Nomograms Based on Serum N-glycome for Diagnosis of Papillary Thyroid Microcarcinoma and Prediction of Lymph Node Metastasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Serum N-glycome Detection and MS Data Processing
2.3. Statistical Analysis
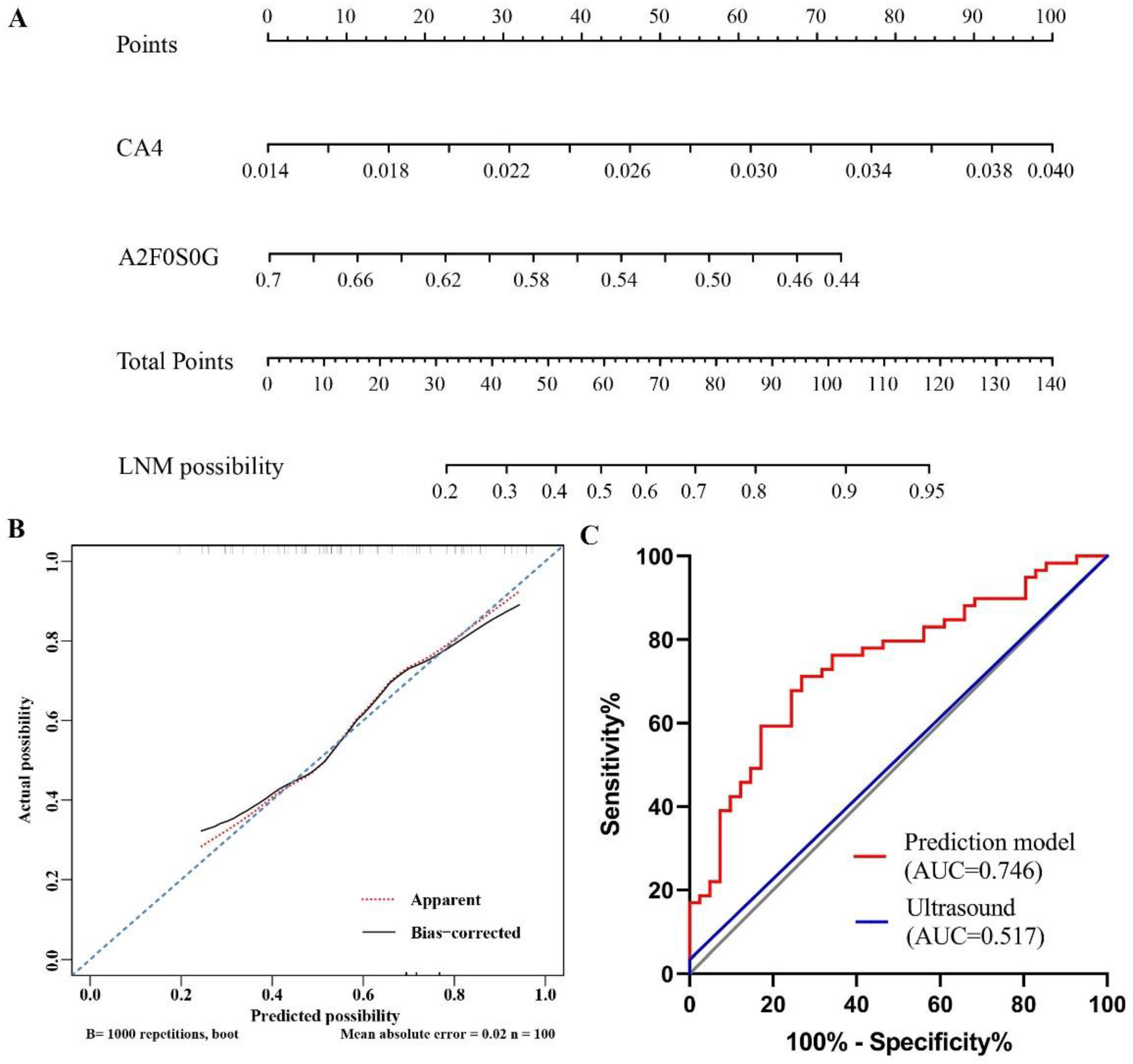
3. Results
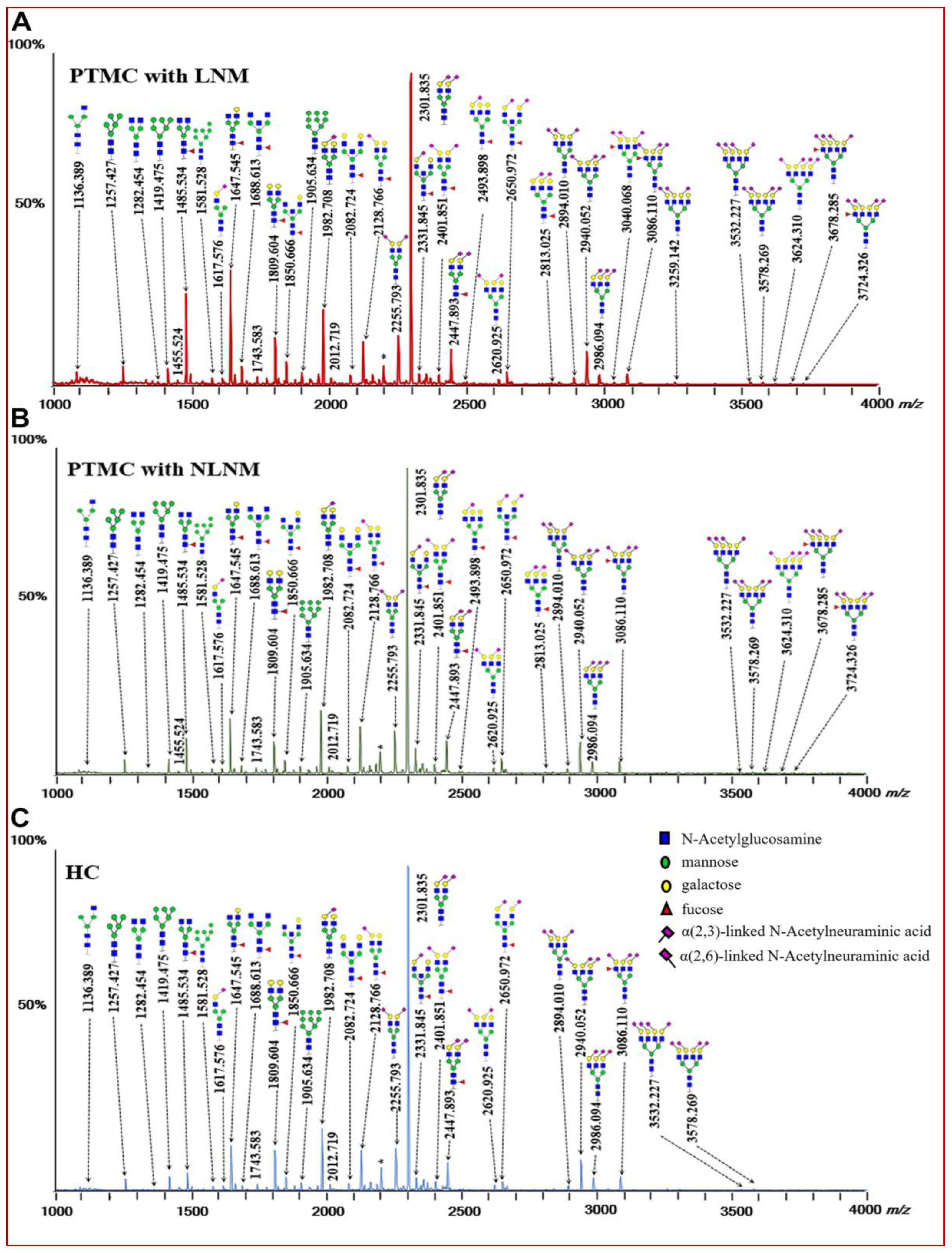
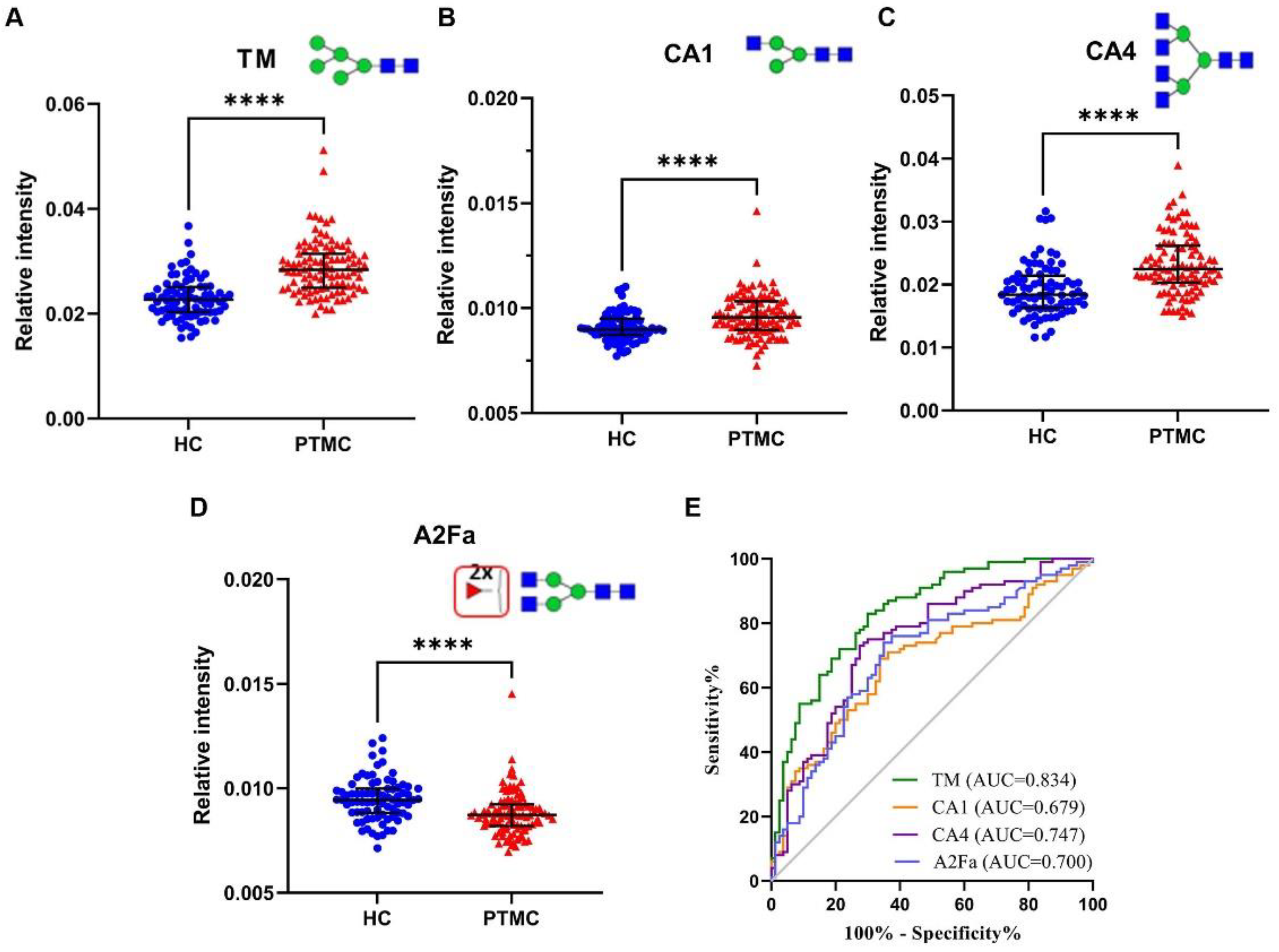
3.1. Identification of Serum N-glycomic Features for Discriminating PTMC from HC
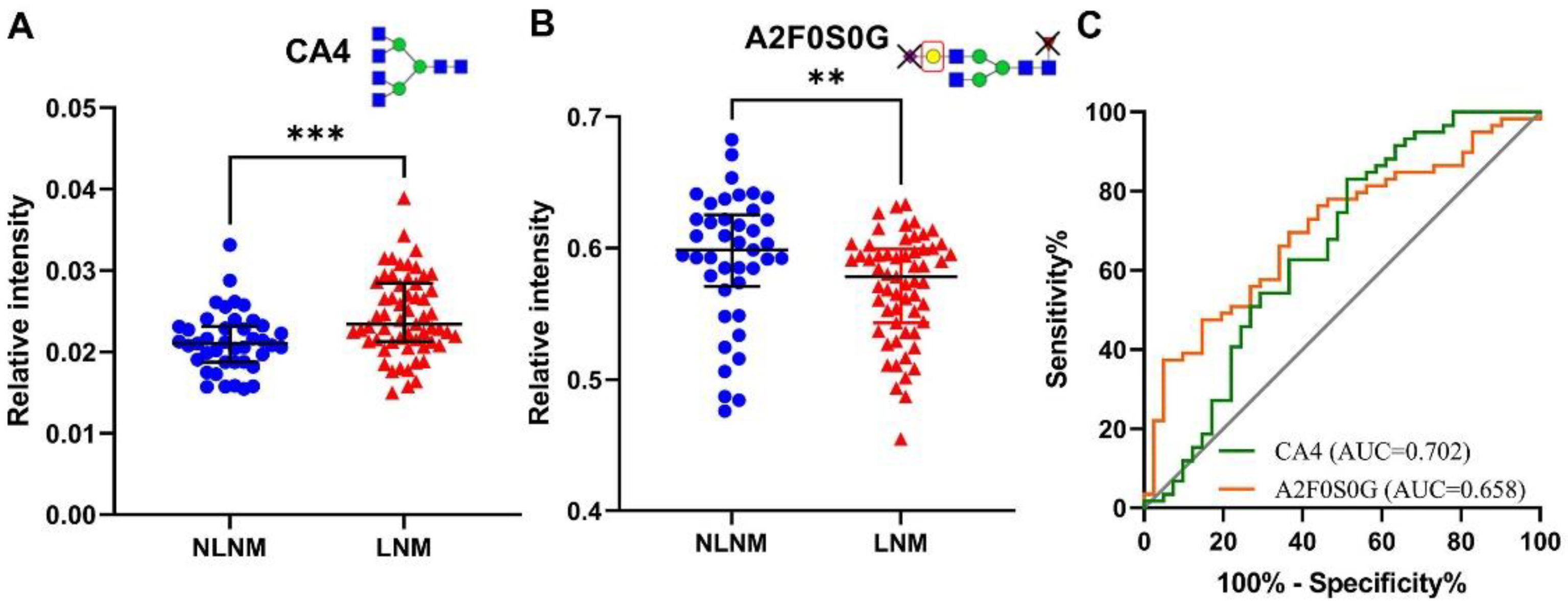
3.2. Identification of Serum N-glycomic Features of PTMC Patients with LNM
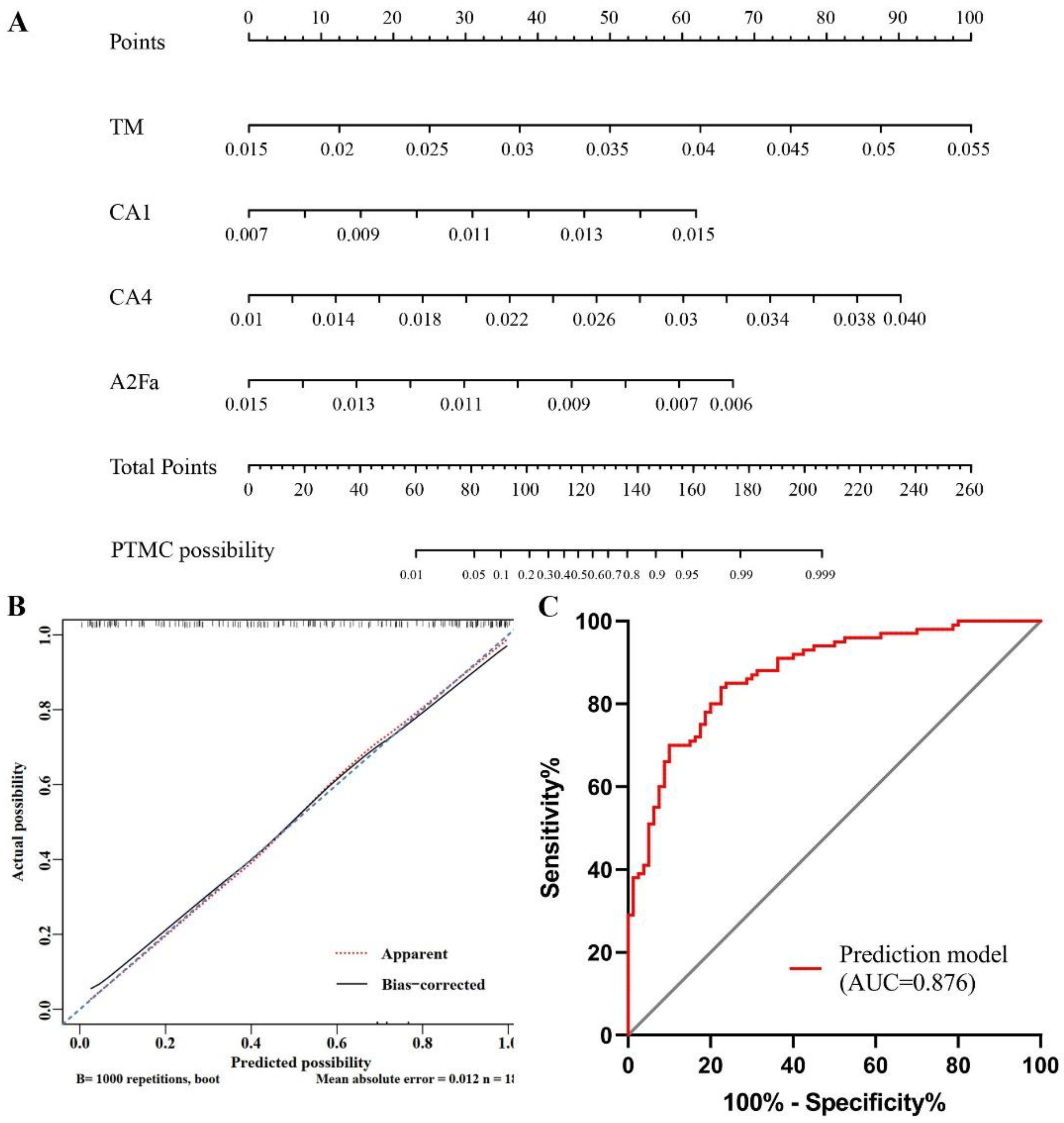
3.3. Establishment of Nomograms Based on Glycan Traits for the Diagnosis of PTMC and Preoperative Prediction of LNM
3.4. Association between Serum N-glycomes and CI of PTMC
3.5. Identification of serum N-glycome differences between PTC and PTMC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, L.; Wang, J. Clinical Predictors of Lymph Node Metastasis and Survival Rate in Papillary Thyroid Microcarcinoma: Analysis of 3607 Patients at a Single Institution. J. Surg. Res. 2018, 221, 128–134. [Google Scholar] [CrossRef]
- Miranda-Filho, A.; Lortet-Tieulent, J.; Bray, F.; Cao, B.; Franceschi, S.; Vaccarella, S.; Dal Maso, L. Thyroid Cancer Incidence Trends by Histology in 25 Countries: A Population-Based Study. Lancet Diabetes Endocrinol. 2021, 9, 225–234. [Google Scholar] [CrossRef]
- Kim, T.Y.; Shong, Y.K. Active Surveillance of Papillary Thyroid Microcarcinoma: A Mini-Review from Korea. Endocrinol. Metab. 2017, 32, 399–406. [Google Scholar] [CrossRef]
- Leboulleux, S.; Tuttle, R.M.; Pacini, F.; Schlumberger, M. Papillary Thyroid Microcarcinoma: Time to Shift from Surgery to Active Surveillance? Lancet Diabetes Endocrinol. 2016, 4, 933–942. [Google Scholar] [CrossRef]
- Chen, A.Y.; Jemal, A.; Ward, E.M. Increasing Incidence of Differentiated Thyroid Cancer in the United States, 1988–2005. Cancer 2009, 115, 3801–3807. [Google Scholar] [CrossRef] [PubMed]
- Simard, E.P.; Ward, E.M.; Siegel, R.; Jemal, A. Cancers with Increasing Incidence Trends in the United States: 1999 through 2008. CA Cancer J. Clin. 2012, 62, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.J.; Kim, W.G.; Choi, Y.M.; Kwon, H.; Lee, Y.M.; Sung, T.Y.; Yoon, J.H.; Chung, K.W.; Hong, S.J.; Kim, T.Y.; et al. Features Predictive of Distant Metastasis in Papillary Thyroid Microcarcinomas. Thyroid 2016, 26, 161–168. [Google Scholar] [CrossRef]
- Lee, C.I.; Kutlu, O.; Khan, Z.F.; Picado, O.; Lew, J.I. Margin Positivity and Survival in Papillary Thyroid Microcarcinoma: A National Cancer Database Analysis. J. Am. Coll. Surg. 2021, 233, 537–544. [Google Scholar] [CrossRef]
- Al-Qurayshi, Z.; Nilubol, N.; Tufano, R.P.; Kandil, E. Wolf in Sheep’s Clothing: Papillary Thyroid Microcarcinoma in the Us. J. Am. Coll. Surg. 2020, 230, 484–491. [Google Scholar] [CrossRef]
- Sugitani, I.; Ito, Y.; Takeuchi, D.; Nakayama, H.; Masaki, C.; Shindo, H.; Teshima, M.; Horiguchi, K.; Yoshida, Y.; Kanai, T.; et al. Indications and Strategy for Active Surveillance of Adult Low-Risk Papillary Thyroid Microcarcinoma: Consensus Statements from the Japan Association of Endocrine Surgery Task Force on Management for Papillary Thyroid Microcarcinoma. Thyroid 2021, 31, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Timler, D.; Tazbir, J.; Matejkowska, M.; Gosek, A.; Czyz, W.; Brzezinski, J. Expression of Proteins: D1 Cyclin and Ki-67 in Papillary Thyroid Carcinomas. Folia Histochem. Cytobiol. 2001, 39 (Suppl. 2), 201–202. [Google Scholar] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Navas-Carrillo, D.; Rodriguez, J.M.; Montoro-García, S.; Orenes-Piñero, E. High-resolution proteomics and metabolomics in thyroid cancer: Deciphering novel biomarkers. Crit. Rev. Clin. Lab. Sci. 2017, 54, 446–457. [Google Scholar] [CrossRef]
- Kuo, E.J.; Goffredo, P.; Sosa, J.A.; Roman, S.A. Aggressive Variants of Papillary Thyroid Microcarcinoma Are Associated with Extrathyroidal Spread and Lymph-Node Metastases: A Population-Level Analysis. Thyroid 2013, 23, 1305–1311. [Google Scholar] [CrossRef]
- Zhang, X.L.; Qian, L.X. Ultrasonic Features of Papillary Thyroid Microcarcinoma and Non-Microcarcinoma. Exp. Ther. Med. 2014, 8, 1335–1339. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, R.; Ma, Y.; Wang, D.; Su, Y.; Diao, C.; Zhang, J.; Qian, J.; Liu, J. Establishment and Validation of the Scoring System for Preoperative Prediction of Central Lymph Node Metastasis in Papillary Thyroid Carcinoma. Sci. Rep. 2018, 8, 6962. [Google Scholar] [CrossRef]
- Wu, X.; Li, B.; Zheng, C.; He, X. Risk Factors for Central Lymph Node Metastases in Patients with Papillary Thyroid Microcarcinoma. Endocr. Pract. 2018, 24, 1057–1062. [Google Scholar] [CrossRef]
- Li, F.; Wu, Y.; Chen, L.; Hu, L.; Liu, X. Evaluation of Clinical Risk Factors for Predicting Insidious Right Central and Posterior Right Recurrent Laryngeal Nerve Lymph Node Metastasis in Papillary Thyroid Microcarcinoma Patients (Cn0): Experience of a Single Center. Ann. Transl. Med. 2019, 7, 8. [Google Scholar] [CrossRef]
- Qu, H.; Sun, G.R.; Liu, Y.; He, Q.S. Clinical Risk Factors for Central Lymph Node Metastasis in Papillary Thyroid Carcinoma: A Systematic Review and Meta-Analysis. Clin. Endocrinol. 2015, 83, 124–132. [Google Scholar] [CrossRef]
- Zhu, M.; Zheng, W.; Xiang, Y.; Gu, J.; Wang, K.; Shang, J. The Relationship between Central Lymph Node Metastasis and the Distance from Tumor to Thyroid Capsule in Papillary Thyroid Microcarcinoma without Capsule Invasion. Gland Surg. 2020, 9, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Hakomori, S. Tumor-Associated Carbohydrate Antigens Defining Tumor Malignancy: Basis for Development of Anti-Cancer Vaccines. Adv. Exp. Med. Biol. 2001, 491, 369–402. [Google Scholar] [PubMed]
- Park, H.M.; Hwang, M.P.; Kim, Y.W.; Kim, K.J.; Jin, J.M.; Kim, Y.H.; Yang, Y.H.; Lee, K.H.; Kim, Y.G. Mass Spectrometry-Based N-Linked Glycomic Profiling as a Means for Tracking Pancreatic Cancer Metastasis. Carbohydr. Res. 2015, 413, 5–11. [Google Scholar] [CrossRef]
- Gudelj, I.; Baciarello, M.; Ugrina, I.; De Gregori, M.; Napolioni, V.; Ingelmo, P.M.; Bugada, D.; De Gregori, S.; Derek, L.; Pucic-Bakovic, M.; et al. Changes in Total Plasma and Serum N-Glycome Composition and Patient-Controlled Analgesia after Major Abdominal Surgery. Sci. Rep. 2016, 6, 31234. [Google Scholar] [PubMed]
- Zhang, Z.; Reiding, K.R.; Wu, J.; Li, Z.; Xu, X. Distinguishing Benign and Malignant Thyroid Nodules and Identifying Lymph Node Metastasis in Papillary Thyroid Cancer by Plasma N-Glycomics. Front. Endocrinol. 2021, 12, 692910. [Google Scholar] [CrossRef]
- Vreeker, G.C.; Hanna-Sawires, R.G.; Mohammed, Y.; Bladergroen, M.R.; Nicolardi, S.; Dotz, V.; Nouta, J.; Bonsing, B.A.; Mesker, W.E.; van Der Burgt, Y.E.; et al. Serum N-Glycome Analysis Reveals Pancreatic Cancer Disease Signatures. Cancer Med. 2020, 9, 8519–8529. [Google Scholar] [CrossRef]
- Saldova, R.; Haakensen, V.D.; Rodland, E.; Walsh, I.; Stockmann, H.; Engebraaten, O.; Borresen-Dale, A.L.; Rudd, P.M. Serum N-Glycome Alterations in Breast Cancer During Multimodal Treatment and Follow-Up. Mol. Oncol. 2017, 11, 1361–1379. [Google Scholar] [CrossRef]
- Reiding, K.R.; Blank, D.; Kuijper, D.M.; Deelder, A.M.; Wuhrer, M. High-Throughput Profiling of Protein N-Glycosylation by Maldi-Tof-Ms Employing Linkage-Specific Sialic Acid Esterification. Anal. Chem. 2014, 86, 5784–5793. [Google Scholar] [CrossRef]
- Zhang, Z.; Westhrin, M.; Bondt, A.; Wuhrer, M.; Standal, T.; Holst, S. Serum Protein N-Glycosylation Changes in Multiple Myeloma. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 960–970. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, J.; Liu, P.; Kang, L.; Xu, X. Diagnostic Potential of Plasma Igg N-Glycans in Discriminating Thyroid Cancer from Benign Thyroid Nodules and Healthy Controls. Front. Oncol. 2021, 11, 658223. [Google Scholar] [CrossRef]
- Watanabe, Y.; Aoki-Kinoshita, K.F.; Ishihama, Y.; Okuda, S. Glycopost Realizes Fair Principles for Glycomics Mass Spectrometry Data. Nucleic Acids Res. 2020, 49, D1523–D1528. [Google Scholar] [CrossRef] [PubMed]
- Jansen, B.C.; Reiding, K.R.; Bondt, A.; Hipgrave Ederveen, A.L.; Palmblad, M.; Falck, D.; Wuhrer, M. Massytools: A High-Throughput Targeted Data Processing Tool for Relative Quantitation and Quality Control Developed for Glycomic and Glycoproteomic Maldi-Ms. J. Proteome Res. 2015, 14, 5088–5098. [Google Scholar] [CrossRef] [PubMed]
- Maass, K.; Ranzinger, R.; Geyer, H.; von der Lieth, C.W.; Geyer, R. De Novo Composition Analysis of Glycoconjugates. Proteomics 2007, 7, 4435–4444. [Google Scholar] [CrossRef] [PubMed]
- Ceroni, A.; Maass, K.; Geyer, H.; Geyer, R.; Dell, A.; Haslam, S.M. Glycoworkbench: A Tool for the Computer-Assisted Annotation of Mass Spectra of Glycans. J. Proteome Res. 2008, 7, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Bladergroen, M.R.; Reiding, K.R.; Ederveen, A.L.H.; Vreeker, G.C.; Clerc, F.; Holst, S.; Bondt, A.; Wuhrer, M.; van der Burgt, Y.E. Automation of High-Throughput Mass Spectrometry-Based Plasma N-Glycome Analysis with Linkage-Specific Sialic Acid Esterification. J. Proteome Res. 2015, 14, 4080–4086. [Google Scholar] [CrossRef]
- Ščupáková, K.; Adelaja, O.T.; Balluff, B.; Ayyappan, V.; Tressler, C.M.; Jenkinson, N.M.; Claes, B.S.; Bowman, A.P.; Cimino-Mathews, A.M.; White, M.J.; et al. Clinical importance of high-mannose, fucosylated, and complex N-glycans in breast cancer metastasis. JCI Insight. 2021, 6, e146945. [Google Scholar] [CrossRef]
- Yamamoto, M.; Harada, Y.; Suzuki, T.; Fukushige, T.; Yamakuchi, M.; Kanekura, T.; Dohmae, N.; Hori, K.; Maruyama, I. Application of High-Mannose-Type Glycan-Specific Lectin from Oscillatoria Agardhii for Affinity Isolation of Tumor-Derived Extracellular Vesicles. Anal. Biochem. 2019, 580, 21–29. [Google Scholar] [CrossRef]
- Holst, S.; Wuhrer, M.; Rombouts, Y. Glycosylation Characteristics of Colorectal Cancer. Adv. Cancer Res. 2015, 126, 203–256. [Google Scholar]
- Chik, J.H.; Zhou, J.; Moh, E.S.; Christopherson, R.; Clarke, S.J.; Molloy, M.P.; Packer, N.H. Comprehensive Glycomics Comparison between Colon Cancer Cell Cultures and Tumours: Implications for Biomarker Studies. J. Proteom. 2014, 108, 146–162. [Google Scholar] [CrossRef]
- Tan, Z.; Cao, L.; Wu, Y.; Wang, B.; Song, Z.; Yang, J.; Cheng, L.; Yang, X.; Zhou, X.; Dai, Z.; et al. Bisecting Glcnac Modification Diminishes the Pro-Metastatic Functions of Small Extracellular Vesicles from Breast Cancer Cells. J. Extracell. Vesicles 2020, 10, e12005. [Google Scholar] [CrossRef]
- Zhan, L.; Chen, L.; Chen, Z. Knockdown of Fut3 Disrupts the Proliferation, Migration, Tumorigenesis and Tgf-Beta Induced Emt in Pancreatic Cancer Cells. Oncol. Lett. 2018, 16, 924–930. [Google Scholar] [PubMed]
- Lu, H.-H.; Lin, S.-Y.; Weng, R.R.; Juan, Y.-H.; Chen, Y.-W.; Hou, H.-H.; Hung, Z.-C.; Oswita, G.A.; Huang, Y.-J.; Guu, S.-Y.; et al. Fucosyltransferase 4 Shapes Oncogenic Glycoproteome to Drive Metastasis of Lung Adenocarcinoma. eBioMedicine 2020, 57, 102846. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, M.; Takimoto, R.; Tamura, F.; Yoshida, M.; Ono, M.; Murase, K.; Sato, Y.; Osuga, T.; Sato, T.; Iyama, S.; et al. Fucosylated Tgf-Beta Receptors Transduces a Signal for Epithelial-Mesenchymal Transition in Colorectal Cancer Cells. Br. J. Cancer 2014, 110, 156–163. [Google Scholar] [CrossRef]
- Qu, N.; Zhang, L.; Ji, Q.H.; Chen, J.Y.; Zhu, Y.X.; Cao, Y.M.; Shen, Q. Risk Factors for Central Compartment Lymph Node Metastasis in Papillary Thyroid Microcarcinoma: A Meta-Analysis. World J. Surg. 2015, 39, 2459–2470. [Google Scholar] [CrossRef] [PubMed]
- Theodoratou, E.; Thaci, K.; Agakov, F.; Timofeeva, M.N.; Stambuk, J.; Pucic-Bakovic, M.; Vuckovic, F.; Orchard, P.; Agakova, A.; Din, F.V.; et al. Glycosylation of Plasma Igg in Colorectal Cancer Prognosis. Sci. Rep. 2016, 6, 28098. [Google Scholar] [CrossRef] [PubMed]
- Kodar, K.; Stadlmann, J.; Klaamas, K.; Sergeyev, B.; Kurtenkov, O. Immunoglobulin G Fc N-Glycan Profiling in Patients with Gastric Cancer by Lc-Esi-Ms: Relation to Tumor Progression and Survival. Glycoconj. J. 2012, 29, 57–66. [Google Scholar] [CrossRef]
- Kanoh, Y.; Mashiko, T.; Danbara, M.; Takayama, Y.; Ohtani, S.; Egawa, S.; Baba, S.; Akahoshi, T. Changes in Serum IgG Oligosaccharide Chains with Prostate Cancer Progression. Anticancer Res. 2004, 24, 3135–3139. [Google Scholar]
- Qian, Y.; Wang, Y.; Zhang, X.; Zhou, L.; Zhang, Z.; Xu, J.; Ruan, Y.; Ren, S.; Xu, C.; Gu, J. Quantitative Analysis of Serum IgG Galactosylation Assists Differential Diagnosis of Ovarian Cancer. J. Proteome Res. 2013, 12, 4046–4055. [Google Scholar] [CrossRef]
- Bones, J.; Byrne, J.C.; O’Donoghue, N.; McManus, C.; Scaife, C.; Boissin, H.; Nastase, A.; Rudd, P.M. Glycomic and Glycoproteomic Analysis of Serum from Patients with Stomach Cancer Reveals Potential Markers Arising from Host Defense Response Mechanisms. J. Proteome Res. 2011, 10, 1246–1265. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Shu, J.; Hou, Y.; Chen, M.; Yu, H.; Ma, T.; Du, H.; Zhang, J.; Qiao, Y.; et al. Abnormal Galactosylated-Glycans Recognized by Bandeiraea Simplicifolia Lectin I in Saliva of Patients with Breast Cancer. Glycoconj. J. 2020, 37, 373–394. [Google Scholar] [CrossRef]
- Pinho, S.S.; Reis, C.A. Glycosylation in Cancer: Mechanisms and Clinical Implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.H.; Telang, S.D.; Shah, P.M.; Patel, P.S. Tissue and Serum Alpha 2-3- and Alpha 2-6-Linkage Specific Sialylation Changes in Oral Carcinogenesis. Glycoconj. J. 2008, 25, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sun, X.; Luo, Y.; Wang, F.; Lyu, Z. Clinical and Pathologic Predictors of Lymph Node Metastasis in Papillary Thyroid Microcarcinomas. Ann. Diagn. Pathol. 2020, 49, 151647. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | PTMC | HC | |
|---|---|---|---|
| with NLNM | with LNM | ||
| Sample size | 41 | 59 | 80 |
| Age at operation (y, X ± S) | 40.34 ± 6.58 | 38.56 ± 7.91 | 38.26 ± 6.54 |
| Sex (n (%)) | |||
| Female | 33 (80.5) | 36 (61.1) | 40 (50.0) |
| Male | 8 (19.5) | 23 (38.9) | 40 (50.0) |
| Family history of thyroid disease (n (%)) | |||
| No | 31 (75.6) | 46 (78.0) | \ |
| Yes | 10 (24.4) | 13 (22.0) | \ |
| Tumor size (cm, X ± S) | 0.69 ± 0.18 | 0.73 ± 0.23 | \ |
| ≤0.5 | 6 (14.6) | 15 (25.4) | \ |
| >0.5 | 35 (85.4) | 44 (74.6) | \ |
| Clinical LNM (n (%)) | |||
| Absent | 41 (100.0) | 57 (96.6) | \ |
| Present | 0 (0.0) | 2 (3.4) | \ |
| Pathological subtype (n (%)) | |||
| Classic | 32 (78.1) | 51 (86.4) | \ |
| Follicular variant | 6 (14.6) | 5 (8.5) | \ |
| Classic and follicular variant | 3 (7.3) | 3 (5.1) | \ |
| Tumor location (n (%)) | |||
| Unifocal | 36 (87.8) | 45 (76.3) | \ |
| Multifocal | 5 (12.2) | 14 (23.7) | \ |
| Tumor calcification (n (%)) | |||
| Absent | 29 (70.7) | 43 (72.9) | \ |
| Present | 12 (29.3) | 16 (27.1) | \ |
| Microscopic capsular invasion (n (%)) | |||
| Absent | 14 (34.1) | 21 (35.6) | \ |
| Present | 27(65.9) | 38 (64.4) | \ |
| Hashimoto’s thyroiditis (n (%)) | |||
| Absent | 30 (73.2) | 47 (79.7) | \ |
| Present | 11 (26.8) | 12 (20.3) | \ |
| Descriptions | Median | p Value | |||
|---|---|---|---|---|---|
| HC | PTMC | Univariate | Multivariate | ||
| Glycan traits—general | |||||
| TM | High-mannose glycans in total spectrum | 0.0227 | 0.0284 | 1.38 × 10−14 | 0.002 |
| MHy | The ratio of high-mannose to hybrid glycans | 1.7240 | 2.0062 | 2.36 × 10−9 | 0.372 |
| CA1 | Monoantennary species (A1) in complex glycans | 0.0090 | 0.0096 | 3.61 × 10−5 | 0.042 |
| CA4 | Tetraantennary species (A4) in complex glycans | 0.0184 | 0.0224 | 1.26 × 10−8 | 0.000 |
| Glycan traits—fucosylation (F) | |||||
| CFa | Antenna-fucosylation in complex glycans | 0.0095 | 0.0090 | 7.73 × 10−5 | 0.597 |
| A2Fa | Antenna-fucosylation in diantennary (A2) | 0.0094 | 0.0087 | 3.94 × 10−6 | 0.010 |
| Glycan traits—bisection (B) | |||||
| A2F0B | GlcNAc with non-fucosylated diantennary | 0.0306 | 0.0342 | 0.0002 | 0.162 |
| A2F0SB | GlcNAc with non-fucosylated sialylated diantennary | 0.0222 | 0.0254 | 0.0001 | 0.307 |
| Descriptions | Median | p Value | |||
|---|---|---|---|---|---|
| NLNM | LNM | Univariate | Multivariate | ||
| Glycan traits—general | |||||
| MHy | The ratio of high-mannose to hybrid glycans | 1.9494 | 2.0865 | 0.0265 | 0.979 |
| MM | Average number of mannoses on high-mannose | 6.8579 | 6.9325 | 0.0334 | 0.752 |
| CA2 | Diantennary species (A2) in complex glycans | 0.8557 | 0.8413 | 0.0130 | 0.853 |
| CA3 | Triantennary species (A3) in complex glycans | 0.1051 | 0.1157 | 0.0485 | 0.888 |
| CA4 | Tetraantennary species (A4) in complex glycans | 0.0210 | 0.0234 | 0.0006 | 0.001 |
| Glycan traits—galactosylation (G) | |||||
| A2F0S0G | In non-fucosylated, non-sialylated diantennary | 0.5986 | 0.5783 | 0.0073 | 0.011 |
| Glycan traits—sialylation (S) | |||||
| A3S | In triantennary (A3) | 0.9001 | 0.9056 | 0.0358 | 0.795 |
| Descriptions | Median of PTMC without CI | Median of PTMC with CI | p Value | |
|---|---|---|---|---|
| Glycan traits—galactosylation (G) | ||||
| CG | In all complex glycans | 0.9496 | 0.9585 | 0.0045 |
| A2G | In diantennary glycans (A2) | 0.8762 | 0.8919 | 0.0120 |
| A2FG | In fucosylated diantennary glycans (A2) | 0.7334 | 0.7668 | 0.0030 |
| A2S0G | In non-sialylated diantennary glycans (A2) | 0.5105 | 0.5447 | 0.0144 |
| A2FS0G | In fucosylated non-sialylated dianntennary glycans (A2) | 0.5066 | 0.5334 | 0.0139 |
| Glycan traits—sialylation (S) | ||||
| CS | In all complex glycans | 0.7970 | 0.8159 | 0.0377 |
| A2FS | In fucosylated diantennary glycans (A2) | 0.3657 | 0.4026 | 0.0159 |
| Glycan traits-α-2,6-linked sialylation (E) | ||||
| A2FE | In fucosylated diantennary glycans (A2) | 0.2907 | 0.3251 | 0.0147 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Cao, Z.; Liu, R.; Li, Z.; Wu, J.; Liu, X.; Wu, M.; Xu, X.; Liu, Z. Nomograms Based on Serum N-glycome for Diagnosis of Papillary Thyroid Microcarcinoma and Prediction of Lymph Node Metastasis. Curr. Oncol. 2022, 29, 6018-6034. https://doi.org/10.3390/curroncol29090474
Zhang Z, Cao Z, Liu R, Li Z, Wu J, Liu X, Wu M, Xu X, Liu Z. Nomograms Based on Serum N-glycome for Diagnosis of Papillary Thyroid Microcarcinoma and Prediction of Lymph Node Metastasis. Current Oncology. 2022; 29(9):6018-6034. https://doi.org/10.3390/curroncol29090474
Chicago/Turabian StyleZhang, Zejian, Zhen Cao, Rui Liu, Zepeng Li, Jianqiang Wu, Xiaoli Liu, Mengwei Wu, Xiequn Xu, and Ziwen Liu. 2022. "Nomograms Based on Serum N-glycome for Diagnosis of Papillary Thyroid Microcarcinoma and Prediction of Lymph Node Metastasis" Current Oncology 29, no. 9: 6018-6034. https://doi.org/10.3390/curroncol29090474
APA StyleZhang, Z., Cao, Z., Liu, R., Li, Z., Wu, J., Liu, X., Wu, M., Xu, X., & Liu, Z. (2022). Nomograms Based on Serum N-glycome for Diagnosis of Papillary Thyroid Microcarcinoma and Prediction of Lymph Node Metastasis. Current Oncology, 29(9), 6018-6034. https://doi.org/10.3390/curroncol29090474






