati, M.; Mehta, L.S.; Pettey, C.; et al. Preventing and experiencing ischemic heart disease as a woman: State of the science: A scientific statement from the American Heart Association. Circulation 2016, 133, 1302–1331. [Google Scholar] [CrossRef]
- Brenner, B.; Bikdeli, B.; Tzoran, I.; Madridano, O.; López-Reyes, R.; Suriñach, J.M.; Blanco-Molina, Á.; Tufano, A.; Núñez, J.J.L.; Trujillo-Santos, J.; et al. Arterial ischemic events are a major complication in cancer patients with venous thromboembolism. Am. J. Med. 2018, 131, 1095–1103. [Google Scholar] [CrossRef]
- Khorana, A.A.; Soff, G.A.; Kakkar, A.K.; Vadhan-Raj, S.; Riess, H.; Wun, T.; Streiff, M.B.; Garcia, D.A.; Liebman, H.A.; Belani, C.P.; et al. Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. N. Engl. J. Med. 2019, 380, 720–728. [Google Scholar] [CrossRef]
| Characteristic | Median | Range | |||
| Age | 67 | 32–88 | |||
| Weight | 73.5 | 43–125 | |||
| BMI | 26 | 15–51.1 | |||
| BSA | 1.8 | 1.3–2.4 | |||
| Months after major surgery | 6 | 1–180 | |||
| Cycles of chemotherapy | 6 | 1–20 | |||
| Characteristic | Total | ATE | p Value | ||
| n | % | Yes (%) | No (%) | ||
| Sex | 0.230 | ||||
| Female | 59 | 16.7 | 0 (0) | 59 (17.3) | |
| Male | 295 | 83.3 | 12(100) | 283 (82.7) | |
| Diabetes | 0.660 | ||||
| Yes | 46 | 13 | 2 (16.7) | 44 (12.9) | |
| No | 308 | 87 | 10 (83.3) | 298 (87.1) | |
| BMI >35 | 0.084 | ||||
| Yes | 18 | 5.1 | 0 (0) | 18 (5.3) | |
| No | 334 | 94.4 | 11 (91.7) | 323 (94.4) | |
| Missing | 2 | 0.5 | 1 (8.3) | 1 (0.3) | |
| BMI > 25 | 0.097 | ||||
| Yes | 208 | 58.8 | 7 (58.3) | 201 (58.8) | |
| No | 144 | 40.7 | 4 (33.3) | 140 (40.9) | |
| Missing | 2 | 0.5 | 1 (8.3) | 1 (0.3) | |
| Anti-platelet therapy | 0.686 | ||||
| Yes | 51 | 14.4 | 2 (16.7) | 49 (14.3) | |
| No | 303 | 85.6 | 10 (83.3) | 293 (85.7) | |
| Anticoagulants | >0.999 | ||||
| Yes | 18 | 5.1 | 0 (0) | 18 (5.3) | |
| No | 336 | 94.9 | 12 (100) | 324 (94.7) | |
| Antihypertensives | 0.773 | ||||
| Yes | 137 | 38.7 | 4 (33.3) | 133 (38.9) | |
| No | 217 | 61.3 | 8 (66.7) | 209 (61.1) | |
| Atrial fibrillation | >0.999 | ||||
| Yes | 12 | 3.4 | 0 (0) | 12 (3.5) | |
| No | 342 | 96.6 | 12 (100) | 330 (96.5) | |
| Cholesterol-lowering medication | >0.999 | ||||
| Yes | 54 | 15.3 | 2 (16.7) | 52 (15.2) | |
| No | 300 | 84.8 | 10 (83.3) | 290 (84.8) | |
| Coronary artery disease | >0.999 | ||||
| Yes | 48 | 13.6 | 1 (8.3) | 47 (13.7) | |
| No | 306 | 86.4 | 11(91.7) | 295 (86.3) | |
| Previous peripheral arterial embolism/thrombosis | 0.336 | ||||
| Yes | 13 | 3.7 | 1 (8.3) | 12 (3.5) | |
| No | 341 | 96.3 | 11 (91.7) | 330 (96.5) | |
| Smoking history | 0.952 | ||||
| Yes | 174 | 49.2 | 6 (50) | 168 (49.1) | |
| No | 180 | 50.9 | 6 (50) | 174 (50.9) | |
| Solid tumour other than UTC | 0.022 | ||||
| Yes | 35 1 | 9.9 | 4 (33.3) | 31 (9.1) | |
| No | 319 | 90.1 | 8 (66.7) | 311 (90.9) | |
| Previous VTE | >0.999 | ||||
| Yes | 29 | 8.2 | 1 (8.3) | 28 (8.2) | |
| No | 325 | 91.8 | 11 (91.7) | 314 (91.8) | |
| Haematologic malignancy | >0.999 | ||||
| Yes | 3 | 0.9 | 0 (0) | 3 (0.9) | |
| No | 351 | 99.1 | 12 (100) | 339 (99.1) | |
| Coagulation disorder | >0.999 | ||||
| Yes | 2 | 0.6 | 0 (0) | 2 (0.6) | |
| No | 352 | 99.4 | 12 (100) | 340 (99.4) | |
| Major Surgery | >0.999 | ||||
| Other major surgery (no cystectomy) | 46 | 13 | 1 (8.3) | 45 (13.2) | |
| Cystectomy | 186 | 52.5 | 7 (58.4) | 179 (52.3) | |
| No major surgery | 122 | 34.5 | 4 (33.3) | 118 (34.5) | |
| Time since UTC diagnosis | >0.999 | ||||
| ≤6 months | 12 | 3.4 | 0 (0) | 12 (3.5) | |
| >6 months | 342 | 96.6 | 12 (100) | 330 (96.5) | |
| Histology | 0.084 | ||||
| TCC | 308 | 87 | 10 (83.3) | 298 (87.1) | |
| Mixed | 34 | 9.6 | 0 (0) | 34 (10) | |
| non-TCC | 11 | 3.1 | 2 (16.7) | 9 (2.6) | |
| Missing | 1 | 0.3 | 0 (0) | 1 (0.3) | |
| Primary site | 0.373 | ||||
| Bladder | 298 | 84.2 | 12 (100) | 286 (83.6) | |
| Bladder/Renal pelvis | 2 | 0.5 | 0 (0) | 2 (0.6) | |
| Renal pelvis | 50 | 14.1 | 0 (0) | 50 (14.6) | |
| Ureter | 3 | 0.9 | 0 (0) | 3 (0.9) | |
| Urethra | 1 | 0.3 | 0 (0) | 1 (0.3) | |
| Performance status | 0.127 | ||||
| 0 | 124 | 35 | 4 (33.3) | 120 (35.1) | |
| 1 | 132 | 37.3 | 8 (66.7) | 124 (36.3) | |
| 2 | 73 | 20.6 | 0 (0) | 73 (21.4) | |
| 3 | 25 | 7.1 | 0 (0) | 25 (7.3) | |
| Number of disease sites | 0.754 | ||||
| 1 | 206 | 58.2 | 9 (75) | 197 (57.6) | |
| 2 | 107 | 30.2 | 3 (25) | 104 (30.4) | |
| 3 | 33 | 9.3 | 0 (0) | 33 (9.7) | |
| 4 | 8 | 2.3 | 0 (0) | 8 (2.3) | |
| Location of disease | 0.350 | ||||
| Pelvis | 237 | 67 | 10 (83.3) | 227 (66.4) | |
| Non-pelvis | 117 | 33 | 2 (16.7) | 115 (33.6) | |
| Type of Chemotherapy | |||||
| Cisplatin | 188 | 53.1 | 5 (41.7) | 183 (53.5) | 0.139 |
| Carboplatin | 150 | 42.4 | 5 (41.7) | 145 (42.4) | |
| Other | 16 | 4.5 | 2 (616.7) | 14 (4.1) | |
| Conventional | 199 | 56.2 | 7 (58.3) | 192 (56.2) | 0.880 |
| Dose-dense | 155 | 43.8 | 5 (41.7) | 150 (43.8) | |
| Gemcitabine | 222 | 62.7 | 8 (66.7) | 214 (62.6) | >0.999 |
| Other | 132 | 37.3 | 4 (33.3) | 128 (37.4) | |
| Anthracycline | 96 | 27.1 | 2 (16.7) | 94 (27.5) | 0.525 |
| Non-anthracycline | 258 | 72.9 | 10 (83.3) | 248 (72.5) | |
| History of neoadjuvant/adjuvant chemotherapy | 0.001 | ||||
| Yes | 73 | 20.6 | 7 (58.3) | 66 (19.3) | |
| No | 281 | 79.4 | 5 (41.9) | 276 (80.7) | |
| History of chemotherapy not for UTC | 0.188 | ||||
| Yes | 6 | 1.7 | 1(8.3) | 5 (1.5) | |
| No | 348 | 98.3 | 11 (91.7) | 337 (98.5) | |
| History of radiation | >0.999 | ||||
| Yes | 83 | 23.5 | 3 (25) | 80 (23.4) | |
| No | 271 | 76.5 | 9 (75) | 262 (76.6) | |
| Radiation field | 0.424 | ||||
| Pelvis | 58 | 16.4 | 3 (25) | 55 (16.1) | |
| Other + no radiation | 296 | 83.6 | 9 (75) | 287 (83.9) | |
| History of hormone therapy | >0.999 | ||||
| Yes | 9 | 2.5 | 0 (0) | 9 (2.6) | |
| No | 345 | 97.5 | 12 (100) | 333 (97.4) | |
| Hormone/anthracycline therapy | 0.524 | ||||
| Yes | 97 | 27.4 | 2 (16.7) | 95 (27.8) | |
| No | 257 | 72.6 | 10 (83.3) | 247 (72.2) | |
| Pre-chemo PLTs > 350,000/μL | >0.999 | ||||
| Yes | 113 | 31.9 | 4 (33.3) | 109 (31.9) | |
| No | 241 | 68.1 | 8 (66.7) | 233 (68.1) | |
| Hgb < 10 g/dL or ESA | >0.999 | ||||
| Yes | 34 | 9.6 | 1 (8.3) | 33 (9.6) | |
| No | 320 | 90.4 | 11 (91.7) | 309 (90.4) | |
| Pre-chemo WBCs > 11,000/μL | 0.704 | ||||
| Yes | 66 | 18.6 | 1 (8.3) | 65 (19) | |
| No | 288 | 81.4 | 11 (91.7) | 277 (81) | |
| ATE | |||||
| Yes | 12 | 3.4 | |||
| No | 342 | 96.6 | |||
| Type of ATE | |||||
| Peripheral arterial thrombosis/embolism | 2 | ||||
| Ischaemic stroke | 7 | ||||
| Unstable angina | 1 | ||||
| MI | 2 | ||||
| Subsequent lines of therapy | 0.588 | ||||
| 0 | 220 | 62.2 | 7 (58.3) | 213 (62.3) | |
| 1 | 82 | 23.1 | 2 (16.7) | 80 (23.4) | |
| 2–5 | 51 | 14.4 | 3 (25) | 48 (14) | |
| missing | 1 | 0.3 | 0 (0) | 1 (0.3) | |
| n (%) | Incidence Function (%) | ||||
|---|---|---|---|---|---|
| 3-Month | 6-Month | 12-Month | 24-Month | ||
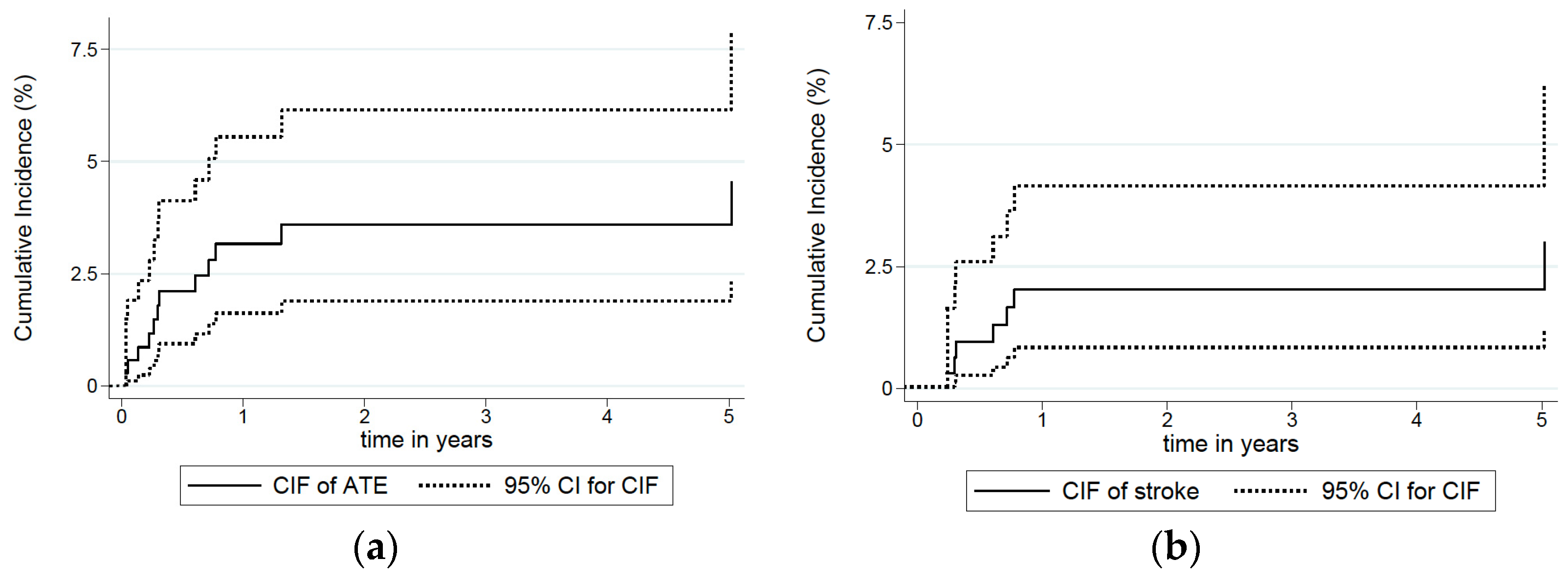
| Total ATE cases | 12 (100) | 1.2 (0.4–2.8) | 2.1 (0.9–4.1) | 3.2 (1.6–5.6) | 3.6 (1.9–6.2) |
| Ischaemic stroke | 7 (58.3) | 0.3 (0.3–1.6) | 1 (0.3–2.6) | 2 (0.8–4.2) | 2 (0.8–4.2) |
| Cisplatin | |||||
| Yes | 188 (53.1) | 1.1 (0.2–3.6) | 2.3 (0.8–5.4) | 2.9 (0.8–5.4) | 2.9 (1.1–6.3) |
| No | 166 (46.9) | 1.3 (0.3–4.1) | 1.9 (0.5–5.1) | 3.5 (1.3–7.4) | 4.4 (1.8–8.8) |
| Dose dense chemotherapy | |||||
| Yes | 155 (43.8) | 1.3 (0.3–4.3) | 2.8 (0.9–6.6) | 3.6 (1.3–7.7) | 3.6 (1.3–7.7) |
| No | 199 (56.2) | 1 (0.2–3.4) | 1.6 (0.4–4.2) | 2.8 (1.1–6.1) | 3.6 (1.5–7.2) |
| Gemcitabine | |||||
| Yes | 222 (62.7) | 1.4 (0.4–3.8) | 2.5 (0.9–5.3) | 3.1 (1.3–6.3) | 3.9 (1.7–7.5) |
| No | 132 (37.3) | 0.8 (0.1–3.8) | 1.6 (0.3–5.1) | 3.2 (1.1–7.5) | 3.2 (1.1–7.5) |
| Histology | |||||
| TCC + mixed | 342 (96.9) | 0.6 (0.1–2.1) | 1.6 (0.6–3.5) | 2.7 (1.3–5) | 3.1 (1.5–5.7) |
| Other | 11 (3.1) | 18.2 (2.9–44.2) | 18.2 (2.9–44.2) | 18.2 (2.9–44.2) | 18.2 (2.9–44.2) |
| Solid tumour other than UTC | |||||
| No | 319 (90.1) | 1.3 (0.4–3.1) | 1.7 (0.6–3.6) | 2.4 (1.1–4.7) | 2.9 (1.3–5.4) |
| Yes | 35 (9.9) | - | 6.5 (1.2–18.7) | 11 (2.7–25.9) | 11 (2.7–25.9) |
| History of adjuvant/neoadjuvant | |||||
| None | 281 (79.4) | 0.8 (0.2–2.5) | 1.2 (0.3–3.1) | 1.6 (0.5–3.8) | 2.1 (0.8–4.7) |
| One at least | 73 (20.6) | 2.8 (0.5–8.6) | 5.7 (1.8–12.8) | 9 (3.7–17.4) | 9 (3.7–17.4) |
| Univariate | Multivariate | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cox Regression Analysis | Competing Risks Analysis | Competing Risks Analysis | ||||||||
| Factor | n | HR | 95% CI | p | SHR | 95% CI | p | SHR | 95% CI | p |
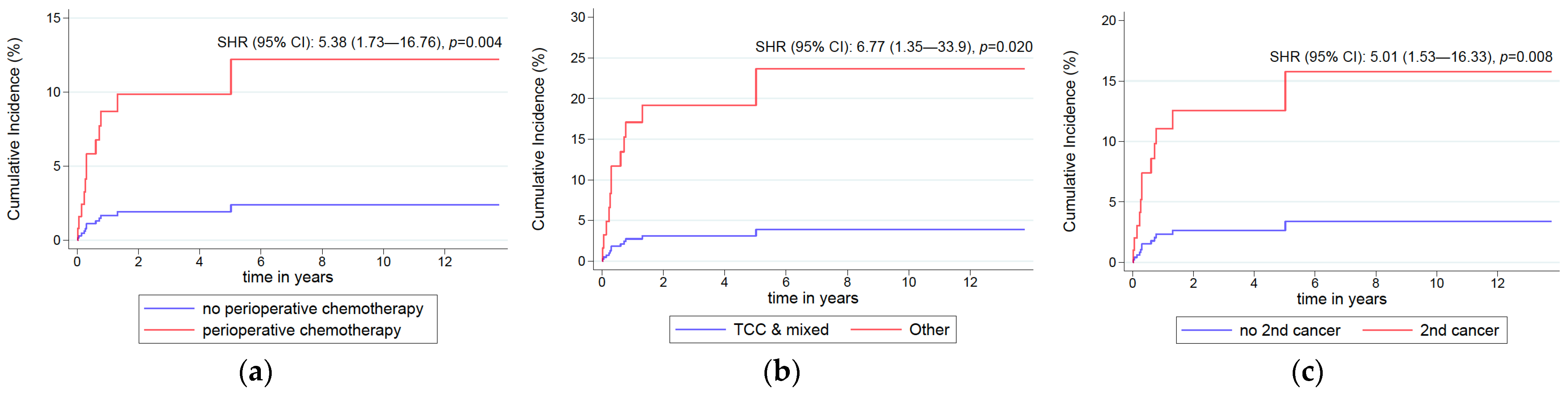
| Solid tumour other than UTC | 0.010 | 0.008 | 0.028 | |||||||
| No | 319 | Ref | Ref | Ref | ||||||
| Yes | 35 | 4.88 | 1.46–16.3 | 5.01 | 1.53–16.33 | 3.71 | 1.15–11.97 | |||
| Histology | 0.003 | 0.020 | 0.028 | |||||||
| TCC + mixed | 342 | Ref | Ref | Ref | ||||||
| Other | 11 | 10.15 | 2.16–47.68 | 6.77 | 1.35–33.9 | 7.79 | 1.25–48.43 | |||
| History of adjuvant/neoadjuvant | 0.005 | 0.004 | 0.010 | |||||||
| None | 281 | Ref | Ref | Ref | ||||||
| One at least | 73 | 5.17 | 1.63–16.41 | 5.38 | 1.73–16.76 | 5.55 | 1.51–20.48 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bamias, A.; Tzannis, K.; Zakopoulou, R.; Sakellakis, M.; Dimitriadis, J.; Papatheodoridi, A.; Rallidis, L.; Halvatsiotis, P.; Tsiara, A.; Kaparelou, M.; et al. Risk for Arterial Thromboembolic Events (ATEs) in Patients with Advanced Urinary Tract Cancer (aUTC) Treated with First-Line Chemotherapy: Single-Center, Observational Study. Curr. Oncol. 2022, 29, 6077-6090. https://doi.org/10.3390/curroncol29090478
Bamias A, Tzannis K, Zakopoulou R, Sakellakis M, Dimitriadis J, Papatheodoridi A, Rallidis L, Halvatsiotis P, Tsiara A, Kaparelou M, et al. Risk for Arterial Thromboembolic Events (ATEs) in Patients with Advanced Urinary Tract Cancer (aUTC) Treated with First-Line Chemotherapy: Single-Center, Observational Study. Current Oncology. 2022; 29(9):6077-6090. https://doi.org/10.3390/curroncol29090478
Chicago/Turabian StyleBamias, Aristotelis, Kimon Tzannis, Roubini Zakopoulou, Minas Sakellakis, John Dimitriadis, Alkistis Papatheodoridi, Loukianos Rallidis, Panagiotis Halvatsiotis, Anna Tsiara, Maria Kaparelou, and et al. 2022. "Risk for Arterial Thromboembolic Events (ATEs) in Patients with Advanced Urinary Tract Cancer (aUTC) Treated with First-Line Chemotherapy: Single-Center, Observational Study" Current Oncology 29, no. 9: 6077-6090. https://doi.org/10.3390/curroncol29090478
APA StyleBamias, A., Tzannis, K., Zakopoulou, R., Sakellakis, M., Dimitriadis, J., Papatheodoridi, A., Rallidis, L., Halvatsiotis, P., Tsiara, A., Kaparelou, M., Kostouros, E., Barbarousi, D., Koutsoukos, K., Fragiadis, E., Dellis, A. E., Anastasiou, I., Stravodimos, K., Pinitas, A., Papatsoris, A., ... Dimopoulos, M.-A. (2022). Risk for Arterial Thromboembolic Events (ATEs) in Patients with Advanced Urinary Tract Cancer (aUTC) Treated with First-Line Chemotherapy: Single-Center, Observational Study. Current Oncology, 29(9), 6077-6090. https://doi.org/10.3390/curroncol29090478








