Donor Age and Non-Relapse Mortality: Study of Their Association after HLA-Matched Allogeneic Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndrome
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Baseline Characteristics
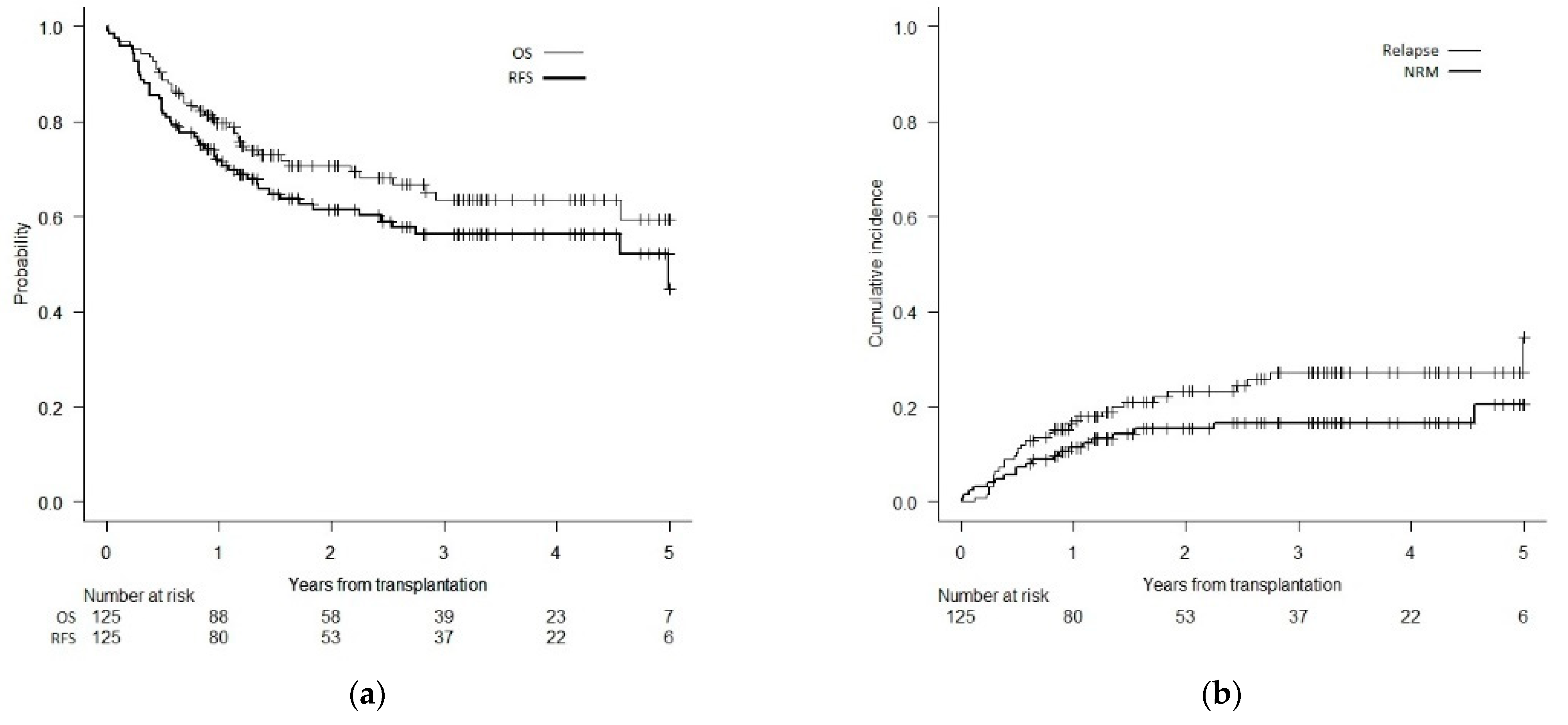
3.2. Survival and Relapse
3.3. Association between Donor Age and Non-Relapse Mortality
3.4. Association between Donor Age, Graft-versus-Host Disease and Non-Relapse Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Souza, A.; Fretham, C.; Lee, S.J.; Arora, M.; Brunner, J.; Chhabra, S.; Devine, S.; Eapen, M.; Hamadani, M.; Hari, P.; et al. Current Use of and Trends in Hematopoietic Cell Transplantation in the United States. Biol. Blood Marrow Transpl. 2020, 26, e177–e182. [Google Scholar] [CrossRef] [PubMed]
- McDonald, G.B.; Sandmaier, B.M.; Mielcarek, M.; Sorror, M.; Pergam, S.A.; Cheng, G.S.; Hingorani, S.; Boeckh, M.; Flowers, M.D.; Lee, S.J.; et al. Survival, Nonrelapse Mortality, and Relapse-Related Mortality after Allogeneic Hematopoietic Cell Transplantation: Comparing 2003–2007 versus 2013–2017 Cohorts. Ann. Intern. Med. 2020, 172, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Passweg, J.R.; Baldomero, H.; Chabannon, C.; Basak, G.W.; de la Cámara, R.; Corbacioglu, S.; Dolstra, H.; Duarte, R.; Glass, B.; Greco, R.; et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: Monitoring of activities and trends over 30 years. Bone Marrow Transpl. 2021, 56, 1651–1664. [Google Scholar] [CrossRef] [PubMed]
- Penack, O.; Peczynski, C.; Mohty, M.; Yakoub-Agha, I.; Styczynski, J.; Montoto, S.; Duarte, R.F.; Kröger, N.; Schoemans, H.; Koenecke, C.; et al. How much has allogeneic stem cell transplant-related mortality improved since the 1980s? A retrospective analysis from the EBMT. Blood Adv. 2020, 4, 6283–6290. [Google Scholar] [CrossRef]
- Lin, R.J.; Artz, A.S. Allogeneic hematopoietic cell transplantation for older patients. Hemat. Am. Soc. Hematol. Educ. Program 2021, 2021, 254–263. [Google Scholar] [CrossRef]
- Shouval, R.; Fein, J.A.; Labopin, M.; Kröger, N.; Duarte, R.F.; Bader, P.; Chabannon, C.; Kuball, J.; Basak, G.W.; Dufour, C.; et al. Outcomes of allogeneic haematopoietic stem cell transplantation from HLA-matched and alternative donors: A European Society for Blood and Marrow Transplantation registry retrospective analysis. Lancet Haematol. 2019, 6, e573–e584. [Google Scholar] [CrossRef]
- Alousi, A.M.; Le-Rademacher, J.; Saliba, R.M.; Appelbaum, F.R.; Artz, A.; Benjamin, J.; Devine, S.M.; Kan, F.; Laughlin, M.J.; Lazarus, H.M.; et al. Who is the better donor for older hematopoietic transplant recipients: An older-aged sibling or a young, matched unrelated volunteer? Blood 2013, 121, 2567–2573. [Google Scholar] [CrossRef]
- Kröger, N.; Zabelina, T.; de Wreede, L.; Berger, J.; Alchalby, H.; van Biezen, A.; Milpied, N.; Volin, L.; Mohty, M.; Leblond, V.; et al. Allogeneic stem cell transplantation for older advanced MDS patients: Improved survival with young unrelated donor in comparison with HLA-identical siblings. Leukemia 2013, 27, 604–609. [Google Scholar] [CrossRef] [Green Version]
- Kollman, C.; Howe, C.W.; Anasetti, C.; Antin, J.H.; Davies, S.M.; Filipovich, A.H.; Hegland, J.; Kamani, N.; Kernan, N.A.; King, R.; et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: The effect of donor age. Blood 2001, 98, 2043–2051. [Google Scholar] [CrossRef]
- Kollman, C.; Spellman, S.R.; Zhang, M.J.; Hassebroek, A.; Anasetti, C.; Antin, J.H.; Champlin, R.E.; Confer, D.L.; DiPersio, J.F.; Fernandez-Viña, M.; et al. The effect of donor characteristics on survival after unrelated donor transplantation for hematologic malignancy. Blood 2016, 127, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Rezvani, A.R.; Storer, B.E.; Guthrie, K.A.; Schoch, H.G.; Maloney, D.G.; Sandmaier, B.M.; Storb, R. Impact of donor age on outcome after allogeneic hematopoietic cell transplantation. Biol. Blood Marrow Transpl. 2015, 21, 105–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastida, J.M.; Cabrero, M.; Lopez-Godino, O.; Lopez-Parra, M.; Sanchez-Guijo, F.; Lopez-Corral, L.; Vazquez, L.; Caballero, D.; Del Cañizo, C. Influence of donor age in allogeneic stem cell transplant outcome in acute myeloid leukemia and myelodisplastic syndrome. Leuk. Res. 2015, 39, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Shouval, R.; Fein, J.A.; Labopin, M.; Cho, C.; Bazarbachi, A.; Baron, F.; Bug, G.; Ciceri, F.; Corbacioglu, S.; Galimard, J.E.; et al. Development and validation of a disease risk stratification system for patients with haematological malignancies: A retrospective cohort study of the European Society for Blood and Marrow Transplantation registry. Lancet Haematol. 2021, 8, e205–e215. [Google Scholar] [CrossRef]
- Atkinson, K.; Farrell, C.; Chapman, G.; Downs, K.; Penny, R.; Biggs, J. Female marrow donors increase the risk of acute graft-versus-host disease: Effect of donor age and parity and analysis of cell subpopulations in the donor marrow inoculum. Br. J. Haematol. 1986, 63, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, N.; Badsberg, J.H.; Lönnqvist, B.; Ringdén, O.; Volin, L.; Rajantie, J.; Nikoskelainen, J.; Keiding, N. Graft-versus-leukaemia activity associated with CMV-seropositive donor, post-transplant CMV infection, young donor age and chronic graft-versus-host disease in bone marrow allograft recipients. The Nordic Bone Marrow Transplantation Group. Bone Marrow Transpl. 1990, 5, 413–418. [Google Scholar]
- Richa, E.M.; Kunnavakkam, R.; Godley, L.A.; Kline, J.; Odenike, O.; Larson, R.A.; Nguyen, V.; Stock, W.; Wickrema, A.; Van Besien, K.; et al. Influence of related donor age on outcomes after peripheral blood stem cell transplantation. Cytotherapy 2012, 14, 707–715. [Google Scholar] [CrossRef]
- Segal, E.; Martens, M.; Wang, H.L.; Brazauskas, R.; Weisdorf, D.; Sandmaier, B.M.; Khoury, H.J.; de Lima, M.; Saber, W. Comparing outcomes of matched related donor and matched unrelated donor hematopoietic cell transplants in adults with B-Cell acute lymphoblastic leukemia. Cancer 2017, 123, 3346–3355. [Google Scholar] [CrossRef] [Green Version]
- Sorror, M.L.; Storb, R.F.; Sandmaier, B.M.; Maziarz, R.T.; Pulsipher, M.A.; Maris, M.B.; Bhatia, S.; Ostronoff, F.; Deeg, H.J.; Syrjala, K.L.; et al. Comorbidity-age index: A clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J. Clin. Oncol. 2014, 32, 3249–3256. [Google Scholar] [CrossRef]
- Shaw, B.E.; Logan, B.R.; Spellman, S.R.; Marsh, S.G.E.; Robinson, J.; Pidala, J.; Hurley, C.; Barker, J.; Maiers, M.; Dehn, J.; et al. Development of an Unrelated Donor Selection Score Predictive of Survival after HCT: Donor Age Matters Most. Biol. Blood Marrow Transpl. 2018, 24, 1049–1056. [Google Scholar] [CrossRef] [Green Version]
- Paz, A.; Rigoni, L.; Fischer, G.; Schittler, M.; Pezzi, A.; Valim, V.; Dahmer, A.; Zambonato, B.; Amorin, B.; Sehn, F.; et al. Donor characteristics and hematopoietic stem cell transplantation outcome: Experience of a single center in Southern Brazil. Hematol. Transfus. Cell Ther. 2018, 40, 136–142. [Google Scholar] [CrossRef]
- Flowers, M.E.; Inamoto, Y.; Carpenter, P.A.; Lee, S.J.; Kiem, H.P.; Petersdorf, E.W.; Pereira, S.E.; Nash, R.A.; Mielcarek, M.; Fero, M.L.; et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood 2011, 117, 3214–3219. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, J.; Raiola, A.M.; Evangelista, A.; Carella, A.M.; Martino, M.; Patriarca, F.; Risitano, A.; Bramanti, S.; Busca, A.; Giaccone, L.; et al. Impact of donor age and kinship on clinical outcomes after T-cell-replete haploidentical transplantation with PT-Cy. Blood Adv. 2020, 4, 3900–3912. [Google Scholar] [CrossRef] [PubMed]
- DeZern, A.E.; Franklin, C.; Tsai, H.L.; Imus, P.H.; Cooke, K.R.; Varadhan, R.; Jones, R.J. Relationship of donor age and relationship to outcomes of haploidentical transplantation with posttransplant cyclophosphamide. Blood Adv. 2021, 5, 1360–1368. [Google Scholar] [CrossRef]
- Karam, E.; Laporte, J.; Solomon, S.R.; Morris, L.E.; Zhang, X.; Holland, H.K.; Bashey, A.; Solh, M.M. Who Is a Better Donor for Recipients of Allogeneic Hematopoietic Cell Transplantation: A Young HLA-Mismatched Haploidentical Relative or an Older Fully HLA-Matched Sibling or Unrelated Donor? Biol. Blood Marrow Transpl. 2019, 25, 2054–2060. [Google Scholar] [CrossRef] [PubMed]
- Ciurea, S.O.; Al Malki, M.M.; Kongtim, P.; Fuchs, E.J.; Luznik, L.; Huang, X.J.; Ciceri, F.; Locatelli, F.; Aversa, F.; Castagna, L.; et al. The European Society for Blood and Marrow Transplantation (EBMT) consensus recommendations for donor selection in haploidentical hematopoietic cell transplantation. Bone Marrow Transpl. 2020, 55, 12–24. [Google Scholar] [CrossRef]
- Baron, F.; Zachee, P.; Maertens, J.; Kerre, T.; Ory, A.; Seidel, L.; Graux, C.; Lewalle, P.; Van Gelder, M.; Theunissen, K.; et al. Non-myeloablative allogeneic hematopoietic cell transplantation following fludarabine plus 2 Gy TBI or ATG plus 8 Gy TLI: A phase II randomized study from the Belgian Hematological Society. J. Hematol. Oncol. 2015, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Azuma, E.; Hirayama, M.; Yamamoto, H.; Komada, Y. The role of donor age in naive T-cell recovery following allogeneic hematopoietic stem cell transplantation: The younger the better. Leuk. Lymphoma 2002, 43, 735–739. [Google Scholar] [CrossRef]
- Hirayama, M.; Azuma, E.; Jiang, Q.; Kobayashi, M.; Iwamoto, S.; Kumamoto, T.; Kisenge, R.; Yamamoto, H.; Komada, Y. The reconstitution of CD45RBhiCD4+ naive T cells is inversely correlated with donor age in murine allogeneic haematopoietic stem cell transplantation. Br. J. Haematol. 2000, 111, 700–707. [Google Scholar] [CrossRef]
- Carreras, E.; Jiménez, M.; Gómez-García, V.; de la Cámara, R.; Martín, C.; Martínez, F.; Iriondo, A.; Sanz, G.; Cañizo, C.; Cabrera, R.; et al. Donor age and degree of HLA matching have a major impact on the outcome of unrelated donor haematopoietic cell transplantation for chronic myeloid leukaemia. Bone Marrow Transpl. 2006, 37, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Mehta, J.; Gordon, L.I.; Tallman, M.S.; Winter, J.N.; Evens, A.M.; Frankfurt, O.; Williams, S.F.; Grinblatt, D.; Kaminer, L.; Meagher, R.; et al. Does younger donor age affect the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies beneficially? Bone Marrow Transpl. 2006, 38, 95–100. [Google Scholar] [CrossRef]
- Guru Murthy, G.S.; Kim, S.; Hu, Z.H.; Estrada-Merly, N.; Abid, M.B.; Aljurf, M.; Bacher, U.; Badawy, S.M.; Beitinjaneh, A.; Bredeson, C.; et al. Relapse and Disease-Free Survival in Patients with Myelodysplastic Syndrome Undergoing Allogeneic Hematopoietic Cell Transplantation Using Older Matched Sibling Donors vs Younger Matched Unrelated Donors. JAMA Oncol. 2022, 8, 404–411. [Google Scholar] [CrossRef] [PubMed]

| Total Number of Subjects | 125 (%) |
| Recipient age, years (median/range/IQR) | 56/18–70/50–62 |
| Donor age, years (median/range/IQR) | 32/18–74/24–52 |
| Donor type | |
| MRD (8/8) | 41 (33) |
| MUD (8/8) | 84 (67) |
| Donor age (years)/type | |
| <50 MRD | 10 (8) |
| <50 MUD | 80 (64) |
| ≥50 MRD | 31 (25) |
| ≥50 MUD | 4 (3) |
| Disease | |
| Acute Myeloid Leukemia | 89 (71) |
| Myelodysplastic syndrome | 36 (29) |
| Disease relapse risk (DRSS) | |
| Low | 23 (19) |
| Intermediate-1 | 72 (58) |
| Intermediate-2 | 16 (13) |
| High | 9 (7) |
| Very high | 4 (3) |
| Recipient Karnofsky Performance Status | |
| <90% | 47 (38) |
| ≥90% | 77 (62) |
| Recipient HCT-CI | |
| 0 | 37 (30) |
| 1–2 | 46 (37) |
| ≥3 | 40 (32) |
| Cell source | |
| Bone marrow | 33 (26) |
| TNC range (×108/kg) | 1.20–6.51 |
| Peripheral blood | 92 (74) |
| CD34+ cell range (×106/kg) | 2.44–14.45 |
| CMV serostatus positive (recipient) | 46 (37) |
| Sex mismatch (female donor to male recipient) | 22 (18) |
| Conditioning regimen intensity | |
| Myeloablative | 112 (90) |
| Reduced intensity | 13 (10) |
| GVHD prophylaxis | |
| MTX/CnI | 103 (82) |
| MMF/CnI | 22 (18) |
| rATG * | 46 (37) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadri, Y.; Phan, M.; Bambace, N.; Bernard, L.; Cohen, S.; Delisle, J.-S.; Kiss, T.; Lachance, S.; Roy, D.-C.; Sauvageau, G.; et al. Donor Age and Non-Relapse Mortality: Study of Their Association after HLA-Matched Allogeneic Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndrome. Curr. Oncol. 2022, 29, 5955-5962. https://doi.org/10.3390/curroncol29080470
Kadri Y, Phan M, Bambace N, Bernard L, Cohen S, Delisle J-S, Kiss T, Lachance S, Roy D-C, Sauvageau G, et al. Donor Age and Non-Relapse Mortality: Study of Their Association after HLA-Matched Allogeneic Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndrome. Current Oncology. 2022; 29(8):5955-5962. https://doi.org/10.3390/curroncol29080470
Chicago/Turabian StyleKadri, Yasmine, Michelle Phan, Nadia Bambace, Léa Bernard, Sandra Cohen, Jean-Sébastien Delisle, Thomas Kiss, Sylvie Lachance, Denis-Claude Roy, Guy Sauvageau, and et al. 2022. "Donor Age and Non-Relapse Mortality: Study of Their Association after HLA-Matched Allogeneic Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndrome" Current Oncology 29, no. 8: 5955-5962. https://doi.org/10.3390/curroncol29080470
APA StyleKadri, Y., Phan, M., Bambace, N., Bernard, L., Cohen, S., Delisle, J.-S., Kiss, T., Lachance, S., Roy, D.-C., Sauvageau, G., Veilleux, O., Roy, J., & Ahmad, I. (2022). Donor Age and Non-Relapse Mortality: Study of Their Association after HLA-Matched Allogeneic Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndrome. Current Oncology, 29(8), 5955-5962. https://doi.org/10.3390/curroncol29080470







