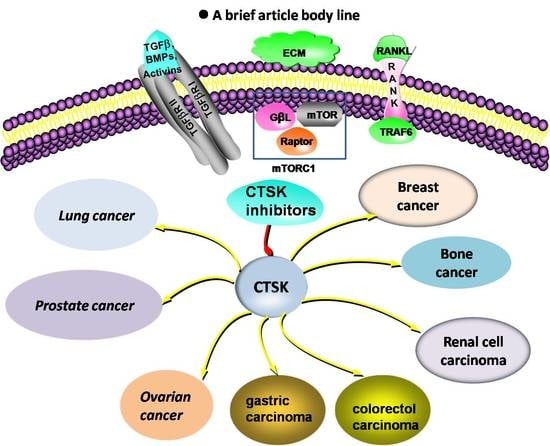
Cathepsin K: A Versatile Potential Biomarker and Therapeutic Target for Various Cancers
Abstract
1. Introduction
2. CTSK and the Cancers Development
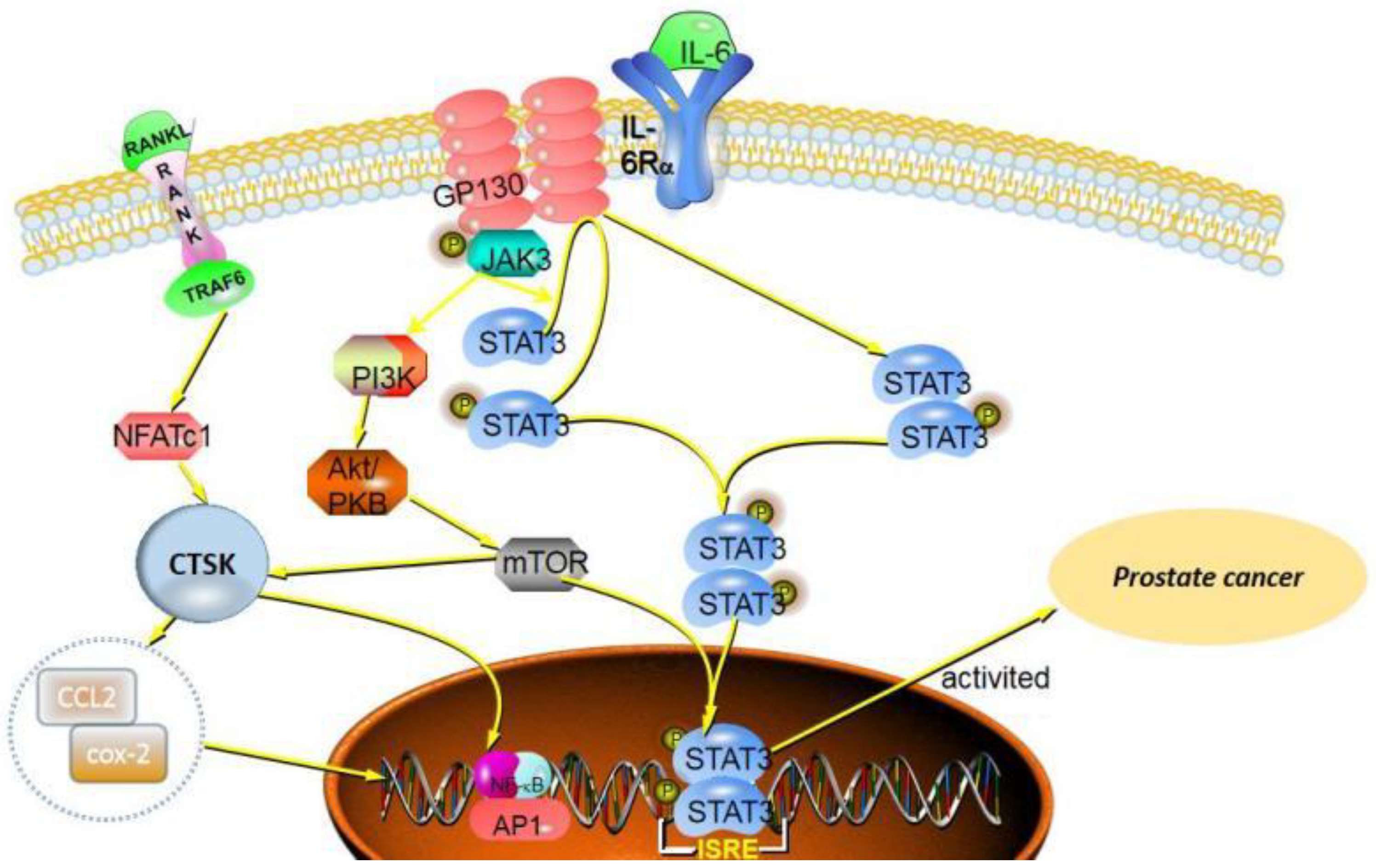
3. CTSK in Prostate Cancer
4. CTSK in Breast Cancer
5. CTSK in Bone Cancer
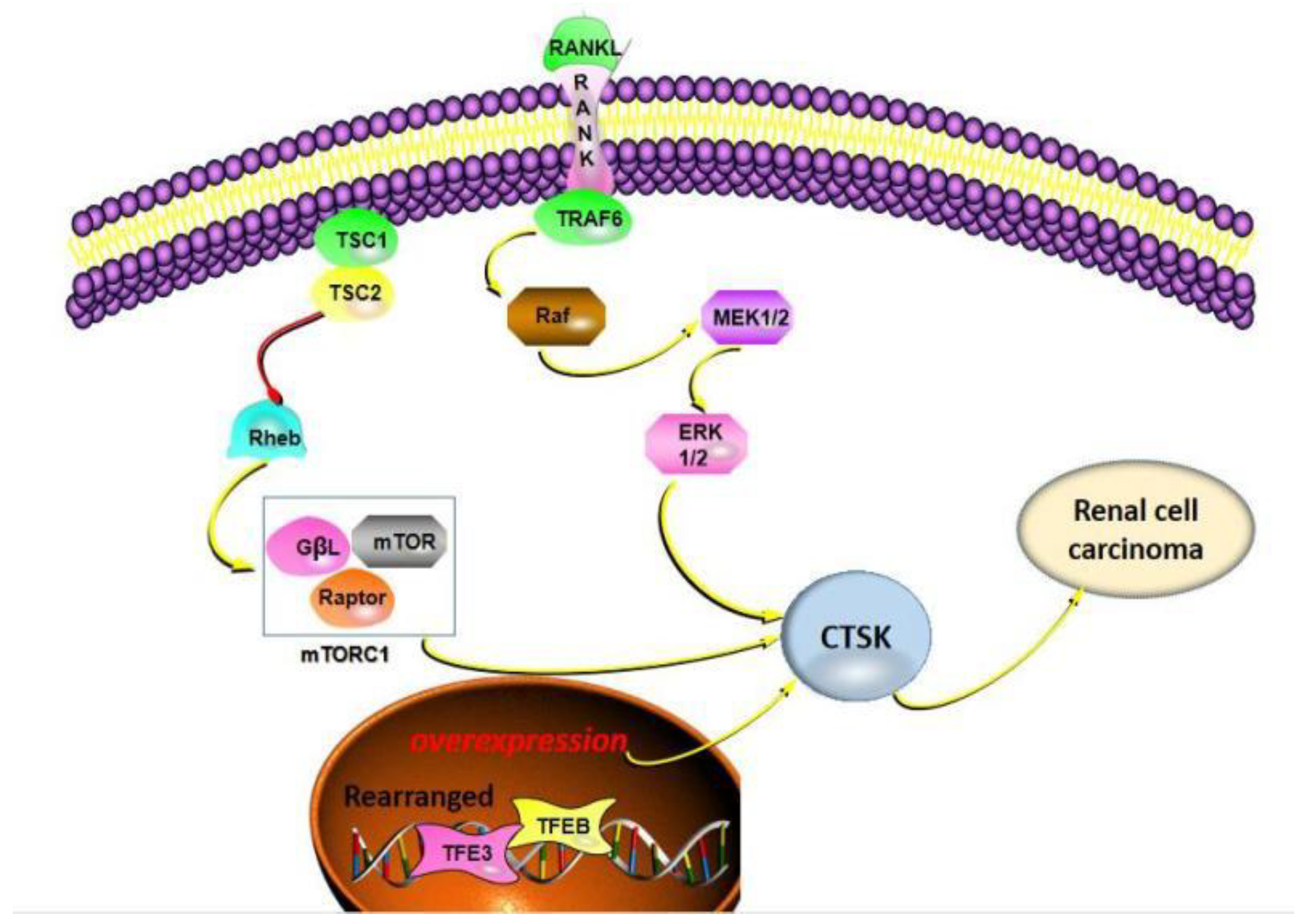
6. CTSK in Renal Carcinoma
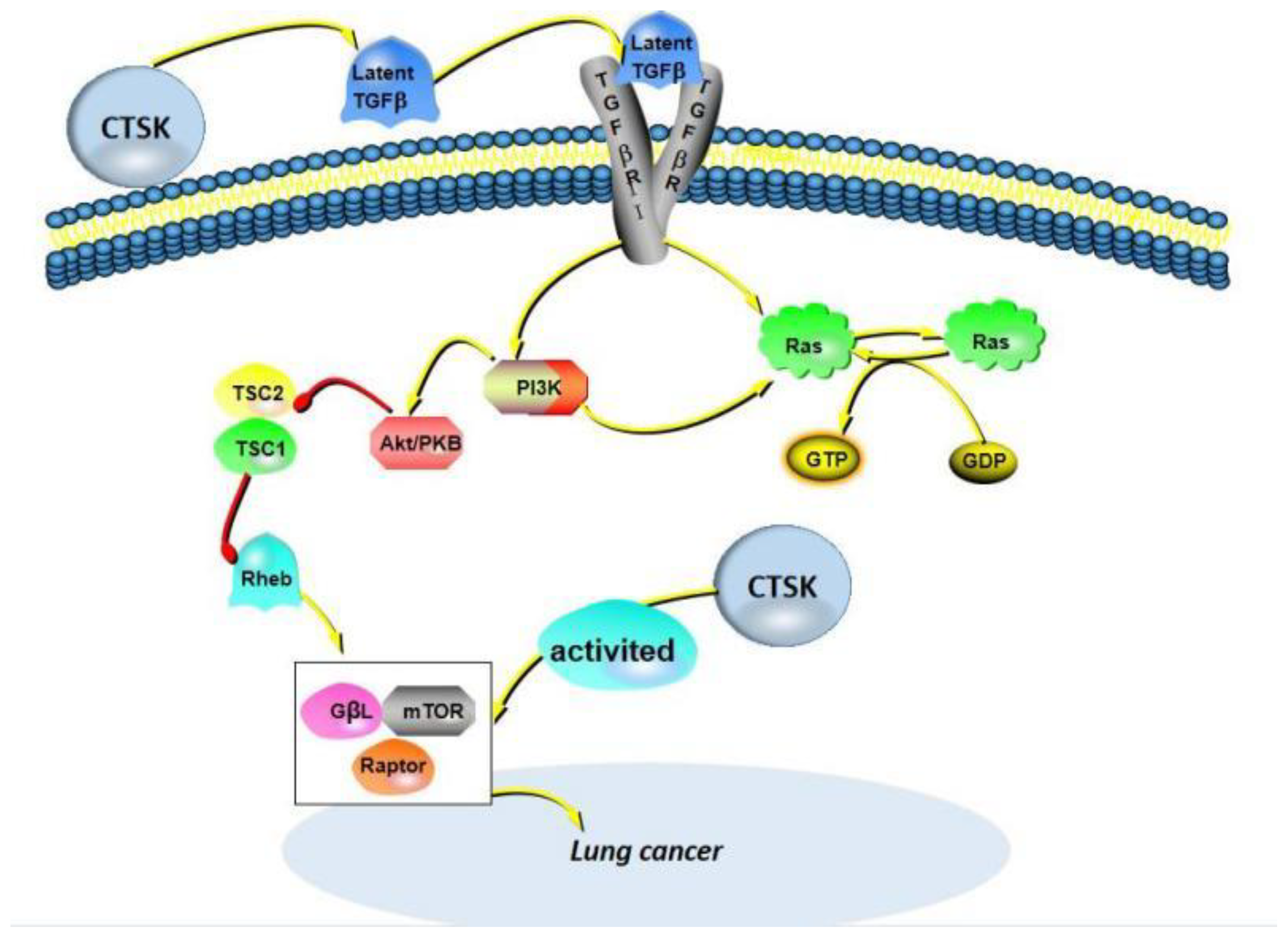
7. CTSK in Lung Cancer
8. CTSK in Colorectal Cancer
9. CTSK in Other Cancers
9.1. Ovarian Cancer
9.2. Gastric Cancer
9.3. Melanoma
10. CTSK Is a Potential Biomarker for Cancers Diagnosis
11. CTSK Is a Potential Therapeutic Target for Cancers
| Compound | Formula | Chemical Structure | Bioactivity | Reference |
|---|---|---|---|---|
| Odanacatib | C25H27F4N3O3S | 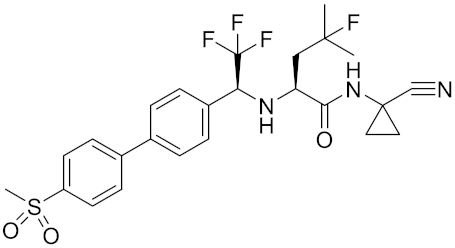 | Inhibitor, IC50 = 0.2 nM (Human), IC50 = 1 nM (Rabbit); odanacatib (30 mg/kg/day, orally) can persistently suppress bone resorption in OVX monkeys. | [155,164,165] |
| MK-0674 | C26H27F6N3O2 | 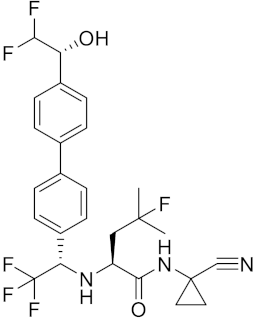 | Inhibitor, IC50 = 0.4 nM | [166] |
| L-873724 | C23H26F3N3O3S |  | Inhibitor, IC50 = 0.2 nM | [167,168] |
| Balicatib/AAE581 | C23H33N5O2 | 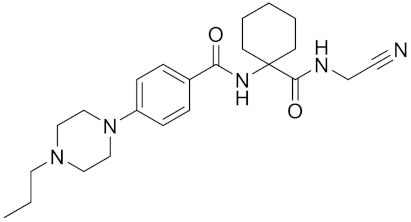 | Inhibitor, IC50 = 1.4 nM; IC50 = 56 nM (Rat); IC50 = 480 nM (mouse) | [169] |
| NC-2300/VEL-0230 | C14H24NO5 - | 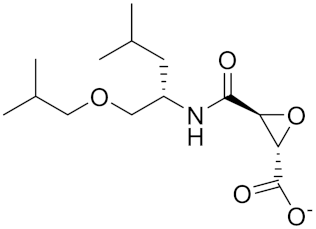 | Inhibitor, IC50 = 46 nM; IC50 = 319 nM (Rat); IC50 = 102 nM (mouse) | [169] |
| Gü1303 | C20H22N4O3 | 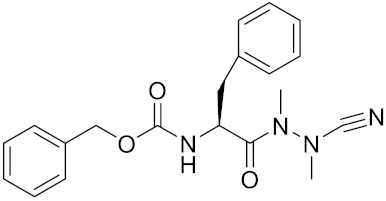 | Inhibitor, Ki = 0.91 nM | [170] |
| Gü2602 | C16H22N4O3 | 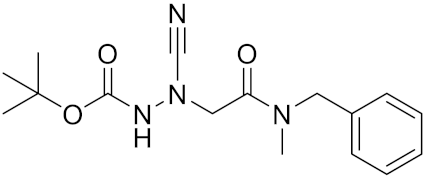 | Inhibitor, Ki = 0.013 nM | [170] |
| Cathepsin K inhibitor 2 | C30H33F4N5O3 | 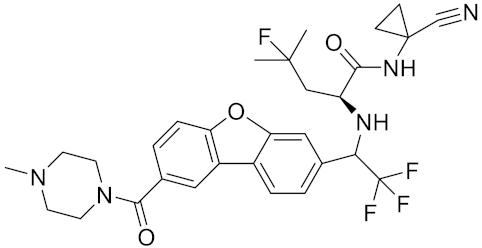 | [171] | |
| Cathepsin inhibitor 1 | C20H24ClN5O2 | 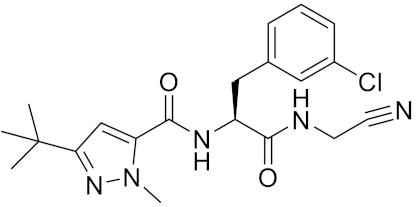 | Inhibitor, IC50 = 5.5 nM | [172] |
| Relacatib/ SB-462795 | C27H32N4O6S | 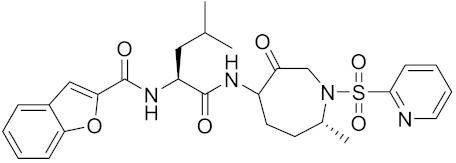 | Inhibitor, Ki = 0.041 nM | [149,173] |
| BML-244 | C11H21NO3 |  | Inhibitor, 1 μM | [174] |
| 4S-7-cis-methylazepanone | C27H32N4O6S |  | Inhibitor, Ki = 0.16 nM (human) | [173] |
| 4S-parent azepanone | C26H30N4O6S | 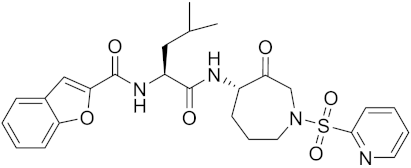 | Inhibitor, Ki = 0.16 nM (human) | [173,175] |
| Compound 24 | C40H47N5O7 | 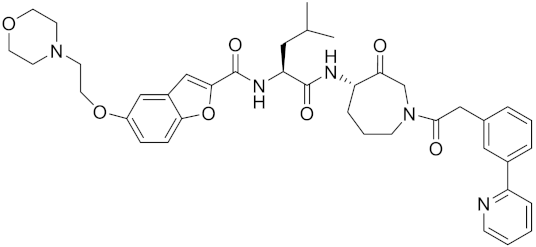 | Inhibitor, Ki = 0.0048 nM (human); Ki = 4.8 nM (rat) | [175] |
| ONO-5334 | C21H34N4O4S | 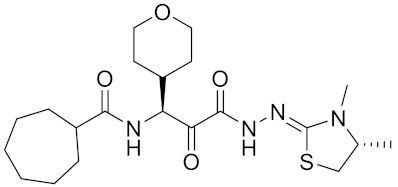 | Inhibitor, Ki = 0.10 nM (human); Ki = 0.049 nM (rabbit); Ki = 0.85 nM (rat) | [176,177] |
| 2-Cyanopyrimidine | C5H3N3 | 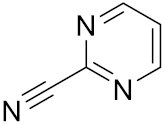 | Inhibitor, IC50 = 170 nM | [178] |
| LHVS | C28H37N3O5S | 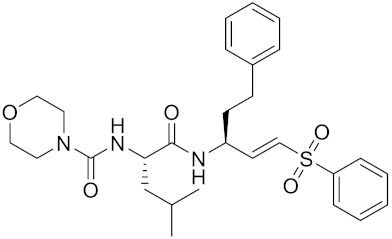 | Inhibitor, 5 μM (Osteoclasts) | [179] |
| L-006235/L-235 | C24H30N6O2S | 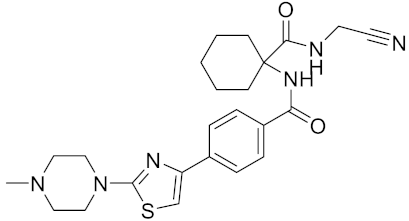 | Inhibitor, IC50 = 5 nM | [142,150,180] |
| calpeptin 1/Cbz-Leu-Nle-H | C20H30N2O4 | 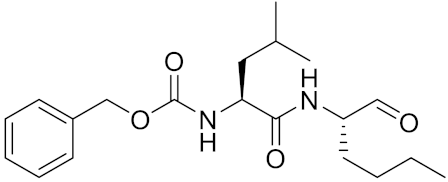 | Inhibitor, IC50 = 0.11 nM | [181] |
| Boc-Nle-H | C11H21NO3 | 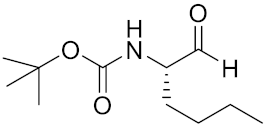 | Inhibitor, IC50 = 51 nM | [181] |
| Inhibitor 9 | 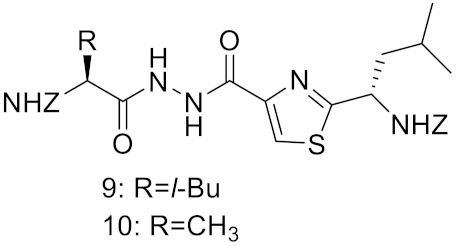 | Inhibitor, Ki = 10 nM | [182] | |
| Inhibitor10 | Inhibitor, Ki = 120 nM | [182] | ||
| Compound rac-34a | C22H23F2N2OS | 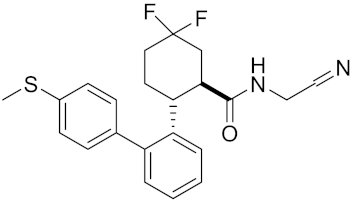 | Inhibitor, IC50 = 0.46 nM | [183] |
| Compound (−)34a | Inhibitor, IC50 = 0.28 | |||
| Compound (+)34a | Inhibitor, IC50 = 7.1 | |||
| Compound rac-34b | C21H19F3N2O |  | Inhibitor, IC50 = 36 | [183] |
| Compound rac-34c | C22H22Cl2N2OS | 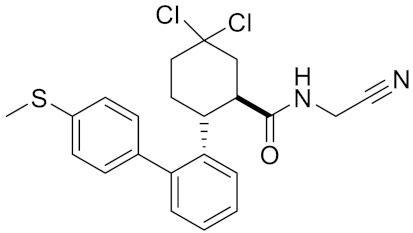 | Inhibitor, IC50 = 0.58 | [183] |
| Compound rac-38a | C22H23FN2OS | 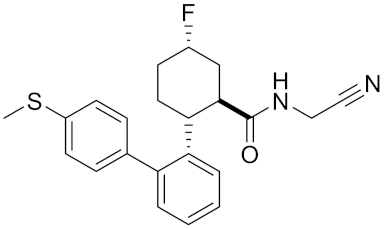 | Inhibitor, IC50 = 4.2 | [183] |
| Compound rac-38b | C22H23FN2OS | 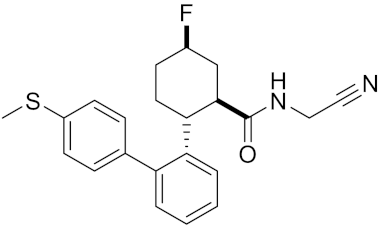 | Inhibitor, IC50 = 3.7 | [183] |
| Compound 1a |  | R = H, Inhibitor, IC50 = 0.47 | [178] | |
| Compound 1b | R = 3-CH3, Inhibitor, IC50 = 0.46 | |||
| Compound 1c | R = 4-CH3, Inhibitor, IC50 = 0.19 | |||
| Compound 1d | R = 3-Cl, Inhibitor, IC50 > 1 | |||
| Compound 1e | R = 4-Cl, Inhibitor, IC50 = 0.35 | |||
| Compound 1f | R = 3-OCH3, Inhibitor, IC50 > 1 | |||
| Compound 1g | R = 4-OCH3, Inhibitor, IC50 = 0.06 | |||
| Tri-Ring P3 Benzamide-Containing Aminonitriles | 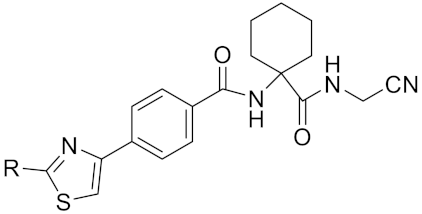 | Inhibitor, Ki < 0.003 nM | [180] | |
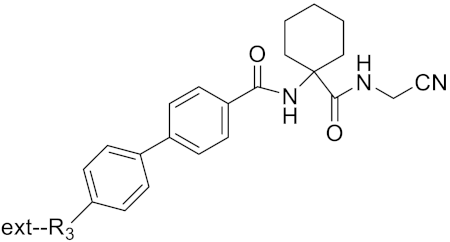 | Inhibitor, Ki < 0.00025 nM | |||
| Nonpeptidic Cyanamides | 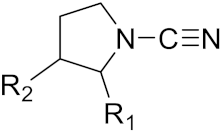 | Inhibitor, IC50 = 0.05–13.7 μM | [142] | |
| Compound 4a |  | R= GlyOMe, Inhibitor, IC50 = 0.1 mM | [184] | |
| Compound 4d | R= L-AsnOMe, Inhibitor, IC50 = 0.4 mM | [184] | ||
| Amentoflavone | 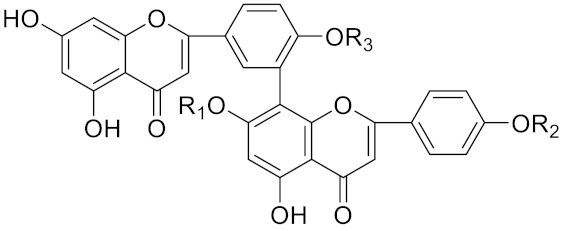 | R1 = R2 = R3 = H, Inhibitor, IC50 = 1.88 μM | [185] | |
| Podocarpusflavone A, | R1= R3 = H, R2 = CH3, Inhibitor, IC50 = 2.51 μM | [185] | ||
| 7′′,4′′′-dimethylamentoflavone | R1= R2 = CH3, R3 = H, Inhibitor, IC50 = 1.57 μM | [185] | ||
| Bilobetin | R1= R2 = H, R3 = CH3, Inhibitor, IC50 = 1.55 μM | [185] | ||
| 2,3-dihydroamentoflavone | C30H18O10 | 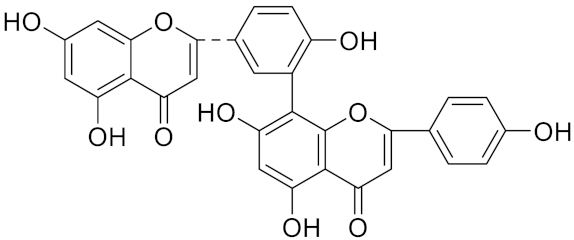 | Inhibitor, IC50 = 1.39 μM | [185] |
| Hinokiflavone | C30H18O10 | 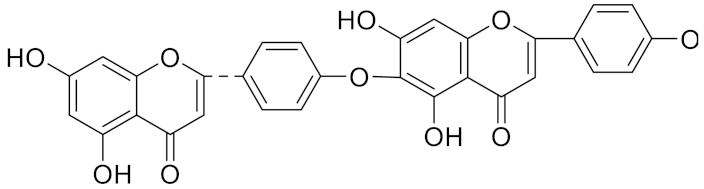 | Inhibitor, IC50 = 8.797 μM | [185] |
| Kushennol F | C25H28O6 | 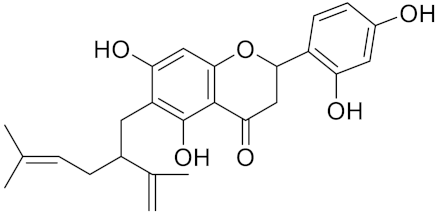 | Inhibitor, IC50 = 27.24 nM | [186] |
| Sophoraflavone G | C25H28O6 | 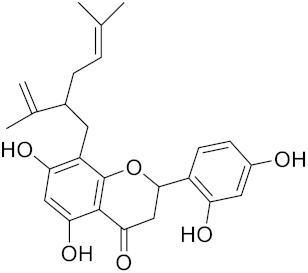 | Inhibitor, IC50 = 1.54 nM | [186] |
| A series ofketoamides with varying P1 moieties | 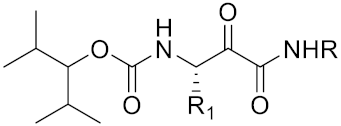 | Inhibitor, IC50 = 0.77–12,000 nM | [187] |
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Islami, F.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global cancer in women: Burden and trends. Cancer Epidemiol. Biomark. Prev. 2017, 26, 444–457. [Google Scholar] [CrossRef]
- Mattiuzzi, C.; Lippi, G. Current cancer epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, K.D.; Thorburn, A. Regulation of apoptosis by autophagy to enhance cancer therapy. Yale J. Biol. Med. 2019, 92, 707–718. [Google Scholar] [PubMed]
- Neville, B.W.; Day, T.A. Oral cancer and precancerous lesions. CA Cancer J. Clin. 2002, 52, 195–215. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Deveraux, Q.; Turk, B.; Sali, A. Comprehensive search for cysteine cathepsins in the human genome. Biol. Chem. 2004, 385, 363–372. [Google Scholar] [CrossRef]
- Abdel-Magid, A.F. Inhibition of cathepsin K: A novel and promising treatment for osteoporosis. ACS Med. Chem. Lett. 2015, 6, 628–629. [Google Scholar] [CrossRef]
- Drake, F.H.; Dodds, R.A.; James, I.E.; Coleman, L.; Rieman, D.; Barthlow, R.; Lee-Rykaczewski, E.; Coleman, L.; Rieman, D.; Barthlow, R.; et al. Cathepsin K, but not cathepsins B, L, or S, is abundantly expressed in human osteoclasts. J. Biol. Chem. 1996, 271, 12511–12516. [Google Scholar] [CrossRef]
- Yang, J.; Shang, G.D.; Zhang, Y.Q. Study of a novel antiosteoporosis screening model targeted on cathepsin K. Biomed. Environ. Sci. 2004, 17, 273–280. [Google Scholar]
- Rünger, T.M.; Quintanilla-Dieck, M.J.; Bhawan, J. Role of cathepsin K in the turnover of the dermal extracellular matrix during scar formation. J. Investig. Dermatol. 2007, 127, 293–297. [Google Scholar] [CrossRef]
- Gelb, B.D.; Shi, G.P.; Chapman, H.A.; Desnick, R.J. Pycnodysostosis, a lysosomal disease caused by cathepsin K deficiency. Science 1996, 273, 1236–1238. [Google Scholar] [CrossRef]
- Garnero, P.; Borel, O.; Byrjalsen, I.; Ferreras, M.; Drake, F.H.; McQueney, M.S.; Foged, N.T.; Delmas, P.D.; Delaissé, J.M. The collagenolytic activity of cathepsin K is unique among mammalian proteinases. J. Biol. Chem. 1998, 273, 32347–32352. [Google Scholar] [CrossRef]
- Cheng, X. The expression and significance of collagenolyitc cathepsin K in the left ventricle remodeling during the hypertensive heart failure. Clin. Med. J. Chin. 2008, 15, 449–453. [Google Scholar]
- Dai, Q.; Xie, F.; Han, Y.; Ma, X.; Zhou, S.; Jiang, L.; Zou, W.; Wang, J. Inactivation of regulatory-associated protein of mTOR (raptor)/mammalian target of rapamycin complex 1 (mTORC1) signaling in osteoclasts increases bone mass by inhibiting osteoclast differentiation in mice. J. Biol. Chem. 2017, 292, 196–204. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Jin, J.; Jin, D.; Cui, L.; Li, X.; Rei, Y.; Jiang, H.; Zhao, G.; Yang, G.; et al. Increased serum cathepsin K in patients with coronary artery disease. Yonsei Med. J. 2014, 55, 912–919. [Google Scholar] [CrossRef]
- Hu, L.; Cheng, X.W.; Song, H.; Inoue, A.; Jiang, H.; Li, X.; Shi, G.P.; Kozawa, E.; Okumura, K.; Kuzuya, M. Cathepsin K activity controls injury-related vascular repair in mice. Hypertension 2014, 63, 607–615. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Liu, D.J.; Guo, Y.Y.; Zhang, J.L.; Zhang, X.L. Role of cathepsin in rheumatoid arthritis. Pract. Pharm. Clin. Remed. 2021, 24, 649–654. [Google Scholar]
- Littlewood-Evans, A.J.; Bilbe, G.; Bowler, W.B.; Farley, D.; Wlodarski, B.; Kokubo, T.; Inaoka, T.; Sloane, J.; Evans, D.B.; Gallagher, J.A. The osteoclast-associated protease cathepsin K is expressed in human breast carcinoma. Cancer Res. 1997, 57, 5386–5390. [Google Scholar]
- Lindeman, J.H.; Hanemaaijer, R.; Mulder, A.; Dijkstra, P.D.; Szuhai, K.; Bromme, D.; Verheijen, J.H.; Hogendoorn, P.C. Cathepsin K is the principal protease in giant cell tumor of bone. Am. J. Pathol. 2004, 165, 593–600. [Google Scholar] [CrossRef]
- Brubaker, K.D.; Vessella, R.L.; True, L.D.; Thomas, R.; Corey, E. Cathepsin K mRNA and protein expression in prostate cancer progression. J. Bone Miner. Res. 2003, 18, 222–230. [Google Scholar] [CrossRef]
- Joyce, J.A.; Baruch, A.; Chehade, K.; Meyer-Morse, N.; Giraudo, E.; Tsai, F.Y.; Greenbaum, D.C.; Hager, J.H.; Bogyo, M.; Hanahan, D. Cathepsin cysteine proteases are effffectors of invasive growth and angiogenesis during multistage tumorigenesis. Cancer Cell 2004, 5, 443–453. [Google Scholar] [CrossRef]
- Kleer, C.G.; Bloushtain-Qimron, N.; Chen, Y.H.; Carrasco, D.; Hu, M.; Yao, J.; Kraeft, S.K.; Collins, L.C.; Sabel, M.S.; Argani, P.; et al. Epithelial and stromal cathepsin K and CXCL14 expression in breast tumor progression. Clin. Cancer Res. 2008, 14, 5357–5367. [Google Scholar] [CrossRef] [PubMed]
- Boutté, A.M.; Friedman, D.B.; Bogyo, M.; Min, Y.; Yang, L.; Lin, P.C. Identifification of a myeloid-derived suppressor cell cystatin-like protein that inhibits metastasis. FASEB J. 2011, 25, 2626–2637. [Google Scholar] [CrossRef] [PubMed]
- Rolli, M.; Fransvea, E.; Pilch, J.; Saven, A.; Felding-Habermann, B. Activated integrin alphavbeta3 cooperates with metalloproteinase MMP-9 in regulating migration of metastatic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 9482–9487. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, S.; Nakamura, K.; Iwai, S.; Murakami, M.; Itoh, T.; Kijima, H.; Shipley, J.M.; Senior, R.M.; Shibuya, M. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell 2002, 2, 289–300. [Google Scholar] [CrossRef]
- Christensen, J.; Shastri, V.P. Matrix-metalloproteinase-9 is cleaved and activated by cathepsin K. BMC Res. Notes 2015, 8, 322. [Google Scholar] [CrossRef]
- Parks, A.N.; Nahata, J.; Edouard, N.E.; Temenoff, J.S.; Platt, M.O. Sequential, but not concurrent, incubation of cathepsin K and L with type I collagen results in extended proteolysis. Sci. Rep. 2019, 9, 5399. [Google Scholar] [CrossRef]
- Corisdeo, S.; Gyda, M.; Zaidi, M.; Moonga, B.S.; Troen, B.R. New insights into the regulation of cathepsin K gene expression by osteoprotegerin ligand. Biochem. Biophys. Res. Commun. 2001, 285, 335–339. [Google Scholar] [CrossRef]
- Podgorski, I.; Linebaugh, B.E.; Koblinski, J.E.; Rudy, D.L.; Herroon, M.K.; Olive, M.B.; Sloane, B.F. Bone marrow-derived cathepsin K cleaves SPARC in bone metastasis. Am. J. Pathol. 2009, 175, 1255–1269. [Google Scholar] [CrossRef]
- Terpos, E.; Confavreux, C.B.; Clézardin, P. Bone antiresorptive agents in the treatment of bone metastases associated with solid tumours or multiple myeloma. Bonekey Rep. 2015, 4, 744. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Rachner, T.D.; Coleman, R.E.; Jakob, F. Endocrine aspects of bone metastases. Lancet Diabetes Endocrinol. 2014, 2, 500–512. [Google Scholar] [CrossRef]
- Coleman, R.; Gnant, M.; Morgan, G.; Clezardin, P. Effects of bone-targeted agents on cancer progression and mortality. J. Natl. Cancer Inst. 2012, 104, 1059–1067. [Google Scholar] [CrossRef]
- Seo, S.U.; Woo, S.M.; Kim, M.W.; Lee, H.S.; Kim, S.H.; Kang, S.C.; Lee, E.W.; Min, K.J.; Kwon, T.K. Cathepsin K inhibition-induced mitochondrial ROS enhances sensitivity of cancer cells to anti-cancer drugs through USP27x-mediated Bim protein stabilization. Redox Biol. 2020, 30, 101422. [Google Scholar] [CrossRef]
- Guri, Y.; Hall, M.N. mTOR Signaling confers resistance to targeted cancer drugs. Trends Cancer 2016, 2, 688–697. [Google Scholar] [CrossRef]
- Kimura, T.; Egawa, S. Epidemiology of prostate cancer in Asian countries. Int. J. Urol. 2018, 25, 524–531. [Google Scholar] [CrossRef]
- Kohaar, I.; Petrovics, G.; Srivastava, S. A rich array of prostate cancer molecular biomarkers: Opportunities and challenges. Int. J. Mol. Sci. 2019, 20, 1813. [Google Scholar] [CrossRef]
- Catalona, W.J.; Richie, J.P.; Ahmann, F.R.; Hudson, M.A.; Scardino, P.T.; Flanigan, R.C.; DeKernion, J.B.; Ratliff, T.L.; Kavoussi, L.R.; Dalkin, B.L.; et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: Results of a multicenter clinical trial of 6630 men. J. Urol. 2017, 197, S200–S207. [Google Scholar] [CrossRef]
- Zhang, X. Interactions between cancer cells and bone microenvironment promote bone metastasis in prostate cancer. Cancer Commun. 2019, 39, 76. [Google Scholar] [CrossRef]
- Eguchi, K.; Akiba, Y.; Akiba, N.; Nagasawa, M.; Cooper, L.F.; Uoshima, K. Insulin-like growth factor binding Protein-3 suppresses osteoblast differentiation via bone morphogenetic protein-2. Biochem. Biophys. Res. Commun. 2018, 507, 465–470. [Google Scholar] [CrossRef]
- Salazar, V.S.; Gamer, L.W.; Rosen, V. BMP signalling in skeletal development, disease and repair. Nat. Rev. Endocrinol. 2016, 12, 203–221. [Google Scholar] [CrossRef]
- Verrecchia, F.; Rédini, F. Transforming growth factor-β signaling plays a pivotal role in the interplay between osteosarcoma cells and their microenvironment. Front. Oncol. 2018, 8, 133. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. Biomed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed]
- Burr, D.B. Changes in bone matrix properties with aging. Bone 2019, 120, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Vidak, E.; Javoršek, U.; Vizovišek, M.; Turk, B. Cysteine cathepsins and their extracellular roles: Shaping the microenvironment. Cells 2019, 8, 264. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Iqbal, M.; Daniyal, M.; Khan, A.U. Awareness and current knowledge of breast cancer. Biol. Res. 2017, 50, 33. [Google Scholar] [CrossRef]
- Samavat, H.; Kurzer, M.S. Estrogen metabolism and breast cancer. Cancer Lett. 2015, 356, 231–243. [Google Scholar] [CrossRef]
- Trabert, B.; Sherman, M.E.; Kannan, N.; Stanczyk, F.Z. Progesterone and breast cancer. Endocr. Rev. 2020, 41, 320–344. [Google Scholar] [CrossRef]
- Tian, J.M.; Ran, B.; Zhang, C.L.; Yan, D.M.; Li, X.H. Estrogen and progesterone promote breast cancer cell proliferation by inducing cyclin G1 expression. Braz. J. Med. Biol. Res. 2018, 51, e5612. [Google Scholar] [CrossRef]
- Le Gall, C.; Bellahcène, A.; Bonnelye, E.; Gasser, J.A.; Castronovo, V.; Green, J.; Zimmermann, J.; Clézardin, P. A cathepsin K inhibitor reduces breast cancer induced osteolysis and skeletal tumor burden. Cancer Res. 2007, 67, 9894–9902. [Google Scholar] [CrossRef]
- Muschler, J.; Streuli, C.H. Cell-matrix interactions in mammary gland development and breast cancer. Cold Spring Harb. Perspect. Biol. 2010, 2, a003202. [Google Scholar] [CrossRef]
- Lugo-Cintrón, K.M.; Gong, M.M.; Ayuso, J.M.; Tomko, L.A.; Beebe, D.J.; Virumbrales-Muñoz, M.; Ponik, S.M. Breast fibroblasts and ECM components modulate breast cancer cell migration through the secretion of MMPs in a 3D microfluidic co-culture model. Cancers 2020, 12, 1173. [Google Scholar] [CrossRef]
- Wu, X.; Li, F.; Dang, L.; Liang, C.; Lu, A.; Zhang, G. RANKL/RANK system-based mechanism for breast cancer bone metastasis and related therapeutic strategies. Front. Cell Dev. Biol. 2020, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.H.; Nakashima, T.; Sanchez, O.H.; Kozieradzki, I.; Komarova, S.V.; Sarosi, I.; Morony, S.; Rubin, E.; Sarao, R.; Hojilla, C.V.; et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature 2006, 440, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.; Zhang, J.; Fix, A.; Brewer, E.; Li, Y.P.; Zhang, Z.Y.; Chen, J. Targeted overexpression of BSP in osteoclasts promotes bone metastasis of breast cancer cells. J. Cell. Physiol. 2009, 218, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Tiedemann, K.; Khalil, J.A.; Russo, C.; Siegel, P.M.; Komarova, S.V. Osteoclast precursors acquire sensitivity to breast cancer derived factors early in differentiation. Bone 2008, 43, 386–393. [Google Scholar] [CrossRef]
- Motyckova, G.; Weilbaecher, K.N.; Horstmann, M.; Rieman, D.J.; Fisher, D.Z.; Fisher, D.E. Linking osteopetrosis and pycnodysostosis: Regulation of cathepsin K expression by the microphthalmia transcription factor family. Proc. Natl. Acad. Sci. USA 2001, 98, 5798–5803. [Google Scholar] [CrossRef]
- Montgomery, N.; Hill, A.; McFarlane, S.; Neisen, J.; O’Grady, A.; Conlon, S.; Jirstrom, K.; Kay, E.W.; Waugh, D.J. CD44 enhances invasion of basal-like breast cancer cells by upregulating serine protease and collagen-degrading enzymatic expression and activity. Breast Cancer Res. 2012, 14, R84. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Hsing, C.H.; Li, C.F.; Chan, C.H.; Chang, M.C.; Yan, J.J.; Chang, M.S. Anti-IL-20 monoclonal antibody suppresses breast cancer progression and bone osteolysis in murine models. J. Immunol. 2012, 188, 1981–1991. [Google Scholar] [CrossRef]
- Gruenwald, N.; Demos, T.C.; Lomasney, L.M.; Rapp, T. Giant-cell tumor. Orthopedics 2006, 29, 94, 167–171. [Google Scholar]
- Wülling, M.; Engels, C.; Jesse, N.; Werner, M.; Delling, G.; Kaiser, E. The nature of giant cell tumor of bone. J. Cancer Res. Clin. Oncol 2001, 127, 467–474. [Google Scholar] [CrossRef]
- Kiviranta, R.; Morko, J.; Uusitalo, H.; Aro, H.T.; Vuorio, E.; Rantakokko, J. Accelerated turnover of metaphyseal trabecular bone in mice overexpressing cathepsin K. J. Bone Miner. Res. 2001, 16, 1444–1452. [Google Scholar] [CrossRef]
- Gowen, M.; Lazner, F.; Dodds, R.; Kapadia, R.; Feild, J.; Tavaria, M.; Bertoncello, I.; Drake, F.; Zavarselk, S.; Tellis, I.; et al. Cathepsin K knockout mice develop osteopetrosis due to a deficit in matrix degradation but not demineralization. J. Bone Miner. Res. 1999, 14, 1654–1663. [Google Scholar] [CrossRef]
- Van der Heijden, L.; Dijkstra, P.D.; van de Sande, M.A.; Kroep, J.R.; Nout, R.A.; van Rijswijk, C.S.; Bovée, J.V.; Hogendoorn, P.C.; Gelderblom, H. The clinical approach toward giant cell tumor of bone. Oncologist 2014, 19, 550–561. [Google Scholar] [CrossRef]
- Liao, T.S.; Yurgelun, M.B.; Chang, S.S.; Zhang, H.Z.; Murakami, K.; Blaine, T.A.; Parisien, M.V.; Kim, W.; Winchester, R.J.; Lee, F.Y. Recruitment of osteoclast precursors by stromal cell derived factor-1 (SDF-1) in giant cell tumor of bone. J. Orthop. Res. 2005, 23, 203–209. [Google Scholar] [CrossRef]
- Branstetter, D.G.; Nelson, S.D.; Manivel, J.C.; Blay, J.Y.; Chawla, S.; Thomas, D.M.; Jun, S.; Jacobs, I. Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone. Clin. Cancer Res. 2012, 18, 4415–4424. [Google Scholar] [CrossRef]
- Chawla, S.; Henshaw, R.; Seeger, L.; Choy, E.; Blay, J.Y.; Ferrari, S.; Kroep, J.; Grimer, R.; Reichardt, P.; Rutkowski, P.; et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: Interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol. 2013, 14, 901–908. [Google Scholar] [CrossRef]
- Murai, M.; Oya, M. Renal cell carcinoma: Etiology, incidence and epidemiology. Curr. Opin. Urol. 2004, 14, 229–233. [Google Scholar] [CrossRef]
- Jonasch, E.; Gao, J.; Rathmell, W.K. Renal cell carcinoma. BMJ 2014, 349, g4797. [Google Scholar] [CrossRef]
- Akgul, M.; Williamson, S.R.; Ertoy, D.; Argani, P.; Gupta, S.; Caliò, A.; Reuter, V.; Tickoo, S.; Al-Ahmadie, H.A.; Netto, G.J.; et al. Diagnostic approach in TFE3-rearranged renal cell carcinoma: A multi-institutional international survey. J. Clin. Pathol. 2021, 74, 291–299. [Google Scholar] [CrossRef]
- Martignoni, G.; Gobbo, S.; Camparo, P.; Brunelli, M.; Munari, E.; Segala, D.; Pea, M.; Bonetti, F.; Illei, P.B.; Netto, G.J.; et al. Differential expression of cathepsin K in neoplasms harboring TFE3 gene fusions. Mod. Pathol. 2011, 24, 1313–1319. [Google Scholar] [CrossRef]
- Martignoni, G.; Pea, M.; Gobbo, S.; Brunelli, M.; Bonetti, F.; Segala, D.; Pan, C.C.; Netto, G.; Doglioni, C.; Hes, O.; et al. Cathepsin-K immunoreactivity distinguishes MiTF/TFE family renal translocation carcinomas from other renal carcinomas. Mod. Pathol. 2009, 22, 1016–1022. [Google Scholar] [CrossRef]
- Wu, H.; He, D.; Biswas, S.; Shafiquzzaman, M.; Zhou, X.; Charron, J.; Wang, Y.; Nayak, B.K.; Habib, S.L.; Liu, H.; et al. mTOR activation initiates renal cell carcinoma development by coordinating ERK and p38MAPK. Cancer Res. 2021, 81, 3174–3186. [Google Scholar] [CrossRef]
- Cho, D.C.; Mier, J.W. Dual inhibition of PI3-kinase and mTOR in renal cell carcinoma. Curr. Cancer Drug Targets 2013, 13, 126–142. [Google Scholar] [CrossRef]
- Coinu, A.; Petrelli, F.; Barni, S. Optimal treatment of poor-risk renal cell carcinoma patients with mTOR inhibitors and anti-VEGFR agents. Expert Rev. Anticancer Ther. 2016, 16, 33–43. [Google Scholar] [CrossRef]
- Iakymenko, O.A.; Delma, K.S.; Jorda, M.; Kryvenko, O.N. Cathepsin K (clone EPR19992) demonstrates uniformly positive immunoreactivity in renal oncocytoma, chromophobe renal cell carcinoma, and distal tubules. Int J. Surg. Pathol. 2021, 29, 600–605. [Google Scholar] [CrossRef]
- Xia, Q.Y.; Wang, X.; Wei, X.; Wang, X.T.; Ma, H.H.; Lu, Z.F.; Rao, Q. Eosinophilic solid and cystic renal cell carcinoma: Clinicopathological analysis and molecular characterization. Zhonghua Bing Li Xue Za Zhi 2019, 48, 840–845. [Google Scholar]
- Chen, Y.B.; Mirsadraei, L.; Jayakumaran, G.; Al-Ahmadie, H.A.; Fine, S.W.; Gopalan, A.; Sirintrapun, S.J.; Tickoo, S.K.; Reuter, V.E. Somatic mutations of TSC2 or MTOR characterize a morphologically distinct subset of sporadic renal cell carcinoma with eosinophilic and vacuolated cytoplasm. Am. J. Surg. Pathol. 2019, 43, 121–131. [Google Scholar] [CrossRef]
- Farcaş, M.; Gatalica, Z.; Trpkov, K.; Swensen, J.; Zhou, M.; Alaghehbandan, R.; Williamson, S.R.; Magi-Galluzzi, C.; Gill, A.J.; Tretiakova, M.; et al. Eosinophilic vacuolated tumor (EVT) of kidney demonstrates sporadic TSC/MTOR mutations: Next-generation sequencing multi-institutional study of 19 cases. Mod. Pathol. 2022, 35, 344–351. [Google Scholar] [CrossRef]
- McDorman, K.S.; Wolf, D.C. Use of the spontaneous TSC2 knockout (Eker) rat model of hereditary renal cell carcinoma for the study of renal carcinogens. Toxicol. Pathol. 2002, 30, 675–680. [Google Scholar] [CrossRef]
- Sahin, K.; Cross, B.; Sahin, N.; Ciccone, K.; Suleiman, S.; Osunkoya, A.O.; Master, V.; Harris, W.; Carthon, B.; Mohammad, R.; et al. Lycopene in the prevention of renal cell cancer in the TSC2 mutant Eker rat model. Arch. Biochem. Biophys. 2015, 572, 36–39. [Google Scholar] [CrossRef]
- Palsgrove, D.N.; Li, Y.; Pratilas, C.A.; Lin, M.T.; Pallavajjalla, A.; Gocke, C.; de Marzo, A.M.; Matoso, A.; Netto, G.J.; Epstein, J.I.; et al. Eosinophilic solid and cystic (ESC) renal cell carcinomas harbor TSC mutations: Molecular analysis supports an expanding clinicopathologic spectrum. Am. J. Surg. Pathol. 2018, 42, 1166–1181. [Google Scholar] [CrossRef]
- Tretiakova, M.S. Eosinophilic solid and cystic renal cell carcinoma mimicking epithelioid angiomyolipoma: Series of 4 primary tumors and 2 metastases. Hum. Pathol. 2018, 80, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Martignoni, G.; Bonetti, F.; Chilosi, M.; Brunelli, M.; Segala, D.; Amin, M.B.; Argani, P.; Eble, J.N.; Gobbo, S.; Pea, M. Cathepsin K expression in the spectrum of perivascular epithelioid cell (PEC) lesions of the kidney. Mod. Pathol. 2012, 25, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; You, H.; Qi, J.; Yang, C.; Ren, Y.; Cheng, H. Autocrine and paracrine STIP1 signaling promote osteolytic bone metastasis in renal cell carcinoma. Oncotarget 2017, 8, 17012–17026. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; WHO Panel; et al. The 2015 World Health Organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef] [PubMed]
- Caliò, A.; Mengoli, M.C.; Cavazza, A.; Rossi, G.; Ghimenton, C.; Brunelli, M.; Pea, M.; Chilosi, M.; Marcolini, L.; Martignoni, G. Cathepsin K expression in clear cell “sugar” tumor (PEComa) of the lung. Virchows Arch. 2018, 473, 55–59. [Google Scholar] [CrossRef]
- Bühling, F.; Peitz, U.; Wex, T.; Küster, D.; Vieth, M.; Gebert, I.; Roessner, A.; Weber, E.; Malfertheiner, P.; Wex, T. Cathepsins K, L, B, X and W are differentially expressed in normal and chronically inflamed gastric mucosa. Biol. Chem. 2004, 385, 439–445. [Google Scholar] [CrossRef]
- Zhang, D.; Leung, N.; Weber, E.; Saftig, P.; Brömme, D. The effect of cathepsin K deficiency on airway development and TGF-β1 degradation. Respir. Res. 2011, 12, 72. [Google Scholar] [CrossRef]
- Li, M.; Amizuka, N.; Takeuchi, K.; Freitas, P.H.; Kawano, Y.; Hoshino, M.; Oda, K.; Nozawa-Inoue, K.; Maeda, T. Histochemical evidence of osteoclastic degradation of extracellular matrix in osteolytic metastasis originating from human lung small carcinoma (SBC-5) cells. Microsc. Res. Tech. 2006, 69, 73–83. [Google Scholar] [CrossRef]
- Naumnik, W.; Niklińska, W.; Ossolińska, M.; Chyczewska, E. Serum cathepsin K and cystatin C concentration in patients with advanced non-small-cell lung cancer during chemotherapy. Folia Histochem. CytoBiol. 2009, 47, 207–213. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, J.; Chen, S.; Lu, M.; Luo, X.; Yao, S.; Liu, S.; Qin, Y.; Chen, H. Tumor-associated macrophages provide a suitable microenvironment for non-small lung cancer invasion and progression. Lung Cancer 2011, 74, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Heyer, J.; Zhao, H.; Liang, S.; Guo, R.; Zhong, L. The Potential Role of Cathepsin K in Non-Small Cell Lung Cancer. Molecules 2020, 25, 4136. [Google Scholar] [CrossRef] [PubMed]
- Belhamidi, M.S.; Sinaa, M.; Kaoukabi, A.; Krimou, H.; Menfaa, M.; Sakit, F.; Choho, A. Profil épidémiologique et anatomopathologique du cancer colorectal: À propos de 36 caswe [Epidemiological and pathological profile of colorectal cancer: About 36 cases]. Pan Afr. Med. J. 2018, 30, 159. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, S.; Haslam, A.; Choy, A.; Diaz-Cano, S.; Galante, J.R.; Mikropoulos, C.; Boussios, S. Microsatellite instability testing in colorectal patients with Lynch syndrome: Lessons learned from a case report and how to avoid such pitfalls. Pers. Med. 2022, 19, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.F.; Ibrahim, A.E.; Arends, M.J. Molecular pathological classification of colorectal cancer. Virchows Arch. 2016, 469, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhou, R.; Wang, H.; Li, W.; Pan, M.; Yao, X.; Zhan, W.; Yang, S.; Xu, L.; Ding, Y.; et al. Gut microbiota-stimulated cathepsin K secretion mediates TLR4-dependent M2 macrophage polarization and promotes tumor metastasis in colorectal cancer. Cell Death Differ. 2019, 26, 2447–2463. [Google Scholar] [CrossRef]
- Arthur, J.C.; Perez-Chanona, E.; Mühlbauer, M.; Tomkovich, S.; Uronis, J.M.; Fan, T.J.; Campbell, B.J.; Abujamel, T.; Dogan, B.; Rogers, A.B.; et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 2012, 338, 120–123. [Google Scholar] [CrossRef]
- Hurtado, C.G.; Wan, F.; Housseau, F.; Sears, C.L. Roles for interleukin 17 and adaptive immunity in pathogenesis of colorectal cancer. Gastroenterology 2018, 155, 1706–1715. [Google Scholar] [CrossRef]
- Li, L.; Zhu, Z.; Zhao, Y.; Zhang, Q.; Wu, X.; Miao, B.; Cao, J.; Fei, S. FN1, SPARC, and SERPINE1 are highly expressed and significantly related to a poor prognosis of gastric adenocarcinoma revealed by microarray and bioinformatics. Sci. Rep. 2019, 9, 7827. [Google Scholar] [CrossRef]
- Makondi, P.T.; Wei, P.L.; Huang, C.Y.; Chang, Y.J. Development of novel predictive miRNA/target gene pathways for colorectal cancer distance metastasis to the liver using a bioinformatic approach. PLoS ONE 2019, 14, e0211968. [Google Scholar] [CrossRef]
- Gaona-Luviano, P.; Medina-Gaona, L.A.; Magaña-Pérez, K. Epidemiology of ovarian cancer. Chin. ClinOncol 2020, 9, 47. [Google Scholar] [CrossRef]
- Cho, K.R.; Shih, I.-M. Ovarian cancer. Annu. Rev. Pathol. 2009, 4, 287–313. [Google Scholar] [CrossRef]
- Xu, H.; Ma, Y.; Lu, B.; Pan, Z.; Lu, Y.; Liu, P.; Lu, B. Identification of cathepsin K in the peritoneal metastasis of ovarian carcinoma using in-silico, gene expression analysis. J. Cancer 2016, 7, 722–729. [Google Scholar] [CrossRef]
- Tingting, Z.; Xiaojing, L.; Junjun, Q.; Keqin, H.; Junjun, Q. The antisense long noncoding RNA AGAP2-AS1 regulates cell proliferation and metastasis in epithelial ovarian cancer. J. Cancer 2020, 11, 5318–5328. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, J.; Zhao, Z.; Gao, F.; Zhu, J.; Feng, Z.; Lv, X.; Zhao, Z. Identification of NDRG1-regulated genes associated with invasive potential in cervical and ovarian cancer cells. Biochem. Biophys. Res. Commun. 2011, 408, 154–159. [Google Scholar] [CrossRef]
- Weiland, F.; Martin, K.; Oehler, M.K.; Hoffmann, P. Deciphering the molecular nature of ovarian cancer biomarker CA125. Int. J. Mol. Sci. 2012, 13, 10568–10582. [Google Scholar] [CrossRef]
- Ang, T.L.; Fock, K.M. Clinical epidemiology of gastric cancer. Singap. Med. J. 2014, 55, 621–628. [Google Scholar] [CrossRef]
- Ren, G.; Tian, Q.; Fan, D.; Feng, B.; Lu, Y.; Liang, J.; Li, K.; Shang, Y.; Nie, Y.; Wang, X.; et al. Coronin 3 promotes gastric cancer metastasis via the up-regulation of MMP-9 and cathepsin K. Mol. Cancer 2012, 11, 67. [Google Scholar] [CrossRef]
- Digklia, A.; Wagner, A.D. Advanced gastric cancer: Current treatment landscape and future perspectives. World J. Gastroenterol. 2016, 22, 2403–2414. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J.; Carlson, A.J.; Matsuoka, L.Y.; Balch, C.M.; Mihm, M.C. Malignant melanoma. Arch. Pathol. Lab. Med 2001, 125, 1295–1306. [Google Scholar] [CrossRef]
- Sánchez-Danés, A.; Blanpain, C. Deciphering the cells of origin of squamous cell carcinomas. Nat. Rev. Cancer 2018, 18, 549–561. [Google Scholar] [CrossRef]
- Boussios, S.; Rassy, E.; Samartzis, E.; Moschetta, M.; Sheriff, M.; Pérez-Fidalgo, J.A.; Pavlidis, N. Melanoma of unknown primary: New perspectives for an old story. Crit. Rev. Oncol. Hematol. 2021, 158, 103208. [Google Scholar] [CrossRef]
- Quintanilla-Dieck, M.J.; Codriansky, K.; Keady, M.; Bhawan, J.; Rünger, T.M. Expression and regulation of cathepsin K in skin fibroblasts. Exp. Dermatol. 2009, 18, 596–602. [Google Scholar] [CrossRef]
- Quintanilla-Dieck, M.J.; Codriansky, K.; Rünger, T.M.; Bhawan, J.; Rünger, T.M. Cathepsin K in melanoma invasion. J. Investig. Dermatol. 2008, 128, 2281–2288. [Google Scholar] [CrossRef]
- Petricevic, S.J.; Pavlovic, A.; Durdov, M.G.; Becic, K.; Durdov, M.G. Cathepsin K expression in melanoma is associated with metastases. Histol. Histopathol. 2017, 32, 711–716. [Google Scholar]
- Rao, Q.; Wang, Y.; Xia, Q.Y.; Shi, S.S.; Shen, Q.; Tu, P.; Shi, Q.L.; Zhou, X.J.; Wu, B. Cathepsin K in the immunohistochemical diagnosis of melanocytic lesions. Int. J. Clin. Exp. Pathol. 2014, 7, 1132–1139. [Google Scholar]
- Yao, C.; Yu, K.P.; Philbrick, W.; Sun, B.H.; Simpson, C.; Zhang, C.; Insogna, K. Breast cancer-associated gene 3 interacts with Rac1 and augments NF-κB signaling in vitro, but has no effect on RANKL-induced bone resorption in vivo. Int. J. Mol. Med. 2017, 40, 1067–1077. [Google Scholar] [CrossRef]
- Jensen, A.B.; Wynne, C.; Ramirez, G.; He, W.; Song, Y.; Berd, Y.; Wang, H.; Mehta, A.; Lombardi, A. The cathepsin K inhibitor odanacatib suppresses bone resorption in women with breast cancer and established bone metastases: Results of a 4-week, double-blind, randomized, controlled trial. Clin. Breast Cancer 2010, 10, 452–458. [Google Scholar] [CrossRef]
- Argani, P.; Hicks, J.; De Marzo, A.M.; Albadine, R.; Illei, P.B.; Ladanyi, M.; Reuter, V.E.; Netto, G.J. Xp11 translocation renal cell carcinoma (RCC): Extended immunohistochemical profile emphasizing novel RCC markers. Am. J. Surg. Pathol. 2010, 34, 1295–1303. [Google Scholar] [CrossRef]
- Mehner, C.; Hockla, A.; Miller, E.; Ran, S.; Radisky, D.C.; Radisky, E.S. Tumor cell-produced matrix metalloproteinase 9 (MMP-9) drives malignant progression and metastasis of basal-like triple negative breast cancer. Oncotarget 2014, 5, 2736–2749. [Google Scholar] [CrossRef]
- Huang, H. Matrix Metalloproteinase-9 (MMP-9) as a cancer biomarker and MMP-9 biosensors: Recent advances. Sensors 2018, 8, 3249. [Google Scholar] [CrossRef] [PubMed]
- Guzińska-Ustymowicz, K. MMP-9 and cathepsin B expression in tumor budding as an indicator of a more aggressive phenotype of colorectal cancer (CRC). Anticancer Res. 2006, 26, 1589–1594. [Google Scholar] [PubMed]
- Ahir, B.K.; Elias, N.M.; Lakka, S.S. SPARC overexpression alters microRNA expression profiles involved in tumor progression. Genes Cancer 2017, 8, 453–471. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shao, H.; Kirkwood, J.M.; Wells, A. Tenascin-C signaling in melanoma. Cell Adhes. Migr. 2015, 9, 125–130. [Google Scholar] [CrossRef]
- Yoshida, T.; Akatsuka, T.; Imanaka-Yoshida, K. Tenascin-C and integrins in cancer. Cell Adhes. Migr. 2015, 9, 96–104. [Google Scholar] [CrossRef]
- Spenlé, C.; Saupe, F.; Midwood, K.; Burckel, H.; Noel, G.; Orend, G. Tenascin-C: Exploitation and collateral damage in cancer management. Cell Adhes. Migr. 2015, 9, 141–153. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.; Lee, S.K.; Cho, E.Y.; Cho, S.Y. TFE3-expressing perivascular epithelioid cell tumor of the breast. J. Pathol. Transl. Med. 2019, 53, 62–65. [Google Scholar] [CrossRef]
- Tan, M.; Wu, A.; Liao, N.; Liu, M.; Guo, Q.; Yi, J.; Wang, T.; Huang, Y.; Qiu, B.; Zhou, W. Inhibiting ROS-TFE3-dependent autophagy enhances the therapeutic response to metformin in breast cancer. Free Radic. Res. 2018, 52, 872–886. [Google Scholar] [CrossRef]
- Bühling, F.; Röcken, C.; Brasch, F.; Hartig, R.; Yasuda, Y.; Saftig, P.; Brömme, D.; Welte, T. Pivotal role of cathepsin K in lung fibrosis. Am. J. Pathol. 2004, 164, 2203–2216. [Google Scholar] [CrossRef]
- Dobashi, Y.; Suzuki, S.; Matsubara, H.; Kimura, M.; Endo, S.; Ooi, A. Critical and diverse involvement of Akt/mammalian target of rapamycin signaling in human lung carcinomas. Cancer 2009, 115, 107–118. [Google Scholar] [CrossRef]
- Dobashi, Y.; Suzuki, S.; Kimura, M.; Matsubara, H.; Tsubochi, H.; Imoto, I.; Ooi, A. Paradigm of kinase-driven pathway downstream of epidermal growth factor receptor/Akt in human lung carcinomas. Hum. Pathol. 2011, 42, 214–226. [Google Scholar] [CrossRef]
- Cordes, C.; Bartling, B.; Simm, A.; Afar, D.; Lautenschläger, C.; Hansen, G.; Silber, R.E.; Burdach, S.; Hofmann, H.S. Simultaneous expression of Cathepsins B and K in pulmonary adenocarcinomas and squamous cell carcinomas predicts poor recurrence-free and overall survival. Lung Cancer 2009, 64, 79–85. [Google Scholar] [CrossRef]
- Hiramatsu, M.; Ninomiya, H.; Inamura, K.; Nomura, K.; Takeuchi, K.; Satoh, Y.; Okumura, S.; Nakagawa, K.; Yamori, T.; Matsuura, M.; et al. Activation status of receptor tyrosine kinase downstream pathways in primary lung adenocarcinoma with reference of KRAS and EGFR mutations. Lung Cancer 2010, 70, 94–102. [Google Scholar] [CrossRef]
- Denisenko, T.V.; Budkevich, I.N.; Zhivotovsky, B. Cell death-based treatment of lung adenocarcinoma. Cell Death Dis. 2018, 9, 117. [Google Scholar] [CrossRef]
- Bühling, F.; Gerber, A.; Häckel, C.; Krüger, S.; Köhnlein, T.; Brömme, D.; Reinhold, D.; Ansorge, S.; Welte, T. Expression of cathepsin K in lung epithelial cells. Am. J. Respir. Cell Mol. Biol. 1999, 20, 612–619. [Google Scholar] [CrossRef]
- Duan, Y.; Tan, Z.; Yang, M.; Li, J.; Liu, C.; Wang, C.; Zhang, F.; Jin, Y.; Wang, Y.; Zhu, L. PC-3-Derived Exosomes Inhibit Osteoclast Differentiation by Downregulating miR-214 and Blocking NF-κB Signaling Pathway. Biomed. Res. Int. 2019, 2019, 8650846. [Google Scholar] [CrossRef]
- Raimondi, L.; De Luca, A.; Amodio, N.; Manno, M.; Raccosta, S.; Taverna, S.; Bellavia, D.; Naselli, F.; Fontana, S.; Schillaci, O.; et al. Involvement of multiple myeloma cell-derived exosomes in osteoclast differentiation. Oncotarget 2015, 6, 13772–13789. [Google Scholar] [CrossRef]
- Li, H.; Xiao, Z.; Quarles, L.D.; Li, W. Osteoporosis: Mechanism, molecular target and current status on drug development. Curr. Med. Chem. 2021, 28, 1489–1507. [Google Scholar] [CrossRef]
- Boonen, S.; Rosenberg, E.; Claessens, F.; Vanderschueren, D.; Papapoulos, S. Inhibition of cathepsin K for treatment of osteoporosis. Curr. Osteoporos. Rep. 2012, 10, 73–79. [Google Scholar] [CrossRef]
- Palermo, C.; Joyce, J.A. Cysteine cathepsin proteases as pharmacological targets in cancer. Trends Pharmacol. Sci. 2008, 29, 22–28. [Google Scholar] [CrossRef]
- Wang, B.; Li, J.; Ye, Z.; Wu, X. N-myc downstream regulated gene 1 acts as a tumor suppressor in ovarian cancer. Oncol. Rep. 2014, 31, 2279–2285. [Google Scholar] [CrossRef]
- Falgueyret, J.P.; Oballa, R.M.; Okamoto, O.; Wesolowski, G.; Aubin, Y.; Rydzewski, R.M.; Prasit, P.; Riendeau, D.; Rodan, S.B.; Percival, M.D. Novel, nonpeptidic cyanamides as potent and reversible inhibitors of human cathepsins K and L. J. Med. Chem. 2001, 44, 94–104. [Google Scholar] [CrossRef]
- Grabowskal, U.; Chambers, T.J.; Shiroo, M. Recent developments in cathepsin K inhibitor design. Curr. Opin. Drug Discov. Dev. 2005, 8, 619–630. [Google Scholar]
- Vashum, Y.; Kottaiswamy, A.; Bupesh, G.; Singh, R.P.; Kalaiselvan, P.; Thiagarajan, K.; Samuel, S. Inhibitory Effects of Cathepsin K Inhibitor (ODN-MK-0822) on the Paracrine Pro-Osteoclast Factors of Breast Cancer Cells. Curr. Mol. Pharmacol. 2021, 14, 1134–1145. [Google Scholar] [CrossRef]
- Duong, L.T.; Wesolowski, G.A.; Leung, P.; Oballa, R.; Pickarski, M. Efficacy of a cathepsin K inhibitor in a preclinical model for prevention and treatment of breast cancer bone metastasis. Mol. Cancer Ther. 2014, 13, 2898–2909. [Google Scholar] [CrossRef]
- Liang, W.; Wang, F.; Chen, Q.; Dai, J.; Escara-Wilke, J.; Keller, E.T.; Zimmermann, J.; Hong, N.; Lu, Y.; Zhang, J. Targeting cathepsin K diminishes prostate cancer establishment and growth in murine bone. J. Cancer Res. Clin. Oncol. 2019, 145, 1999–2012. [Google Scholar] [CrossRef] [PubMed]
- Rapa, I.; Volante, M.; Cappia, S.; Rosas, R.; Scagliotti, G.V.; Papotti, M. Cathepsin K is selectively expressed in the stroma of lung adenocarcinoma but not in bronchioloalveolar carcinoma: A useful marker of invasive growth. Am. J. Clin. Pathol. 2006, 125, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Stoch, S.A.; Zajic, S.; Stone, J.A.; Miller, D.L.; van Bortel, L.; Lasseter, K.C.; Pramanik, B.; Cilissen, C.; Liu, Q.; Liu, L.; et al. Odanacatib, a selective cathepsin K inhibitor to treat osteoporosis: Safety, tolerability, pharmacokinetics and pharmacodynamics—Results from single oral dose studies in healthy volunteers. Br. J. Clin. Pharmacol. 2013, 75, 1240–1254. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dare, L.; Vasko-Moser, J.A.; James, I.E.; Blake, S.M.; Rickard, D.J.; Hwang, S.M.; Tomaszek, T.; Yamashita, D.S.; Marquis, R.W.; et al. A highly potent inhibitor of cathepsin K (relacatib) reduces biomarkers of bone resorption both in vitro and in an acute model of elevated bone turnover in vivo in monkeys. Bone 2007, 40, 122–131. [Google Scholar] [CrossRef]
- Pennypacker, B.L.; Duong, L.T.; Cusick, T.E.; Masarachia, P.J.; Gentile, M.A.; Gauthier, J.Y.; Black, W.C.; Scott, B.B.; Samadfam, R.; Smith, S.Y.; et al. Cathepsin K inhibitors prevent bone loss in estrogen-deficient rabbits. J. Bone Miner. Res. 2011, 26, 252–262. [Google Scholar] [CrossRef]
- Jerome, C.; Missbach, M.; Gamse, R. Balicatib, a cathepsin K inhibitor, stimulates periosteal bone formation in monkeys. Osteoporos. Int. 2012, 23, 339–349. [Google Scholar] [CrossRef]
- Cusick, T.; Chen, C.M.; Pennypacker, B.L.; Pickarski, M.; Kimmel, D.B.; Scott, B.B.; Duong, L.T. Odanacatib treatment increases hip bone mass and cortical thickness by preserving endocortical bone formation and stimulating periosteal bone formation in the ovariectomized adult rhesus monkey. J. Bone Miner. Res. 2012, 27, 524–537. [Google Scholar] [CrossRef]
- Ochi, Y.; Yamada, H.; Mori, H.; Kawada, N.; Kayasuga, R.; Nakanishi, Y.; Tanaka, M.; Imagawa, A.; Ohmoto, K.; Kawabata, K. ONO-5334, a cathepsin K inhibitor, improves bone strength by preferentially increasing cortical bone mass in ovariectomized rats. J. Bone Miner. Metab. 2014, 32, 645–652. [Google Scholar] [CrossRef]
- Brixen, K.; Chapurlat, R.; Cheung, A.M.; Keaveny, T.M.; Fuerst, T.; Engelke, K.; Recker, R.; Dardzinski, B.; Verbruggen, N.; Ather, S.; et al. Bone density, turnover, and estimated strength in postmenopausal women treated with odanacatib: A randomized trial. J. Clin. Endocrinol. Metab. 2013, 98, 571–580. [Google Scholar] [CrossRef]
- Yi, C.; Hao, K.Y.; Ma, T.; Lin, Y.; Ge, X.Y.; Zhang, Y. Inhibition of cathepsin K promotes osseointegration of titanium implants in ovariectomised rats. Sci. Rep. 2017, 7, 44682. [Google Scholar] [CrossRef]
- Kassahun, K.; McIntosh, I.; Koeplinger, K.; Sun, L.; Talaty, J.E.; Miller, D.L.; Dixon, R.; Zajic, S.; Stoch, S.A. Disposition and metabolism of the cathepsin K inhibitor odanacatib in humans. Drug Metab. Dispos. 2014, 42, 818–827. [Google Scholar] [CrossRef]
- Anderson, M.S.; Gendrano, I.N.; Liu, C.; Jeffers, S.; Mahon, C.; Mehta, A.; Mostoller, K.; Zajic, S.; Morris, D.; Lee, J.; et al. Odanacatib, a selective cathepsin K inhibitor, demonstrates comparable pharmacodynamics and pharmacokinetics in older men and postmenopausal women. J. Clin. Endocrinol. Metab. 2014, 99, 552–560. [Google Scholar] [CrossRef][Green Version]
- Stone, J.A.; McCrea, J.B.; Witter, R.; Zajic, S.; Stoch, S.A. Clinical and translational pharmacology of the cathepsin K inhibitor odanacatib studied for osteoporosis. Br. J. Clin. Pharmacol. 2019, 85, 1072–1083. [Google Scholar] [CrossRef]
- Bonnick, S.; de Villiers, T.; Odio, A.; Palacios, S.; Chapurlat, R.; DaSilva, C.; Scott, B.B.; Le Bailly De Tilleghem, C.; Leung, A.T.; Gurner, D. Effects of odanacatib on BMD and safety in the treatment of osteoporosis in postmenopausal women previously treated with alendronate: A randomized placebo-controlled trial. J. Clin. Endocrinol. Metab. 2013, 98, 4727–4735. [Google Scholar] [CrossRef]
- Costa, A.G.; Cusano, N.E.; Silva, B.C.; Cremers, S.; Bilezikian, J.P. Cathepsin K: Its skeletal actions and role as a therapeutic target in osteoporosis. Nat. Rev. Rheumatol. 2011, 7, 447–456. [Google Scholar] [CrossRef]
- Dai, R.; Wu, Z.; Chu, H.Y.; Lu, J.; Lyu, A.; Liu, J.; Zhang, G. Cathepsin K: The Action in and Beyond Bone. Front. Cell Dev. Biol. 2020, 8, 433. [Google Scholar] [CrossRef]
- Hua, Y.; Xu, X.; Shi, G.P.; Chicco, A.J.; Ren, J.; Nair, S. Cathepsin K knockout alleviates pressure overload-induced cardiac hypertrophy. Hypertension 2013, 61, 1184–1192. [Google Scholar] [CrossRef]
- Coluzzi, F.; Mandatori, I.; Mattia, C. Emerging therapies in metastatic bone pain. Expert Opin. Emerg. Drugs 2011, 16, 441–458. [Google Scholar] [CrossRef]
- Gauthier, J.Y.; Chauret, N.; Cromlish, W.; Desmarais, S.; Duong, L.T.; Falgueyret, J.P.; Kimmel, D.B.; Lamontagne, S.; Léger, S.; LeRiche, T.; et al. The discovery of odanacatib (MK-0822), a selective inhibitor of cathepsin K. Bioorg. Med. Chem. Lett. 2008, 18, 923–928. [Google Scholar] [CrossRef]
- Leung, P.; Pickarski, M.; Zhuo, Y.; Masarachia, P.J.; Duong, L.T. The effects of the cathepsin K inhibitor odanacatib on osteoclastic bone resorption and vesicular trafficking. Bone 2011, 49, 623–635. [Google Scholar] [CrossRef]
- Isabel, E.; Bateman, K.P.; Chauret, N.; Cromlish, W.; Desmarais, S.; Duong, L.T.; Falgueyret, J.P.; Gauthier, J.Y.; Lamontagne, S.; Lau, C.K.; et al. The discovery of MK-0674, an orally bioavailable cathepsin K inhibitor. Bioorg. Med. Chem. Lett. 2010, 20, 887–892. [Google Scholar] [CrossRef]
- Li, C.S.; Deschenes, D.; Desmarais, S.; Falgueyret, J.P.; Gauthier, J.Y.; Kimmel, D.B.; Léger, S.; Massé, F.; McGrath, M.E.; McKay, D.J.; et al. Identification of a potent and selective non-basic cathepsin K inhibitor. Bioorg. Med. Chem. Lett. 2006, 16, 1985–1989. [Google Scholar] [CrossRef]
- Zhuo, Y.; Gauthier, J.Y.; Black, W.C.; Percival, M.D.; Duong, L.T. Inhibition of bone resorption by the cathepsin K inhibitor odanacatib is fully reversible. Bone 2014, 67, 269–280. [Google Scholar] [CrossRef]
- Desmarais, S.; Massé, F.; Percival, M.D. Pharmacological inhibitors to identify roles of cathepsin K in cell-based studies: A comparison of available tools. Biol. Chem. 2009, 390, 941–948. [Google Scholar] [CrossRef]
- Benýšek, J.; Buša, M.; Rubešová, P.; Fanfrlík, J.; Lepšík, M.; Brynda, J.; Matoušková, Z.; Bartz, U.; Horn, M.; Gütschow, M.; et al. Highly potent inhibitors of cathepsin K with a differently positioned cyanohydrazide warhead: Structural analysis of binding mode to mature and zymogen-like enzymes. J. Enzym. Inhib. Med. Chem. 2022, 37, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yin, G.; Pu, X.; Wang, J.; Liao, X.; Huang, Z. Inhibitory Effects of Combined Bone Morphogenetic Protein 2, Vascular Endothelial Growth Factor, and Basic Fibroblast Growth Factor on Osteoclast Differentiation and Activity. Tissue Eng. Part A 2021, 27, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Asaad, N.; Bethel, P.A.; Coulson, M.D.; Dawson, J.E.; Ford, S.J.; Gerhardt, S.; Grist, M.; Hamlin, G.A.; James, M.J.; Jones, E.V.; et al. Dipeptidyl nitrile inhibitors of Cathepsin L. Bioorg. Med. Chem. Lett. 2009, 19, 4280–4283. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, D.S.; Marquis, R.W.; Xie, R.; Nidamarthy, S.D.; Oh, H.J.; Jeong, J.U.; Erhard, K.F.; Ward, K.W.; Roethke, T.J.; Smith, B.R.; et al. Structure activity relationships of 5-, 6-, and 7-methyl-substituted azepan-3-one cathepsin K inhibitors. J. Med. Chem. 2006, 49, 1597–1612. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Yin, W.; Yang, L.; Xue, L.; Ren, J.; Wei, W.; Lu, Q.; Ding, H.; Liu, Z.; Nabar, N.R.; et al. Inhibition of Ctsk alleviates periodontitis and comorbid rheumatoid arthritis via downregulation of the TLR9 signalling pathway. J. Clin. Periodontol. 2019, 46, 286–296. [Google Scholar] [CrossRef]
- Marquis, R.W.; Ru, Y.; LoCastro, S.M.; Zeng, J.; Yamashita, D.S.; Oh, H.J.; Erhard, K.F.; Davis, L.D.; Tomaszek, T.A.; Tew, D.; et al. Azepanone-based inhibitors of human and rat cathepsin K. J. Med. Chem. 2001, 44, 1380–1395. [Google Scholar] [CrossRef]
- Ochi, Y.; Yamada, H.; Mori, H.; Nakanishi, Y.; Nishikawa, S.; Kayasuga, R.; Kawada, N.; Kunishige, A.; Hashimoto, Y.; Tanaka, M.; et al. Effects of ONO-5334, a novel orally-active inhibitor of cathepsin K, on bone metabolism. Bone 2011, 49, 1351–1356. [Google Scholar] [CrossRef]
- Riva, L.; Yuan, S.; Yin, X.; Martin-Sancho, L.; Matsunaga, N.; Pache, L.; Burgstaller-Muehlbacher, S.; De Jesus, P.D.; Teriete, P.; Hull, M.V.; et al. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature 2020, 586, 113–119. [Google Scholar] [CrossRef]
- Altmann, E.; Renaud, J.; Green, J.; Farley, D.; Cutting, B.; Jahnke, W. Arylaminoethyl amides as novel non-covalent cathepsin K inhibitors. J. Med. Chem. 2002, 45, 2352–2354. [Google Scholar] [CrossRef]
- Wilson, S.R.; Peters, C.; Saftig, P.; Brömme, D. Cathepsin K activity-dependent regulation of osteoclast actin ring formation and bone resorption. J. Biol. Chem. 2009, 284, 2584–2592. [Google Scholar] [CrossRef]
- Palmer, J.T.; Bryant, C.; Wang, D.X.; Davis, D.E.; Setti, E.L.; Rydzewski, R.M.; Venkatraman, S.; Tian, Z.Q.; Burrill, L.C.; Mendonca, R.V.; et al. Design and synthesis of tri-ring P3 benzamide-containing aminonitriles as potent, selective, orally effective inhibitors of cathepsin K. J. Med. Chem. 2005, 48, 7520–7534. [Google Scholar] [CrossRef]
- Catalano, J.G.; Deaton, D.N.; Long, S.T.; McFadyen, R.B.; Miller, L.R.; Payne, J.A.; Wells-Knecht, K.J.; Wright, L.L. Design of small molecule ketoamide-based inhibitors of cathepsin K. Bioorg. Med. Chem. Lett. 2004, 14, 719–722. [Google Scholar] [CrossRef]
- Thompson, S.K.; Halbert, S.M.; Bossard, M.J.; Tomaszek, T.A.; Levy, M.A.; Zhao, B.; Smith, W.W.; Abdel-Meguid, S.S.; Janson, C.A.; D’Alessio, K.J.; et al. Design of potent and selective human cathepsin K inhibitors that span the active site. Proc. Natl. Acad. Sci. USA 1997, 94, 14249–14254. [Google Scholar] [CrossRef]
- Crane, S.N.; Black, W.C.; Palmer, J.T.; Davis, D.E.; Setti, E.; Robichaud, J.; Paquet, J.; Oballa, R.M.; Bayly, C.I.; McKay, D.J.; et al. Beta-substituted cyclohexanecarboxamide: A nonpeptidic framework for the design of potent inhibitors of cathepsin K. J. Med. Chem. 2006, 49, 1066–1079. [Google Scholar] [CrossRef]
- Goričan, T.; Ciber, L.; Petek, N.; Svete, J.; Novinec, M. Synthesis and kinetic characterization of hyperbolic inhibitors of human cathepsins K and S based on a succinimide scaffold. Bioorg. Chem. 2021, 115, 105213. [Google Scholar] [CrossRef]
- Zeng, G.Z.; Pan, X.L.; Tan, N.H.; Xiong, J.; Zhang, Y.M. Natural biflavones as novel inhibitors of cathepsin B and K. Eur. J. Med. Chem. 2006, 41, 1247–1252. [Google Scholar] [CrossRef]
- Qiu, Z.C.; Dong, X.L.; Dai, Y.; Xiao, G.K.; Wang, X.L.; Wong, K.C.; Wong, M.S.; Yao, X.S. Discovery of a New Class of Cathepsin K Inhibitors in Rhizoma Drynariae as Potential Candidates for the Treatment of Osteoporosis. Int. J. Mol. Sci. 2016, 17, 2116. [Google Scholar] [CrossRef]
- Tavares, F.X.; Boncek, V.; Deaton, D.N.; Hassell, A.M.; Long, S.T.; Miller, A.B.; Payne, A.A.; Miller, L.R.; Shewchuk, L.M.; Wells-Knecht, K.; et al. Design of potent, selective, and orally bioavailable inhibitors of cysteine protease cathepsin k. J. Med. Chem. 2004, 47, 588–599. [Google Scholar] [CrossRef]



| Cancer Type | Research Samples | Effects | Mechanism | Refs. |
|---|---|---|---|---|
| Prostate | ||||
| Prostate cancer | LNCaP cells; C4-2B cells; PC3 cells; Patient tissue; Patient serum | CTSK promotes invasion and metastasis of prostate cancer; CTSK promotes cytokine Release; | CTSK mediates bone matrix degradation; CTSK is involved in CCL2- and Cox-2-driven pathways; | [44] |
| Breast | ||||
| Breast cancer | MCF-7 cells; MDA-MB-231 cells; Hs578T cells | CTSK is highly expressed in mammary fibroblasts; CTSK promotes breast cancer cells to metastasize to other sites | CTSK interacts with RANKL/RANK; CTSK degrades ECM and activates MMP-9 to promote breast cancer metastasis; CTSK promoter selectively overexpresses BCA3, which interacts with Rac1 and is associated with NF-κB signaling. | [21,54,127,128] |
| Breast cancer with bone metastasis | MDA-MB-231Hi cells | CTSK is strongly expressed after bone metastases in breast cancer; CTSK degrade extracellular matrix; | Both RANKL and TGF-β can induce the transcription factor NFATc1 to accumulate in the nucleus, and NFATc1 binds to the promoter and directly induces the expression of Src and CTSK; The high expression of IL-20 upregulates CTSK and MMP-9, and promotes bone metastasis of breast cancer | [66] |
| Bone | ||||
| Giant cell tumor of bone | osteoclast-like giant cells; multinucleated giant cells | CTSK is abundantly expressed in the multinucleated giant cells and its activity, which was more than 100-fold higher than activities found in other tissues expressing CTSK | CTSK degrades collagen matrix; CTSK/V-ATPase system is primarily proteolytic factors leading to osteolysis of GCT of bone | [18] |
| Kidney | ||||
| CRC | Xp11TRC cells; Renal cells | CTSK is positive; CTSK can be used as an immunodiagnostic marker | CTSK is a transcriptional target of Mitf and TFE3; TFE3 gene rearrangement and TFEB gene amplification lead to the invasion and migration of renal cell carcinoma, and the expression level of CTSK is higher; t (6;11) (p21; q12) translocation; TSC1/TSC2 mutation promotes renal cell carcinoma development; CTSK inhibitor significantly reduced mTOR phosphorylation at S2448 in Caki cells and inhibited renal cell carcinoma progression. | [76,77,78,119,122] |
| CCRCC | CCRCC cells | CTSK is positive | CTSK and mTOR expression is dysregulated | [81,82] |
| PRCC | PRCC cells | CTSK is positive | CTSK and mTOR expression is dysregulated | [28] |
| CRCC | CRCC cells | CTSK is positive | CTSK and mTOR expression is dysregulated | [28] |
| Sporadic RCC | RCC cells | CTSK is positive | CTSK and mTOR expression is dysregulated | [28,123] |
| Lung | ||||
| NSCLC | SBC-5 cells; A549 cells; Patient tissues; Patient serum; Mice tissues | CTSK maintains airway structural integrity; CTSK strongly expressed; | CTSK binds to TGF-β1 and activates the pathway to promote tumor metastasis; CTSK promotes NSCLC invasion and metastasis by activating mTOR signaling pathway; mTOR/S6K/rS6; EGFR/Akt/mTOR pathway plays a role; | [129,130,131,132,133] |
| ADC | Patient tissues | CTSK is positive | TGF-β1 acts as a potent substrate for CTSK. | [133] |
| ADC | Patient tissues | CTSK is positive | CTSK activates mTOR signaling pathway mTOR/S6K/rS6 axis comes into play; | [134] |
| SqCC | Patient tissues | CTSK is positive | Increased expression of p-mTOR, so it associated with the mTOR pathway | [133,135] |
| LCC | Patient tissues | Not mentioned | Not mentioned | |
| Others | ||||
| Colorectal cancer | MC38 cells; SW480 cells; RKO cells; Mice serum | Serum CTSK levels were significantly elevated in mice with intestinal flora imbalance; CTSK contributes to the aggressive phenotype of CRC cells in vitro and in vivo; CTSK as a prognostic biomarker | APC, TP53, KRAS, SMAD4 and PIK3CA mutations promote the occurrence of colorectal cancer; CTSK can stimulate M2-TAMs to secrete cytokines such as IL-10 and IL-17, and then promote the invasion and metastasis of colorectal cancer cells through the NF-κB pathway; CTSK binds to TLR4 and stimulates M2 polarization in tumor-associated macrophages through mTOR pathway. TLR signaling leads to phosphorylation and activation of microphthalmia transcription factors through p38, acting on the CTSK promoter | [94,95,97,99] |
| Ovarian cancer | OCa cells; Patient serum | CTSK promotes the metastasis of ovarian cancer; The expression of CTSK in peritoneal metastatic ovarian cancer was significantly higher than that in primary ovarian cancer; CTSK is a useful marker for the diagnosis of primary OCs with specific enhancement in combination with CA25 and HE4 | AGAP2-AS1 inhibits cell metastasis and proliferation by inhibiting epithelial-mesenchymal transition by downregulating CTSK; Downregulation of NDRG1 reduces the expression of pro-invasive genes CTSK, MMP-7 and TMPRSS4 | [101,104,105,106] |
| Gastric cancer | AGS cells; Gastric epithelial cells and macrophages; Gastric parietal cells | high expression of CTSK in gastric cancer cells; CTSK promotes the metastasis of gastric cancer; | CTSK promotes gastric cancer metastasis as a downstream factor of cytoskeletal protein Coronin 3; CTSK rapidly degrades ECM; CTSK cleaves and activates MMP-9 to promote gastric cancer cell proliferation and migration; | [108] |
| Melanoma | Melanoma cells | CTSK is significantly expressed in skin and fibroblasts; CTSK is positive in most primary melanomas and all cutaneous melanoma metastases; | Through the secretion of MMP and CTSK through the lymph and blood, the internal collagen is cut off, which promotes the penetration of melanoma cells into the dermis and achieves distant metastasis; CTSK may mediate the degradation of matrix proteins after phagocytosis and promote the invasion and metastasis of melanocytes | [113,114,115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, D.; He, L.; Zhang, Q.; Li, W.; Tang, D.; Wu, C.; Yang, F.; Li, K.; Zhang, H. Cathepsin K: A Versatile Potential Biomarker and Therapeutic Target for Various Cancers. Curr. Oncol. 2022, 29, 5963-5987. https://doi.org/10.3390/curroncol29080471
Qian D, He L, Zhang Q, Li W, Tang D, Wu C, Yang F, Li K, Zhang H. Cathepsin K: A Versatile Potential Biomarker and Therapeutic Target for Various Cancers. Current Oncology. 2022; 29(8):5963-5987. https://doi.org/10.3390/curroncol29080471
Chicago/Turabian StyleQian, Die, Lisha He, Qing Zhang, Wenqing Li, Dandan Tang, Chunjie Wu, Fei Yang, Ke Li, and Hong Zhang. 2022. "Cathepsin K: A Versatile Potential Biomarker and Therapeutic Target for Various Cancers" Current Oncology 29, no. 8: 5963-5987. https://doi.org/10.3390/curroncol29080471
APA StyleQian, D., He, L., Zhang, Q., Li, W., Tang, D., Wu, C., Yang, F., Li, K., & Zhang, H. (2022). Cathepsin K: A Versatile Potential Biomarker and Therapeutic Target for Various Cancers. Current Oncology, 29(8), 5963-5987. https://doi.org/10.3390/curroncol29080471