Identifying Breast Cancer Recurrence in Administrative Data: Algorithm Development and Validation
Abstract
:1. Introduction
- (1)
- Develop a novel algorithm for measuring SBCE rates (recurrences and second primary breast cancers) in a population using routinely collected administrative data;
- (2)
- Validate the algorithm’s diagnostic accuracy using the results of a manual record review in a large sub-cohort of patients.
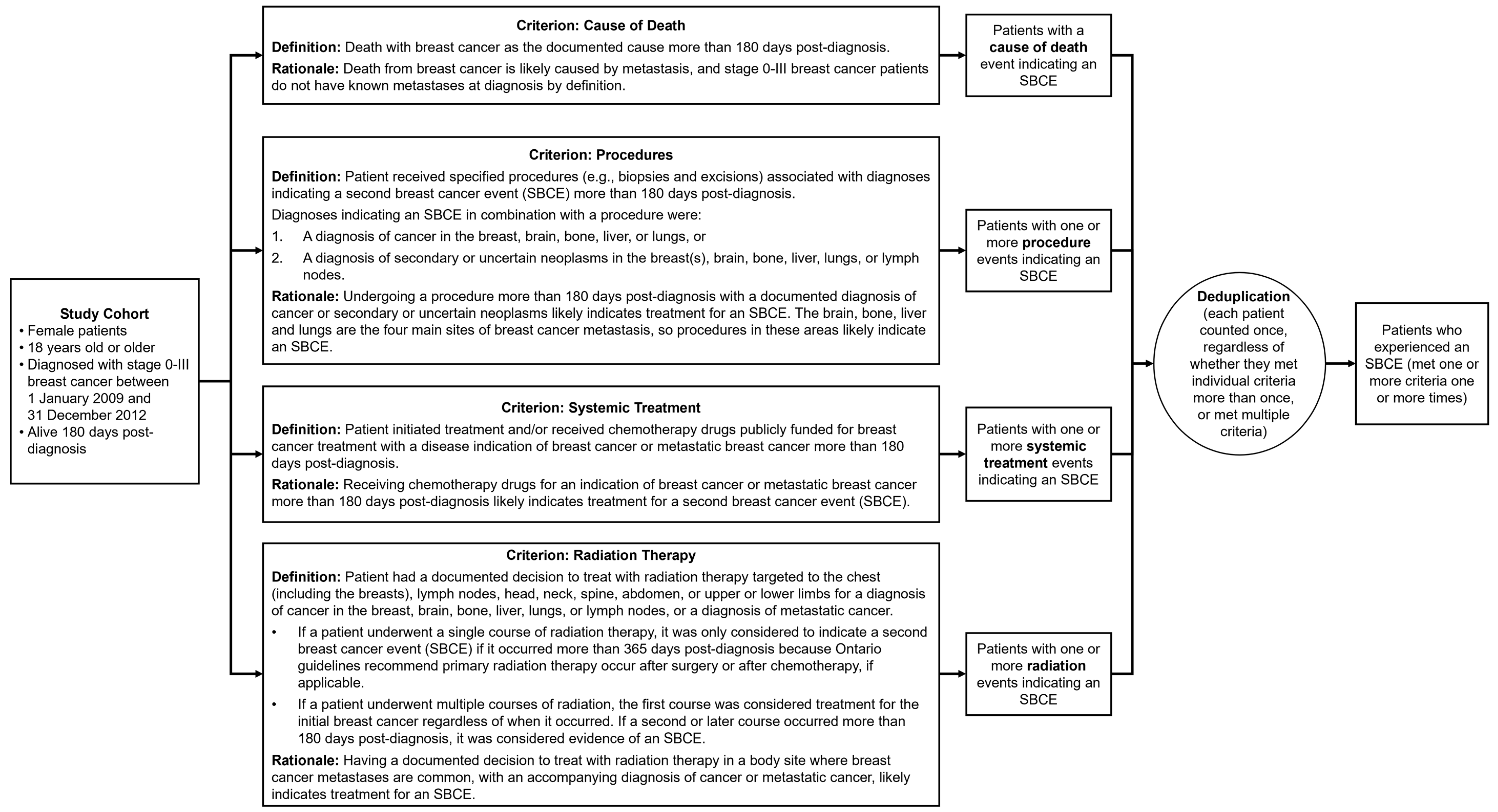
2. Materials and Methods
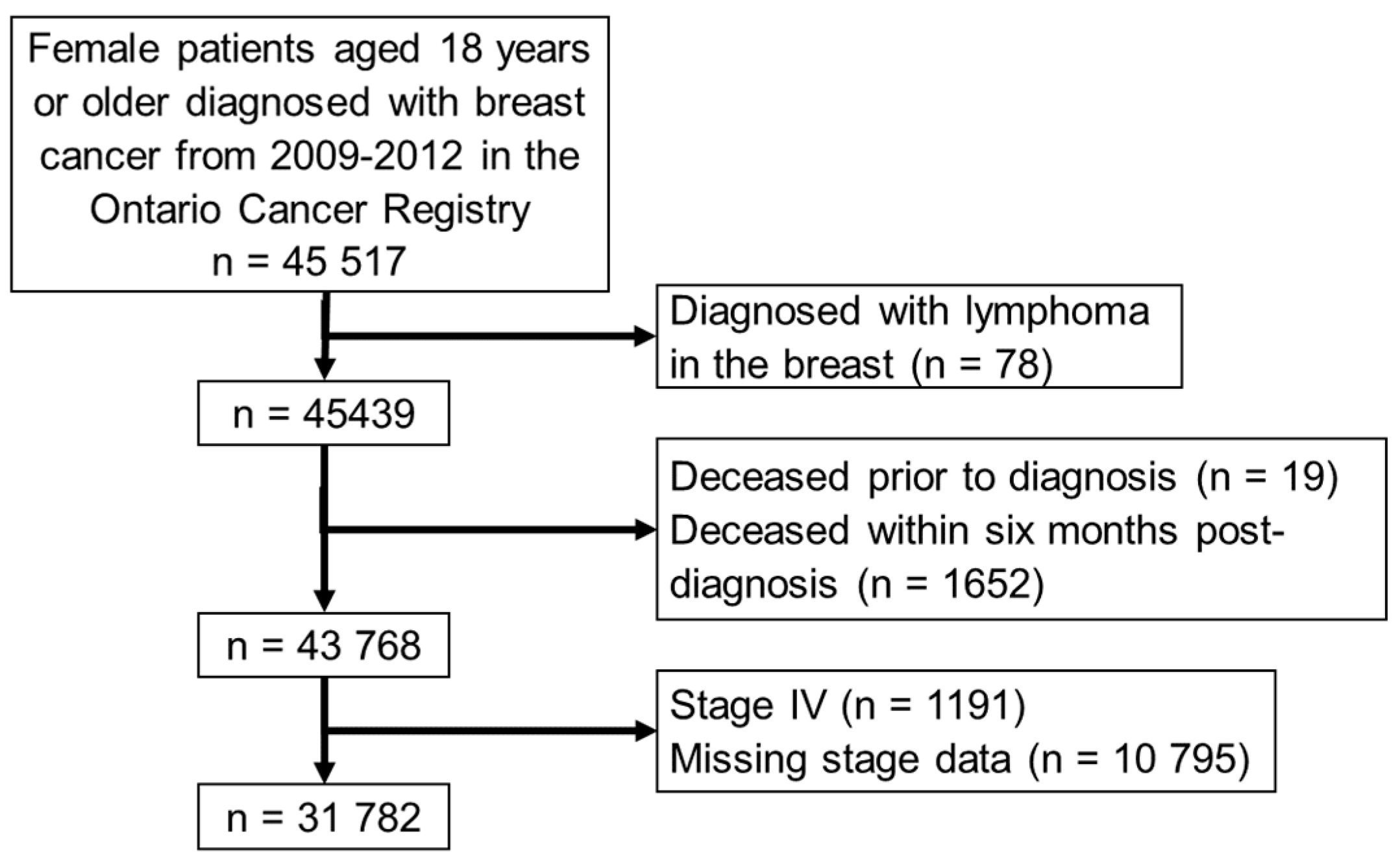
2.1. Patient Selection and Data Sources
2.2. Index Test: Developing the Algorithm
2.3. Manual Record Review
2.4. Statistical Methods
3. Results
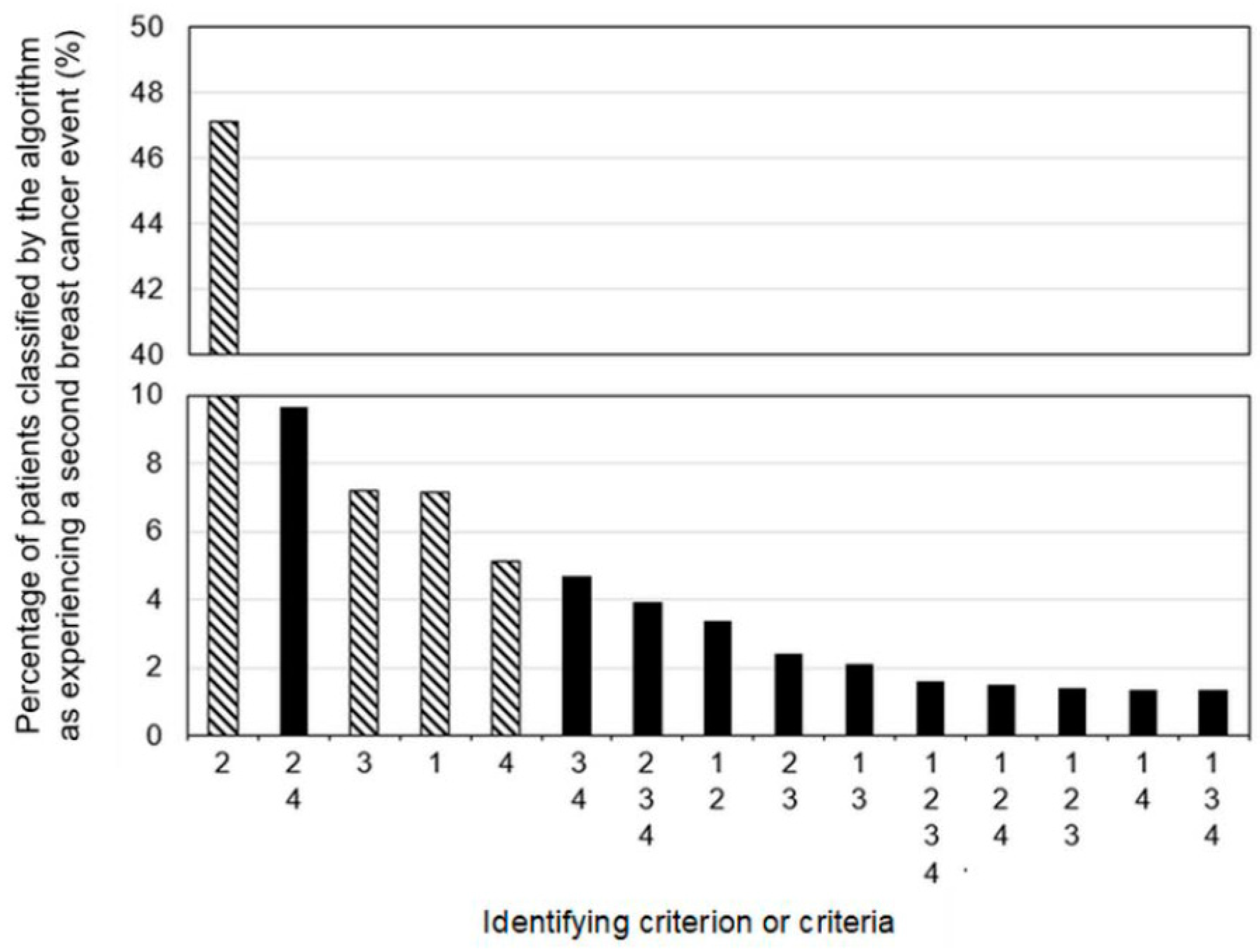
3.1. Cohort Characteristics and Algorithm Classifications
3.2. Exclusions during Manual Review and Validation Sub-Cohort Characteristics
3.3. Algorithm Diagnostic Accuracy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Algorithm Criteria Codes
Appendix A.2. Death from Breast Cancer Criterion
- Data Source(s): Death records from the Ontario Registrar General.
- Coding system: International Classification of Diseases, version 10 (ICD10).
| Code(s) | Code Description |
|---|---|
| C509 | Malignant neoplasm of breast, unspecified |
Appendix A.3. Procedure and Diagnosis Criterion
Appendix A.3.1. Procedures
- Data Source(s): Discharge Abstract Database, National Ambulatory Care Reporting System.
- Coding system: Canadian Classification of Health Interventions, versions 2009, 2012, and 2015.
| Canadian Classification of Health Interventions Code | Canadian Classification of Health Interventions Code Description |
|---|---|
| 1AA80SZXXL | Repair mening brn cranial flap OA xenogr |
| 1AA87SZ | Excision partial, meninges and dura mater of brain using apposition technique [e.g., suture] |
| 1AA87SZXXN | Excis prt mening brn cranial flap OA synth mat |
| 1AC27JX | Radiation, ventricles of brain using focused beam [e.g., gamma knife, cyber knife stereotactic radiosurgery] |
| 1AC52MBSJ | Drainage, ventricles of brain burr hole technique drainage to skin (of head) catheter or shunt (temporarily) left in situ |
| 1AC52SE | Drainage, ventricles of brain burr hole technique drainage without shunt or catheter left in situ |
| 1AF87DAGX | Excision partial, pituitary region endoscopic (via sinus) approach with device NEC |
| 1AJ87SZAZ | Excision partial, cerebellum open [craniotomy flap] approach with ultrasonic aspirator [e.g., CUSA] |
| 1AJ87SZGX | Excision partial, cerebellum open [craniotomy flap] approach with device NEC |
| 1AN27JA | Radiation, brain using external beam [for teletherapy NEC] |
| 1AN27JX | Radiation, brain using focused beam [e.g., gamma knife, cyber knife stereotactic radiosurgery] |
| 1AN53SEFT | Implantation of internal device, brain burr hole technique for access of [semipermeable] catheter [e.g., for chemical palliative infusion] |
| 1AN53SZFT | Implantation of internal device, brain craniotomy [or craniectomy] flap technique for access of [semipermeable] catheter [e.g., for chemical palliative infusion] |
| 1AN87SEAZ | Excision partial, brain burr hole technique for access with ultrasonic aspirator [e.g., CUSA] |
| 1AN87SZAG | Excision partial, brain craniotomy [or craniectomy] flap technique for access with laser |
| 1AN87SZAZ | Excision partial, brain craniotomy [or craniectomy] flap technique for access with ultrasonic aspirator [e.g., CUSA] |
| 1AN87SZGX | Excision partial, brain craniotomy [or craniectomy] flap technique for access with device NEC |
| 1AW27JA | Radiation, spinal cord using external beam [for teletherapy NEC] |
| 1AX35HAM0 | Pharmacotherapy (local), spinal canal and meninges Percutaneous (needle) approach using antineoplastic agent NEC |
| 1AX35HAP1 | Pharmacotherapy (local), spinal canal and meninges percutaneous [needle] approach using anesthetic agent |
| 1AX52MESJ | Drainage, spinal canal and meninges open approach shunt terminating in abdominal cavity [e.g., lumboperitoneal shunt] |
| 1AX87LAGX | Excision partial, spinal canal and meninges using extradural incision technique [e.g., for space occupying lesion of canal] open approach with combined sources of tissue for closure with device NEC |
| 1AX87WKGX | Excision partial, spinal canal and meninges using intradural incision technique [e.g., for meningeal mass] open approach with apposition technique [e.g., suturing] with device NEC |
| 1EA27JA | Radiation, cranium using external beam |
| 1EA87LANW | Excision partial, cranium open approach no tissue used [for closure of wound] using plate, screw device (with or without wire or mesh) |
| 1EA87LANWN | Excise prt cranium OA &plate/scrw synth mater |
| 1EA92LYXXA | Exc rad w reconstruct cranium cranial base oth appr autogr |
| 1EQ27JA | Radiation, soft tissue of head and neck using external beam |
| 1FM87VW | Excision partial, parotid gland using open approach with preservation of facial nerve technique |
| 1GM59BAGX | Destruction, bronchus NEC using endoscopic per orifice approach and device NEC |
| 1GR87DA | Excision partial, lobe of lung using endoscopic approach [VATS] |
| 1GR87QB | Excision partial, lobe of lung using open thoracic approach |
| 1GR89DA | Excision total, lobe of lung using endoscopic approach [VATS] |
| 1GR89QB | Excision total, lobe of lung using open thoracic approach |
| 1GR91QB | Excision radical, lobe of lung open thoracic approach with simple closure |
| 1GR91QBXXN | Excise rad lobe lung thor OA synth mater |
| 1GT27JA | Radiation, lung NEC using external beam |
| 1GT80LA | Repair, lung NEC using open approach |
| 1GT87DA | Excision partial, lung NEC using endoscopic approach [VATS] |
| 1GT87QB | Excision partial, lung NEC using open thoracic approach |
| 1GT89DA | Excise tot lung EA |
| 1GV52DA | Drainage, pleura using endoscopic approach [VATS] |
| 1GV52DATS | Drainage, pleura using endoscopic approach and leaving drainage tube in situ |
| 1GV52HA | Drainage, pleura using percutaneous (needle) approach |
| 1GV52HAHE | Drainage, pleura using percutaneous catheter (intracostal) with underwater seal drainage system |
| 1GV52HATK | Drainage, pleura using percutaneous catheter with suction pump, (under water seal or negative pressure) |
| 1GV52LA | Drainage, pleura using open approach |
| 1GV52LATS | Drainage, pleura using open approach and leaving drainage tube in situ |
| 1GV54JATS | Management of internal device, pleura of drainage tube [e.g., thoracotomy or pleural cavity drain] using external approach |
| 1GV59DAGX | Destruction, pleura using endoscopic approach [VATS] and device NEC |
| 1GV59DAZ9 | Destruction, pleura using endoscopic approach and chemical agent NEC |
| 1GV59HAZ9 | Destruction, pleura using percutaneous instillation of agent NEC (e.g., blood, talc) |
| 1GV87DA | Excision partial, pleura using endoscopic approach [VATS] |
| 1GV89DA | Excision total, pleura using endoscopic approach [VATS] |
| 1GZ31CAND | Ventilation, respiratory system NEC invasive per orifice approach by endotracheal intubation and positive pressure |
| 1GZ31CBND | Ventilation, respiratory system NEC non-invasive approach and positive pressure ventilation (e.g., CPAP, BIPAP) |
| 1GZ32CAMY | Oxygenation, respiratory system NEC using bulk storage manifold system |
| 1HA87LA | Excision partial, pericardium using open approach |
| 1MC87LA | Excision partial, lymph node(s), cervical using open approach with no tissue |
| 1MC87LAXXE | Excise prt lymph nd neck OA loc flp |
| 1MC89LA | Excision total, lymph node(s), cervical using open approach with no tissue |
| 1MC91LA | Excision radical, lymph node(s), cervical without tissue radical neck dissection |
| 1MC91VB | Excision radical, lymph node(s), cervical without tissue modified radical neck dissection |
| 1MD27JA | Radiation, lymph node(s), axillary using external beam |
| 1MD87LA | Excision partial, lymph node(s), axillary using open approach |
| 1MD89LA | Excision total, lymph node(s), axillary using open approach |
| 1MD89LAXXE | Excise tot axil lymph nd OA loc flp |
| 1MD89LAXXG | Excise tot axil lymph nd OA ped flp |
| 1ME87DA | Excision partial, lymph node(s), mediastinal using endoscopic approach |
| 1ME89DA | Excision total, lymph node(s), mediastinal using endoscopic approach |
| 1MF27JA | Radiation, lymph node(s), intrathoracic NEC using external beam |
| 1MF87LA | Excision partial, lymph node(s), intrathoracic NEC using open approach |
| 1MH27JA | Radiation, lymph node(s), pelvic using external beam |
| 1MZ27JA | Radiation, lymphatic system NEC using external beam |
| 1NF90LAXXG | Exc tot w reconstr stom OA w jejnm |
| 1NK87RF | Excision partial, small intestine open approach enteroenterostomy anastomosis technique |
| 1NQ57CJ | Extraction, rectum using per orifice approach and manual technique |
| 1NQ87TF | Excision partial, rectum open abdominal [e.g., anterior] approach colostomy (or ileostomy) with closure of rectal stump [e.g., Hartmann technique] or submucous fistula |
| 1OA27JA | Radiation liver using external beam |
| 1OA59HAAW | Destruction, liver percutaneous approach using radiofrequency |
| 1OA87DA | Excision partial, liver using endoscopic (laparoscopic)approach |
| 1OA87LA | Excision partial, liver using open approach |
| 1OA87LAAZ | Excision partial, liver using ultrasonic aspirator device (for dissection) and open approach |
| 1OE50BANR | Dilate bile dct EPO retro &stent |
| 1OE52GPTS | Drainage, bile ducts using percutaneous transluminal approach [e.g., transhepatic] leaving catheter (tube) in situ |
| 1OE89UF | Excision total, bile ducts using open approach and hepaticojejunostomy technique [for anastomosis] |
| 1OT52HATS | Drain abd cav perc app &tube NOS |
| 1PE52HH | Drainage, renal pelvis using percutaneous approach with insertion of tube (e.g., nephrostomy, pyelostomy) |
| 1PE59BAAG | Destruction, renal pelvis endoscopic per orifice approach Using laser (tissue ablation) |
| 1PM52BATS | Drain bladder EPO &tube NOS |
| 1PM87BA | Excision partial, bladder using endoscopic per orifice approach |
| 1PV52HA | Drainage, surgically created urinary tract using percutaneous needle aspiration |
| 1RD89DA | Excision total, ovary with fallopian tube using endoscopic [laparoscopic] approach |
| 1RD89LA | Excise tot ovary w fallop OA |
| 1RM89AA | Excision total, uterus and surrounding structures using combined laparoscopic and vaginal approach |
| 1SC27JA | Radiation, spinal vertebrae using external beam |
| 1SC74PFNW | Fixation, spinal vertebrae open posterior approach [Includes: posterolateral approach] using screw, screw with plate or rod |
| 1SC75LLKDN | Fuse sp vert ant OA &wire/staple synth mater |
| 1SC75PFGXN | Fuse sp vert post OA &dev NEC synth mater |
| 1SC75PFNWA | Fuse sp vert post OA &plate/scrw autogr |
| 1SC75PFNWN | Fuse sp vert post OA &plate/scrw synth mater |
| 1SC75PFNWQ | Fuse sp vert post OA &plate/scrw combo tis |
| 1SC80HABDN | Repair sp vert perc app w balloon & synth mat |
| 1SC80HAXXN | Repair sp vert perc injct synth mater |
| 1SC80PF | Repair, spinal vertebrae using posterior approach |
| 1SC89LLNWA | Excise tot sp vert ant OA &plate/scrw autogr |
| 1SC89LLNWK | Excise tot sp vert ant OA &plate/scrw homogr |
| 1SC89LLNWN | Excise tot sp vert ant OA &plate/scrw synth mat |
| 1SC89LLNWQ | Excise tot sp vert ant OA &plate/scrw combo tis |
| 1SC89LNNWN | Excis tot sp vert ant w post &plate/scrw syn mat |
| 1SC89PFGX | Excision total, spinal vertebrae posterior approach [posterolateral approach] no tissue used (device only) using device NEC |
| 1SC89PFNWN | Excise tot sp vert post OA &plate/scrw synth mater |
| 1SF74HANW | Fixation, sacrum and coccyx using percutaneous approach and screw, screw with plate |
| 1SH87LAXXE | Excise prt s t back OA loc flp |
| 1SQ27JA | Radiation, pelvis using external beam |
| 1SQ87LAPMN | Excise prt pelvis OA &hip endoprosth synth mat |
| 1SY80LA | Repair m chest & abd OA apposition |
| 1SY87LA | Excision partial, muscles of the chest and abdomen using simple apposition technique [e.g., suture, staple] (for closure of surgical defect) |
| 1SY87LAXXE | Excise prt m chest & abd OA loc flp |
| 1SY87LAXXF | Excise prt m chest & abd non viable free flp |
| 1SZ27JA | Radiation, soft tissue of the chest and abdomen using external beam |
| 1SZ87LA | Excision partial, soft tissue of the chest and abdomen using open approach and apposition [suture, staple] (to close surgical defect) |
| 1SZ87LAXXA | Excise prt s t chest & abd OA autogr |
| 1SZ87LAXXE | Excise prt s t chest & abd OA loc flp |
| 1SZ87LAXXG | Excise prt s t chest & abd OA ped flp |
| 1TK74HALQ | Fixation, humerus percutaneous approach [e.g., with closed or no reduction] fixation device alone using intramedullary nail |
| 1TK74LALQ | Fixation, humerus open approach fixation device alone using intramedullary nail |
| 1TK74LANW | Fixation, humerus open approach fixation device alone using plate, screw |
| 1TK80LAXXN | Repair humerus OA synth mater |
| 1TK87LANWN | Excise prt humerus OA &plate/scrw synth mater |
| 1TV87LA | Excision partial, radius and ulna no tissue used (for closure of defect) using no fixative device |
| 1TZ27JA | Radiation, arm NEC using external beam |
| 1VA74HANV | Fixation, hip joint percutaneous approach [e.g., with closed reduction or no reduction] fixation device alone using pin, nail |
| 1VA74LALQ | Fixation, hip joint open approach fixation device alone using intramedullary nail |
| 1VA74LALQN | Fix hip OA & intramed nail synth mater |
| 1VA74LANV | Fixation, hip joint open approach fixation device alone using pin, nail |
| 1VA74LANW | Fixation, hip joint open approach fixation device alone using plate, screw |
| 1VC74HALQ | Fixation, femur percutaneous approach [e.g., with closed reduction or no reduction] fixation device alone using intramedullary nail |
| 1VC74LALQ | Fixation, femur open approach fixation device alone using intramedullary nail |
| 1VC74LALQN | Fix femur OA &intramed nail synth mater |
| 1VC74LANWQ | Fix femur OA &plate/scrw combo tis |
| 1VC80LAKDQ | Repair femur OA &fix dev NEC combo tis |
| 1VC87LALQ | Excision partial, femur no tissue used (for closure of defect) using intramedullary nail |
| 1VC87LANVN | Excise prt femur OA &pin/nail synth mater |
| 1VC87LANW | Excision partial, femur with synthetic tissue [bone cement, paste] using screw, plate and screw |
| 1VC87LAPMN | Excise prt femur OA &endoprosth synth mat |
| 1VC91LAPNN | Excise rad femur OA &dual comp prosth synth mater |
| 1VD87LAXXA | Excise prt m hip & thigh OA autogr |
| 1VQ74LALQ | Fixation, tibia and fibula open approach fixation device alone using intramedullary nail |
| 1VQ87LANWN | Excise prt tib & fib OA &plate/scrw synth mater |
| 1VZ27JA | Radiation, leg NEC using external beam |
| 1YA87LA | Excision partial, scalp open [excisional] approach Without tissue repair |
| 1YK84LAXXE | Re/construct nipple OA loc flp |
| 1YK84LAXXQ | Re/construct nipple OA combo tis |
| 1YK87LA | Excision partial, nipple using open excisional approach |
| 1YK87LAXXE | Excise prt nipple OA loc flp |
| 1YK89LA | Excision total, nipple using open approach |
| 1YK90LAXXE | Exc tot w reconstr nipple OA loc flp |
| 1YK90LAXXQ | Exc tot w reconstr nipple OA combo tis |
| 1YL87LA | Excision partial, lactiferous duct using open approach |
| 1YL89LA | Excision total, lactiferous duct using open approach |
| 1YM27JA | Radiation, breast using external beam |
| 1YM52HA | Drainage, breast using needle aspiration |
| 1YM52HAAV | Drainage, breast using percutaneous approach with probe |
| 1YM52LA | Drainage, breast using incisional approach |
| 1YM53HAEM | Implantation of internal device, breast of brachytherapy applicator using percutaneous approach |
| 1YM53LAEM | Implantation of internal device, breast of brachytherapy applicator using open approach |
| 1YM54HAG2 | Management of internal device, breast using percutaneous (needle) approach with synthetic agent [e.g., silicone] |
| 1YM54HAW1 | Management of internal device, breast using percutaneous (needle) approach with augmentation agent [e.g., saline, soya] |
| 1YM55LATP | Removal of device, breast without capsulectomy of tissue expander |
| 1YM55WJPM | Removal of device, breast with capsulectomy (with or without inframammary fold repair) of breast implant [prosthesis] |
| 1YM72LA | Release breast OA |
| 1YM74LA | Fixation, breast using open approach |
| 1YM78LAXXE | Repair decr sz breast loc flp |
| 1YM78VQ | Repair by decreasing size, breast using peri areolar round block excisional technique |
| 1YM79LAPM | Repair by increasing size, breast open approach without tissue with implantation of prosthesis |
| 1YM79LATP | Repair by increasing size, breast open approach without tissue with implantation of tissue expander |
| 1YM79LATPG | Augment breast OA w tiss expandr &ped flp |
| 1YM80LA | Repair, breast open approach without tissue with no implantation of device |
| 1YM80LAPM | Repair, breast open approach without tissue with implantation of breast prosthesis |
| 1YM80LAPMA | Repair breast w prosth autogr |
| 1YM80LAPMF | Repair breast OA w prosth free flp |
| 1YM80LAPMG | 2009: Repair, breast using distant pedicled flap (1) with implantation of breast prosthesis 2012: Repair, breast open approach using distant pedicled flap with implantation of breast prosthesis |
| 1YM80LATP | Repair, breast open approach without tissue with implantation of tissue expander |
| 1YM80LATPE | Repair breast w tiss expandr loc flp |
| 1YM80LATPG | 2009: Repair, breast using distant pedicled flap (1) with implantation of tissue expander 2012: Repair, breast open approach using distant pedicled flap with implantation of tissue expander |
| 1YM80LATPK | Repair breast OA w tiss expandr homogr |
| 1YM80LAXXA | 2009: Repair, breast using autograft with no implantation of device 2012: Repair, breast open approach using autograft with no implantation of device |
| 1YM80LAXXE | Repair breast w loc flp |
| 1YM80LAXXF | 2009: Repair, breast using free flap with no implantation of device 2012: Repair, breast open approach using free flap with no implantation of device |
| 1YM80LAXXG | 2009: Repair, breast using distant pedicled flap with no implantation of device 2012: Repair, breast open approach using distant pedicled flap with no implantation of device |
| 1YM87DA | Excision partial, breast using endoscopic approach with simple apposition |
| 1YM87GB | Excision partial, breast using endoscopic guide wire (or needle hook) excision technique with simple apposition of tissue |
| 1YM87LA | Excision partial, breast using open approach with simple apposition of tissue (e.g., suturing) |
| 1YM87LAXXA | Excise prt breast OA autogr |
| 1YM87LAXXE | Excise prt breast OA loc flp |
| 1YM87UT | Excision partial, breast using open guide wire (or needle hook) excision technique and simple apposition of tissue |
| 1YM88LAPM | Excision partial with reconstruction, breast without tissue with implantation of prosthesis |
| 1YM88LAPME | Exc prt breast w prosth loc flp reconst |
| 1YM88LAPMF | Exc prt breast w prosth free flp reconstr |
| 1YM88LAPMG | Exc prt breast w prosth ped flp reconstr |
| 1YM88LAQF | Exc prt breast w prosth/tis expand reconstr |
| 1YM88LAQFE | Exc prt breast w prosth/tis expand loc flp reconst |
| 1YM88LATP | Excision partial with reconstruction, breast without tissue with implantation of tissue expander |
| 1YM88LATPE | Exc prt breast w tiss expandr &loc flp reconst |
| 1YM88LATPF | Exc prt breast w tiss expand free flp reconstr |
| 1YM88LATPG | Exc prt breast w tiss expand ped flp reconstr |
| 1YM88LATPK | Exc prt breast w tiss expand homogr reconstr |
| 1YM88LAXXE | Exc prt breast w loc flp reconstr |
| 1YM88LAXXF | Exc prt breast w free flp reconstr |
| 1YM88LAXXG | Exc prt breast w ped flp reconstr |
| 1YM89LA | Excision total, breast using open approach |
| 1YM89LAXXA | Excise tot breast w autogr |
| 1YM89LAXXE | Excise tot breast OA loc flp |
| 1YM90LAPM | Excision total with reconstruction, breast simple mastectomy with no node dissection without tissue with implantation of breast prosthesis |
| 1YM90LAPME | Exc tot breast prosth loc flp reconstr |
| 1YM90LAPMF | Exc tot breast prosthesis free flp reconstr |
| 1YM90LAPMG | Exc tot breast prosth ped flp reconstr |
| 1YM90LAQF | Exc tot breast prosth w tiss expand reconstr |
| 1YM90LAQFE | Exc tot breast prosth tis expand loc flp reconst |
| 1YM90LAQFG | Exc tot breast prosth tis expand ped flp reconst |
| 1YM90LATP | Excision total with reconstruction, breast simple mastectomy with no node dissection without tissue with implantation of tissue expander |
| 1YM90LATPF | Exc tot breast tiss expand free flp reconstr |
| 1YM90LATPG | Exc tot breast tiss expand ped flp reconstr |
| 1YM90LAXXF | Exc tot breast free flp reconstr |
| 1YM90LAXXG | Exc tot breast ped flp reconstr |
| 1YM90LAXXQ | Exc tot w reconstr breast OA combo tis |
| 1YM91LA | Excision radical, breast without tissue modified or NOS |
| 1YM91LATP | Excision radical, breast with implantation of tissue expander modified or NOS |
| 1YM91LAXXA | 2009: Excision radical (modified), breast using autograft 2012: Excision radical, breast using autograft modified or NOS |
| 1YM91LAXXE | 2009: Excision (modified) radical, breast using local flap 2012: Excision radical, breast using local flap modified or NOS |
| 1YM91TR | Excision radical, breast without tissue extended [Urban] |
| 1YM91TRXXE | 2009: Excision extended radical, breast using local flap 2012: Excision radical, breast using local flap extended [Urban] |
| 1YM92LAPME | Mod rad mastectmy w prosth loc flp reconst |
| 1YM92LAPMF | Mod rad mastectmy w prosth free flp reconst |
| 1YM92LAPMG | Mod rad mastectmy w prosth ped flp reconst |
| 1YM92LAQFE | Mod rad mastectmy w prosth tiss expand loc flp |
| 1YM92LAQFG | Mod rad mastectmy w prosth tiss expand ped flp |
| 1YM92LATPE | Mod rad mastectmy w tiss expandr loc flp reconst |
| 1YM92LATPF | Mod rad mastectmy w tiss expand free flp reconst |
| 1YM92LATPG | Mod rad mastectmy w tiss expand ped flp reconst |
| 1YM92LAXXF | Mod rad mastectmy w free flp reconst |
| 1YM92LAXXG | Mod rad mastectmy w ped flp reconst |
| 1YM92LAXXQ | 2009: Excision radical with reconstruction, breast modified or NOS with no implanted device using combined sources of tissue (e.g., free and pedicled TRAM flap) 2012: Excision radical with reconstruction, breast modified or NOS using combined sources of tissue (e.g., free and pedicled TRAM flap) with no implanted device |
| 1YM92TRPME | Ext rad mastectmy w prosth loc flp reconst |
| 1YM92TRTPE | Ext rad mastectmy wtiss expand loc flp reconst |
| 1YM92TRXXQ | Exc rad w reconstr breast OA w ext rad excisn combo tis |
| 1YR87LA | Excision partial, skin of axillary region open [excisional] approach with apposition technique (e.g., suture, glue) for closure |
| 1YR87LAXXB | Excise prt sk axilla &splt gr |
| 1YS87LA | Excision partial, skin of abdomen and trunk open [excisional] approach with apposition technique (suture, glue) for closure |
| 1YS87LAXXE | Excise prt sk abd & trunk &loc flp |
| 1ZZ35CAM0 | Pharmacotherapy, total body antineoplastic and immunomodulating agents per orifice (oral) approach antineoplastic agent NOS |
| 1ZZ35CAM2 | Pharmacotherapy, total body antineoplastic and immunomodulating agents per orifice (oral) approach antimetabolite |
| 1ZZ35CAM4 | Pharmacotherapy, total body antineoplastic and immunomodulating agents per orifice (oral) approach cytotoxic antibiotic and related substance |
| 1ZZ35CAM5 | Pharmacotherapy, total body antineoplastic and immunomodulating agents per orifice (oral) approach other antineoplastic |
| 1ZZ35HAK7 | Pharm tx NEC perc app ¯olide/lincosamide |
| 1ZZ35HAM0 | Pharmacotherapy, total body antineoplastic and immunomodulating agents percutaneous needle approach [intramuscular, intravenous, subcutaneous, intradermal] antineoplastic agent NOS |
| 1ZZ35HAM3 | Pharmacotherapy, total body antineoplastic and immunomodulating agents percutaneous approach [intramuscular, intravenous, subcutaneous, intradermal] plant alkaloid and other natural product |
| 1ZZ35HAM4 | Pharmacotherapy, total body antineoplastic and immunomodulating agents percutaneous approach [intramuscular, intravenous, subcutaneous, intradermal] cytotoxic antibiotic and related substance |
| 1ZZ35HAM5 | Pharmacotherapy, total body antineoplastic and immunomodulating agents percutaneous approach [intramuscular, intravenous, subcutaneous, intradermal] other antineoplastic |
| 1ZZ35HAM9 | Pharmacotherapy, total body antineoplastic and immunomodulating agents percutaneous approach [intramuscular, intravenous, subcutaneous, intradermal] Combination [multiple] antineoplastic agents |
| 1ZZ35HAN5 | Pharmacotherapy, total body musculoskeletal system agents percutaneous approach [intramuscular, intravenous, subcutaneous, intradermal] drug for treatment of bone disease |
| 2AX13HA | Specimen collection (diagnostic), spinal canal and meninges using percutaneous (needle) approach |
| 2EQ71HA | Biopsy s t head & neck perc ndle app |
| 2FU71HA | Biopsy thyr gl perc ndle app |
| 2GM71BA | Biopsy, bronchus using endoscopic per orifice approach |
| 2GM71BP | Biopsy, bronchus using endoscopic per orifice approach with needle aspiration |
| 2GM71BR | Biopsy, bronchus using endoscopic per orifice approach with brushing/washing |
| 2GT71BA | Biopsy, lung using endoscopic per orifice approach |
| 2GT71BP | Biopsy, lung using endoscopic per orifice approach and needle aspiration |
| 2GT71HA | Biopsy, lung using percutaneous (needle) approach |
| 2GW71DA | Biopsy mediast endo app |
| 2HZ24JAXJ | ECG NOS (ext applic record electrode) |
| 2ME71BP | Biopsy, mediastinal lymph nodes endoscopic per orifice, with needle aspiration |
| 2ME71DA | Biopsy, mediastinal lymph nodes using endoscopic approach |
| 2ME71LA | Biopsy, mediastinal lymph nodes using open approach |
| 2MZ71HA | Biopsy lymph sys perc ndle app |
| 2NF71BA | Biopsy stomach EPO app |
| 2NK70BABL | Inspect sm intest EPO app & gastroscope |
| 2OT71DA | Biopsy, abdominal cavity using endoscopic [laparoscopic] approach |
| 2SZ71HA | Biopsy s t chest & abd perc ndle app |
| 2WY71HA | Biopsy bone marrow perc ndle app |
| 2YK71HA | Biopsy, nipple using percutaneous approach (needle, punch) |
| 2YK71LA | Biopsy, nipple using open [incisional] approach |
| 2YM70LA | Inspection, breast NOS using open approach |
| 2YM71HA | Biopsy, breast NOS using percutaneous (needle) aspiration |
| 2YM71HAGX | Biopsy, breast NOS percutaneous approach using device NEC |
| 2YM71LA | Biopsy, breast NOS incisional biopsy |
| 2ZZ02ZX | Assessment (examination), total body for determining candidacy for treatment |
| 2ZZ13RA | Specimen collect NEC vn puncture |
| 3AN40WE | MRI brain with & without enhancement |
| 3ER20WC | CT head with enhancement |
| 3OG10WZ | Xray b dct w pancr w endo retrograde injct contr |
| 3OT30DA | U/S abd cav alone |
| 3SC40WE | MRI sp vert with & without enhancement |
| 3WZ70CC | Nuclear study msk sys SPECT tomo |
| 3YM30DA | U/S breast u/s only |
| 7SC08PL | Ministrate NEC personal care chronic pain |
Appendix A.3.2. Diagnoses
- Data Source(s): Discharge Abstract Database, National Ambulatory Care Reporting System.
- Coding system: International Classification of Diseases, version 10 (ICD10), 2015.
| International Classification of Diseases (Version 10) Codes | International Classification of Diseases (Version 10) Code Descriptions |
|---|---|
| C50 | Malignant neoplasm of breast |
| C22 | Malignant neoplasm of liver and intrahepatic bile ducts (excluding biliary tract NOS, secondary malignant neoplasm of liver) |
| C34 | Malignant neoplasm of bronchus and lung |
| C41 | Malignant neoplasm of bone and articular cartilage of other and unspecified sites |
| D43 | Neoplasm of uncertain or unknown behaviour of brain and central nervous system (excluding peripheral nerves and autonomic nervous system) |
| C71 | Malignant neoplasm of brain (excluding cranial nerves, retrobulbar tissue) |
| C77 | Secondary and unspecified malignant neoplasm of lymph nodes (excluding malignant neoplasm of lymph nodes, specified as primary) |
| C78 | Secondary malignant neoplasm of respiratory and digestive organs |
| C78.0 | Secondary malignant neoplasm of lung |
| C78.3 | Secondary malignant neoplasm of other and unspecified respiratory organs |
| C78.7 | Secondary malignant neoplasm of liver and intrahepatic bile duct |
| D48 | Neoplasm of uncertain or unknown behaviour of other and unspecified sites (excluding neurofibromatosis (nonmalignant)) |
| D48.0 | Bone and articular cartilage (excluding articular cartilage and cartilage of the ear, larynx, and nose; the connective tissue of the eyelid; and synovia). |
| D48.6 | Breast (including connective tissue of breast, cystosarcoma phyllodes; excluding skin of breast) |
| D37 | Neoplasm of uncertain or unknown behaviour of oral cavity and digestive organs |
| D37.6 | Liver, gallbladder and bile ducts |
| D38 | Neoplasm of uncertain or unknown behaviour of middle ear and respiratory and intrathoracic organs (excluding heart) |
| D38.1 | Trachea, bronchus and lung |
| C79 | Secondary malignant neoplasm of other and unspecified sites |
| C79.3 | Secondary malignant neoplasm of brain and cerebral meninges |
| C79.4 | Secondary malignant neoplasm of other and unspecified parts of nervous system |
| C79.5 | Secondary malignant neoplasm of bone and bone marrow |
Appendix A.4. Systemic Therapy Criterion
- Data Source(s): Activity Level Reporting database.
- Coding system: Not applicable.
| Data Type Analyzed by Algorithm | Description |
|---|---|
| Drug description | PAMIDRONATE |
| CLODRONATE | |
| VINORELBINE | |
| PACLITAXEL | |
| ERIBULIN | |
| PERTUZUMAB | |
| TRASTUZUMAB EMTANSINE |
- Data Source(s): New Drug Funding Program database.
- Coding system: Proprietary to Ontario Health.
| Disease Indication | Policy Name/Drug Name |
|---|---|
| Metastatic or Incurable Locally Advanced—Breast Cancer | Eribulin |
| Unresectable Locally Recurrent or Metastatic—Breast Cancer | Pertuzumab with Trastuzumab |
| Trastuzumab Emtansine | |
| Unresectable Locally Advanced or Metastatic Breast Cancer as Third or Subsequent Line of Treatment (Time-Limited) | Trastuzumab Emtansine |
| Metastatic Breast Cancer | Clodronate (IV) |
| Docetaxel | |
| Nab-Paclitaxel | |
| Paclitaxel | |
| Pamidronate | |
| Trastuzumab in combination with Docetaxel | |
| Trastuzumab in combination with Paclitaxel | |
| Trastuzumab in combination with Vinorelbine | |
| Trastuzumab with First Line Docetaxel | |
| Trastuzumab—Single Agent | |
| Vinorelbine | |
| Second Line—Metastatic Breast Cancer | Trastuzumab |
Appendix A.5. Radiation Treatment Criterion
Appendix A.6. Body Regions Where Radiation Was Applied
- Data Source(s): Activity Level Reporting database.
- Coding system: Proprietary to Ontario Health.
| Body Region Group | Body Region Code | Body Region Code Description |
|---|---|---|
| ABDOMEN | ABDL | Left abdomen |
| ABDOMEN (continued) | ABDO | Whole abdomen |
| ABDR | Right abdomen | |
| ABLB | Lower abdomen | |
| ABLL | Left lower abdomen | |
| ABLR | Right lower abdomen | |
| ABUB | Upper abdomen | |
| ABUL | Left upper abdomen | |
| ABUR | Right upper abdomen | |
| ADRL | Left adrenal | |
| ADRR | Right adrenal | |
| BILE | Bile duct | |
| COLN | Colon | |
| EPIG | Epigastrium | |
| GALL | Gall bladder | |
| INVY | Inverted ‘y’ (dog-leg, hockey-stick) | |
| KIDL | Left kidney | |
| KIDR | Right kidney | |
| LIVR | Liver | |
| PANC | Pancreas | |
| PARA | Para-aortic nodes | |
| SPLE | Spleen | |
| STOM | Stomach | |
| CHEST | AXIL | Left axilla |
| AXIR | Right axilla | |
| BREB | Bilateral breast | |
| BREL | Left breast | |
| BRER | Right breast | |
| CHEB | Bilateral chest lung & area involve | |
| CHEL | Left chest | |
| CHER | Right chest | |
| CHWB | Bilateral chest wall (w/o breast) | |
| CHWL | Left chest wall | |
| CHWR | Right chest wall | |
| CLAB | Bilateral clavicle | |
| CLAL | Left clavicle | |
| CLAR | Right clavicle | |
| ESOI | Lower esophagus | |
| ESOM | Middle esophagus | |
| ESOS | Upper esophagus | |
| ESOW | Entire esophagus | |
| HEML | Left hemimantle | |
| HEMR | Right hemimantle | |
| HERT | Heart | |
| CHEST (continued) | IMCB | Bilateral internal mammary chain |
| LUNB | Bilateral lung | |
| LUNL | Left lung | |
| LUNR | Right lung | |
| MANT | Mantle | |
| MEDI | Mediastinum | |
| PLEL | Left pleura (as in mesothelioma) | |
| PLER | Right pleura | |
| RIBL | Left ribs | |
| RIBR | Right ribs | |
| SCAB | Bilateral scapula | |
| SCAL | Left scapula | |
| SCAR | Right scapula | |
| SCNB | Bilateral supraclavicular nodes | |
| SCNL | Left supraclavicular nodes | |
| SCNR | Right supraclavicular nodes | |
| STER | Sternum | |
| HEAD | ANTB | Bilateral antrum (bull’s eye) |
| ANTL | Left antrum | |
| ANTR | Right antrum | |
| BRAI | Brain | |
| CHKL | Left cheek | |
| CHKR | Right cheek | |
| EARL | Left ear | |
| EARR | Right ear | |
| ETHM | Ethmoid sinus | |
| EYEB | Bilateral eyes | |
| EYEL | Left eye | |
| EYER | Right eye | |
| FACB | Bilateral face | |
| FACL | Left face | |
| FACR | Right face | |
| FLOO | Floor of mouth (boosts) | |
| FOSS | Posterior fossa | |
| GING | Gingiva | |
| HEAD | Head | |
| LACB | Bilateral lacrimal gland | |
| LACL | Left lacrimal gland | |
| LACR | Right lacrimal gland | |
| LIPB | Both lip(s) | |
| HEAD (continued) | LIPI | Lower lip |
| LIPS | Upper lip | |
| MANB | Bilateral mandible | |
| MANL | Left mandible | |
| MANR | Right mandible | |
| MAXB | Bilateral maxilla | |
| MAXL | Left maxilla | |
| MAXR | Right maxilla | |
| NASA | Nasal fossa | |
| NASO | Nasopharynx | |
| ORAL | Oral cavity/buccal mucosa | |
| ORBB | Bilateral orbit | |
| ORBL | Left orbit | |
| ORBR | Right orbit | |
| OROP | Oropharynx | |
| PALH | Hard palate | |
| PALS | Soft palate | |
| PALX | Palate unspecified | |
| PARL | Left parotid | |
| PARR | Right parotid | |
| PITU | Pituitary | |
| SALL | Left salivary gland | |
| SALR | Right salivary gland | |
| SKUL | Skull | |
| SPHE | Sphenoid sinus | |
| SUBM | Submandibular glands | |
| TONG | Tongue | |
| TONS | Tonsil | |
| UVUL | Uvula | |
| LOWER LIMB | ANKB | Bilateral ankle |
| ANKL | Left ankle | |
| ANKR | Right ankle | |
| FEMB | Bilateral femur | |
| FEML | Left femur | |
| FEMR | Right femur | |
| FIBL | Left fibula | |
| FIBR | Right fibula | |
| LOWER LIMB (continued) | FOOB | Bilateral feet |
| FOOL | Left foot | |
| FOOR | Right foot | |
| HEEB | Bilateral heel | |
| HEEL | Left heel | |
| HEER | Right heel | |
| HIPB | Bilateral hip | |
| HIPL | Left hip | |
| HIPR | Right hip | |
| KNEB | Bilateral knee | |
| KNEL | Left knee | |
| KNER | Right knee | |
| LEGB | Bilateral leg | |
| LEGL | Left leg | |
| LEGR | Right leg | |
| LELB | Lower bilateral leg | |
| LELL | Lower left leg | |
| LELR | Lower right leg | |
| LEUB | Upper bilateral leg | |
| LEUL | Upper left leg | |
| LEUR | Upper right leg | |
| TIBL | Left tibia | |
| TIBR | Right tibia | |
| TOEL | Left toes | |
| TOER | Right toes | |
| NECK | HYPO | Hypopharynx |
| LARP | Larygopharynx | |
| LARY | Larynx | |
| NECB | Bilateral neck includes nodes | |
| NECL | Left neck includes nodes | |
| NECR | Right neck includes nodes | |
| PYRI | Pyriform fossa (sinuses) | |
| THYB | Thyroid | |
| TRAC | Trachea | |
| SPINE | COCC | Coccyx |
| SACR | Sacrum | |
| SPCT | Cervical & thoracic spine | |
| SPIC | Cervical spine | |
| SPIL | Lumbar spine | |
| SPIT | Thoracic spine | |
| SPIW | Whole spine | |
| SPLS | Lumbo-sacral spine | |
| SPTL | Thoracic & lumbar spine | |
| UPPER LIMB | ARLL | Lower left arm |
| ARLR | Lower right arm | |
| ARMB | Bilateral arms | |
| ARML | Left arm | |
| ARMR | Right arm | |
| ARUL | Upper left arm | |
| ARUR | Upper right arm | |
| FING | Finger (including thumbs) | |
| HANB | Bilateral hand | |
| HANL | Left hand | |
| HANR | Right hand | |
| HUML | Left humerus | |
| HUMR | Right humerus | |
| RADL | Left radius | |
| RADR | Right radius | |
| SHOB | Bilateral shoulder | |
| SHOL | Left shoulder | |
| SHOR | Right shoulder | |
| ULNL | Left ulna | |
| ULNR | Right ulna |
Appendix A.7. Diagnoses Associated with Radiation
- Data Source(s): Activity Level Reporting database.
- Coding system: International Classification of Diseases, version 10 (ICD10), 2015.
| Codes | Code Description (ICD-10 Version 2015) |
|---|---|
| C50 | Malignant neoplasm of breast |
| C34 | Malignant neoplasm of bronchus and lung |
| C40 | Malignant neoplasm of bone and articular cartilage of limbs |
| C71 | Malignant neoplasm of brain |
| C77 | Secondary and unspecified malignant neoplasm of lymph nodes |
| C78 | Secondary malignant neoplasm of respiratory and digestive organs |
| C79 | Secondary malignant neoplasm of other and unspecified sites |
Appendix B
Exclusions during Manual Record Review and Comparison to Final Validation Sub-Cohort
| Patient Group | Stage at Diagnosis | |||
|---|---|---|---|---|
| Stage 1 N (%) | Stage 2 N (%) | Stage 3 N (%) | Total N | |
| Remaining validation sub-cohort | 701 (31.2%) | 812 (36.2%) | 732 (32.6%) | 2245 |
| Excluded during manual review | 347 (34.3%) | 322 (31.8%) | 344 (34.0%) | 1013 |
| Stage at Diagnosis | Patient Group | Algorithm SBCE Classification | |
|---|---|---|---|
| SBCE N (Row%) | No SBCE N (Row%) | ||
| Stage 1 | Remaining validation sub-cohort | 48 (6.8%) | 653 (93.2%) |
| Excluded during manual review | 27 (7.8%) | 320 (92.2%) | |
| Stage 2 | Remaining validation sub-cohort | 107 (13.2%) | 705 (86.8%) |
| Excluded during manual review | 61 (18.9%) | 261 (81.1%) | |
| Stage 3 | Remaining validation sub-cohort | 216 (29.5%) | 516 (70.5%) |
| Excluded during manual review | 114 (33.1%) | 230 (66.9%) | |
Appendix C
Algorithm Diagnostic Accuracy by Prior Cancer History
| Patients’ Cancer Status Prior to Cohort Entry | N | Agreement Statistic % (95% Confidence Interval) | ||||||
|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | Accuracy | Kappa 1 | Prevalence-Adjusted Bias-Adjusted Kappa 1 | ||
| Remaining validation sub-cohort | 2245 | 85.3 (80.7–89.1) | 93.8 (92.6–94.8) | 67.1 (62.1–71.9) | 97.7 (96.9–98.3) | 92.7 (91.5–93.7) | 70.9 (66.7–75.0) | 85.3 (83.0–87.4) |
| No prior breast cancer (no prior cancer and prior non-breast cancer) | 2182 | 85.9 (81.3–89.8) | 93.7 (92.5–94.8) | 66.5 (61.3–71.4) | 97.9 (97.1–98.5) | 92.7 (91.5–93.8) | 70.8 (66.5–75.0) | 85.4 (83.1–87.5) |
| Any prior cancer (prior breast cancer, non-breast cancer, or both) | 167 | 79.3 (60.3–92.0) | 93.5 (88.0–97.0) | 71.9 (53.3–86.3) | 95.6 (90.6–98.4) | 91.0 (85.6–94.9) | 69.9 (55.7–84.1) | 82.0 (71.2–89.8) |
| No prior cancer | 2078 | 85.9 (81.1–89.9) | 93.8 (92.6–94.8) | 66.7 (61.4–71.7) | 97.9 (97.1–98.5) | 92.8 (91.6–93.9) | 70.9 (66.6–75.3) | 85.6 (83.2–87.7) |
References
- Chubak, J.; Yu, O.; Pocobelli, G.; Lamerato, L.; Webster, J.; Prout, M.N.; Ulcickas Yood, M.; Barlow, W.E.; Buist, D.S.M. Administrative data algorithms to identify second breast cancer events following early-stage invasive breast cancer. JNCI J. Natl. Cancer Inst. 2012, 104, 931–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Kong, S.; Cheung, W.Y.; Bouchard-Fortier, A.; Dort, J.C.; Quan, H.; Buie, E.M.; McKinnon, G.; Quan, M.L. Development and validation of case-finding algorithms for recurrence of breast cancer using routinely collected administrative data. BMC Cancer 2019, 19, 210. [Google Scholar] [CrossRef]
- Ritzwoller, D.P.; Hassett, M.J.; Uno, H.; Cronin, A.M.; Carroll, N.M.; Hornbrook, M.C.; Kushi, L.C. Development, validation, and dissemination of a breast cancer recurrence detection and timing informatics algorithm. JNCI J. Natl. Cancer Inst. 2017, 110, 273–281. [Google Scholar] [CrossRef] [PubMed]
- In, H.; Simon, C.A.; Phillips, J.L.; Posner, M.C.; Ko, C.Y.; Winchester, D.P. The quest for population-level cancer recurrence data; current deficiencies and targets for improvement. J. Surg. Oncol. 2015, 111, 657–662. [Google Scholar] [CrossRef]
- Maishman, T.; Cutress, R.I.; Hernandez, A.; Gerty, S.; Copson, E.R.; Durcan, L.; Eccles, D.M. Local recurrence and breast oncological surgery in young women with breast cancer: The POSH observational cohort study. Ann. Surg. 2017, 266, 165–172. [Google Scholar] [PubMed] [Green Version]
- Pilewskie, M.; Morrow, M. Margins in breast cancer: How much is enough? Cancer 2018, 124, 1335–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pivot, X.; Asmar, L.; Hortobagyi, G.N.; Theriault, R.; Pastorini, F.; Buzdar, A. A retrospective study of first indicators of breast cancer recurrence. Oncology 2000, 58, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Gray, R.; Braybrooke, J.; Davies, C.; Taylor, C.; McGale, P.; Peto, R.; Pritchard, K.I.; Bergh, J.; Dowsett, M.; et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N. Engl. J. Med. 2017, 377, 1836–1846. [Google Scholar] [CrossRef] [Green Version]
- Will, B.P.; Berthelot, J.-M.; Le Petit, C.; Tomiak, E.M.; Verma, S.; Evans, W.K. Estimates of the lifetime costs of breast cancer treatment in Canada. Eur. J. Cancer 2000, 36, 724–735. [Google Scholar] [CrossRef]
- Hawley, S.T.; Janz, N.K.; Griffith, K.A.; Jagsi, R.; Friese, C.R.; Kurian, A.W.; Hamilton, A.S.; Ward, K.C.; Morrow, M.; Wallner, L.P.; et al. Recurrence risk perception and quality of life following treatment of breast cancer. Breast Cancer Res. Treat. 2017, 161, 557–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tewari, A.; Chagpar, A.B. Worry about breast cancer recurrence: A population-based analysis. Am. Surg. 2014, 80, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Geurts, Y.M.; Witteveen, A.; Bretveld, R.; Poortmans, P.M.; Sonke, G.S.; Strobbe, L.J.A.; Siesling, S. Patterns and predictors of first and subsequent recurrence in women with early breast cancer. Breast Cancer Res. Treat. 2017, 165, 709–720. [Google Scholar] [CrossRef]
- Soerjomataram, I.; Louwman, M.W.J.; Ribot, J.G.; Roukema, J.A.; Coebergh, J.W.W. An overview of prognostic factors for long-term survivors of breast cancer. Breast Cancer Res. Treat. 2008, 107, 309–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beatty, J.D.; Sun, Q.; Markowitz, D.; Chubak, J.; Huang, B.; Etzioni, R. Identifying breast cancer recurrence histories via patient-reported outcomes. J. Cancer Surviv. 2022, 16, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Hassett, M.J.; Ritzwoller, D.P.; Taback, N.; Carroll, N.; Cronin, A.M.; Ting, G.V.; Schrag, D.; Warren, J.L.; Hornbrook, M.C.; Weeks, J.C. Validating billing/encounter codes as indicators of lung, colorectal, breast, and prostate cancer recurrence using 2 large contemporary cohorts. Med. Care 2014, 52, e65–e73. [Google Scholar] [CrossRef] [Green Version]
- Whyte, J.L.; Engel-Nitz, N.M.; Teitelbaum, A.; Gomez Rey, G.; Kallich, J.D. An evaluation of algorithms for identifying metastatic breast, lung, or colorectal cancer in administrative claims data. Med. Care 2015, 53, e49–e57. [Google Scholar] [CrossRef] [PubMed]
- Cronin-Fenton, D.; Kjærsgaard, A.; Nørgaard, M.; Amelio, J.; Liede, A.; Hernandez, R.K.; Sørensen, H.T. Breast cancer recurrence, bone metastases, and visceral metastases in women with stage II and III breast cancer in Denmark. Breast Cancer Res. Treat. 2018, 167, 517–528. [Google Scholar] [CrossRef]
- Henriques Abreu, P.; Santos, M.; Henriques Abreu, M.; Aveleira Andrade, B.; Silva, D. Predicting breast cancer recurrence using machine learning techniques: A systematic review. ACM Comput. Surv. 2016, 49, 1–40. [Google Scholar]
- Haque, R.; Shi, J.; Schottinger, J.E.; Ahmed, S.A.; Chung, J.; Avila, C.; Lee, V.S.; Cheetham, T.C.; Habel, L.A.; Fletcher, S.W.; et al. A hybrid approach to identify subsequent breast cancer using pathology and automated health information data. Med. Care 2015, 53, 380–385. [Google Scholar] [PubMed]
- How We Collect Cancer Registry Data. Available online: https://www.cancercareontario.ca/en/data-research/accessing-data/technical-information/cancer-registry-data-collection (accessed on 8 July 2022).
- Apply for OHIP and Get a Health Card. Available online: https://www.ontario.ca/page/apply-ohip-and-get-health-card#section-0 (accessed on 8 July 2022).
- Access Data. Available online: https://www.ccohealth.ca/en/access-data (accessed on 8 July 2022).
- Ontario Cancer Statistics 2016. Available online: https://www.cancercareontario.ca/en/statistical-reports/ontario-cancer-statistics-2016 (accessed on 8 July 2022).
- Agresti, A. An Introduction to Categorical Data Analysis, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Byrt, T.; Bishop, J.; Carlin, J.B. Bias, prevalence and kappa. J. Clin. Epidemiol. 1993, 46, 423–429. [Google Scholar] [CrossRef]
- Sim, J.; Wright, C.C. The Kappa statistic in reliability studies: Use, interpretation, and sample size requirements. Phys. Ther. 2005, 85, 257–268. [Google Scholar] [CrossRef] [Green Version]
- Marrie, R.A.; Fisk, J.D.; Yu, B.N.; Leung, S.; Elliott, L.; Caetano, P.; Warren, S.; Evans, C.; Wolfson, C.; Svenson, L.W.; et al. Mental comorbidity and multiple sclerosis: Validating administrative data to support population-based surveillance. BMC Neurol. 2013, 13, 16. [Google Scholar]
- Fleiss, J.L.; Cohen, J.; Everitt, B.S. Large sample standard errors of kappa and weighted kappa. Psychol. Bull. 1969, 72, 323–327. [Google Scholar]
- Kroenke, C.H.; Chubak, J.; Johnson, L.; Castillo, A.; Weltzien, E.; Caan, B.J. Enhancing breast cancer recurrence algorithms through selective use of medical record data. JNCI J. Natl. Cancer Inst. 2015, 108, djv336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livaudais-Toman, J.; Egorova, N.; Franco, R.; Prasad-Hayes, M.; Howell, E.A.; Wisnivesky, J.; Bickell, N.A. A validation study of administrative claims data to measure ovarian cancer recurrence and secondary debulking surgery. EGEMS 2016, 4, 1208. [Google Scholar] [CrossRef] [Green Version]
| Characteristic | Stage at Diagnosis, N (% of Stage Total) | |||
|---|---|---|---|---|
| Stage 0 N = 1528 | Stage I N = 13,575 | Stage II N = 12,141 | Stage III N = 4538 | |
| Death during follow-up | 6 (0.4%) | 271 (2.0%) | 583 (4.8%) | 490 (10.8%) |
| Median follow-up in months (IQR) | 30.3 (22.4, 40.5) | 35.0 (23.4, 46.4) | 34.0 (22.9, 46.4) | 32.9 (21.5, 45.0) |
| Median age at diagnosis (IQR) | 60.0 (52.0, 68.0) | 63.0 (54.0, 71.0) | 61.0 (50.0, 73.0) | 58.0 (48.0, 71.0) |
| Substage at diagnosis | ||||
| 0 | 1528 (100.0%) | |||
| I | 3552 (26.2%) | |||
| IA | 9508 (70.0%) | |||
| IB | 515 (3.8%) | |||
| II | 277 (2.3%) | |||
| IIA | 7774 (64.0%) | |||
| IIB | 4090 (33.7%) | |||
| III | 275 (6.1%) | |||
| IIIA | 2538 (55.9%) | |||
| IIIB | 785 (17.3%) | |||
| IIIC | 898 (19.8%) | |||
| IIINOS | 42 (0.9%) | |||
| Median tumor size, mm (IQR) | 15.0 (7.0, 25.0) | 12.0 (9.0, 16.0) | 26.0 (22.0, 35.0) | 45.0 (28.0, 65.0) |
| Patients missing tumor size data | 1502 (98.3%) | 4245 (31.3%) | 3783 (31.2%) | 1696 (37.4%) |
| Year of diagnosis | ||||
| 2009 1 | 17 (1.1%) | 2869 (21.1%) | 2776 (22.9%) | 1055 (23.2%) |
| 2010 | 528 (34.6%) | 3620 (26.7%) | 3141 (25.9%) | 1210 (26.7%) |
| 2011 | 506 (33.1%) | 3612 (26.6%) | 3117 (25.7%) | 1153 (25.4%) |
| 2012 | 477 (31.2%) | 3474 (25.6%) | 3107 (25.6%) | 1120 (24.7%) |
| Laterality of original breast cancer diagnosis | ||||
| Right | 715 (46.8%) | 6925 (51.0%) | 6141 (50.6%) | 2292 (50.5%) |
| Left | 818 (53.5%) | 6849 (50.5%) | 6193 (51.0%) | 2309 (50.9%) |
| Tumor morphology | ||||
| Ductal carcinoma | 49 (3.2%) | 8069 (59.4%) | 6657 (54.8%) | 2296 (50.6%) |
| Lobular carcinoma | <6 | 549 (4.0%) | 730 (6.0%) | 313 (6.9%) |
| Mixed carcinoma | 0 | 1030 (7.6%) | 896 (7.4%) | 315 (6.9%) |
| Sarcoma | 0 | <6 | 43 (0.4%) | <6 |
| Other | <6 | 41–45 | 95 (0.8%) | 19–24 |
| Invasive cancer, missing morphology | 1477 (96.7%) | 3881 (28.6%) | 3720 (30.6%) | 1589 (35.0%) |
| Tumor estrogen receptor | ||||
| Borderline or positive | 8 (0.5%) | 8193 (60.4%) | 6438 (53.0%) | 2140 (47.2%) |
| Negative | 8 (0.5%) | 1041 (7.7%) | 1620 (13.3%) | 706 (15.6%) |
| Missing 2 | 1512 (99.0%) | 4341 (32.0%) | 4083 (33.6%) | 1692 (37.3%) |
| Tumor progesterone receptor | ||||
| Borderline or positive | <6 | 7461 (55.0%) | 5795 (47.7%) | 1858 (40.9%) |
| Negative | 9–13 | 1765 (13.0%) | 2258 (18.6%) | 980 (21.6%) |
| Missing 2 | 1514 (99.1%) | 4349 (32.0%) | 4088 (33.7%) | 1700 (37.5%) |
| Tumor human epidermal growth factor receptor 2 (HER2) status | ||||
| Negative or equivocal | <6 | 7266 (53.5%) | 6066 (50.0%) | 1944 (42.8%) |
| Positive | <6 | 727 (5.4%) | 997 (8.2%) | 543 (12.0%) |
| Missing 2 | 1523 (99.7%) | 5582 (41.1%) | 5078 (41.8%) | 2051 (45.2%) |
| Characteristic | Stage at Diagnosis, N (% of Stage Total) | |||
|---|---|---|---|---|
| Stage 0 N = 1528 | Stage I N = 13,575 | Stage II N = 12,141 | Stage III N = 4538 | |
| Algorithm classifications | ||||
| Patients with SBCEs | 62 (4.1%) | 760 (5.6%) | 1635 (13.5%) | 1339 (29.5%) |
| Patients with probable contralateral second primary breast cancers 1 | 24 (1.6%) | 122 (0.9%) | 146 (1.2%) | 86 (1.9%) |
| Algorithm classifications by data type (criterion) | ||||
| Cause of death data | ||||
| Patients with SBCEs | <6 | 65 (0.5%) | 301 (2.5%) | 381 (8.4%) |
| Procedure and associated diagnosis data | ||||
| Patients with SBCEs | 56 (3.7%) | 625 (4.6%) | 1158 (9.5%) | 867 (19.1%) |
| Events | 59 | 654 | 1238 | 961 |
| Contralateral events | 23 | 104 | 99 | 55 |
| Systemic treatment data | ||||
| Patients with SBCEs | 7 (0.5%) | 82 (0.6%) | 356 (2.9%) | 486 (10.7%) |
| Events | 7 | 92 | 402 | 549 |
| Radiation therapy data | ||||
| Patients with SBCEs | 12 (0.8%) | 188 (1.4%) | 492 (4.1%) | 425 (9.4%) |
| Events | 15 | 220 | 615 | 545 |
| Contralateral events | 7 | 50 | 66 | 45 |
| Manual record review location | ||||
| No review | 1528 (100.0%) | 12,874 (94.8%) | 11,329 (93.3%) | 3806 (83.9%) |
| Juravinski Cancer Centre | 0 (0%) | 433 (3.2%) | 474 (3.9%) | 416 (9.2%) |
| Odette Cancer Centre | 0 (0%) | 268 (2.0%) | 338 (2.8%) | 316 (7.0%) |
| Death during follow-up | 6 (0.4%) | 271 (2.0%) | 583 (4.8%) | 490 (10.8%) |
| Median follow-up in months (IQR) | 30.3 (22.4, 40.5) | 35.0 (23.4, 46.4) | 34.0 (22.9, 46.4) | 32.9 (21.5, 45.0) |
| History of primary cancer before cohort entry | ||||
| Prior breast and non-breast cancer | 7 (0.5%) | 66 (0.5%) | 37 (0.3%) | 15 (0.3%) |
| Prior breast cancer only | 84 (5.5%) | 623 (4.6%) | 405 (3.3%) | 115 (2.5%) |
| Prior non-breast cancer only | 76 (5.0%) | 825 (6.1%) | 685 (5.6%) | 227 (5.0%) |
| No prior cancer | 1361 (89.1%) | 12,061 (88.8%) | 11,014 (90.7%) | 4181 (92.1%) |
| Characteristic | Stage, N (%) | |||
|---|---|---|---|---|
| Stage I N = 701 | Stage II N = 812 | Stage III N = 732 | Total N = 2245 | |
| Death during follow-up | 14 (2.0%) | 31 (3.8%) | 73 (10.0%) | 118 (5.3%) |
| Median follow-up in months (IQR) | 34.8 (23.5, 47.5) | 36.1 (23.5, 47.8) | 31.2 (21.3, 44.4) | 34.1 (22.8, 46.6) |
| Median age at diagnosis (IQR) | 59.0 (51.0, 68.0) | 58.0 (49.0, 68.0) | 55.5 (47.0, 66.0) | 57.0 (49.0, 67.0) |
| History of primary cancer before cohort entry | ||||
| Prior breast cancer (alone or with non-breast cancer) | 24 (3.4%) | 25 (3.0%) | 14 (1.9%) | 63 (2.8%) |
| Prior non-breast cancer | 32 (4.6%) | 36 (4.4%) | 36 (4.9%) | 104 (4.6%) |
| No prior cancer | 645 (92.0%) | 751 (92.5%) | 682 (93.2%) | 2078 (92.6%) |
| Year of diagnosis | ||||
| 2009 | 165 (23.5%) | 205 (25.2%) | 167 (22.8%) | 537 (23.9%) |
| 2010 | 169 (24.1%) | 216 (26.6%) | 177 (24.2%) | 562 (25.0%) |
| 2011 | 187 (26.7%) | 191 (23.5%) | 190 (26.0%) | 568 (25.3%) |
| 2012 | 180 (25.7%) | 200 (24.6%) | 198 (27.0%) | 578 (25.7%) |
| Substage at diagnosis | ||||
| I | 201 (28.7%) | 201 (9.0%) | ||
| IA | 472 (67.3%) | 472 (21.0%) | ||
| IB | 28 (4.0%) | 28 (1.2%) | ||
| II | 21 (2.6%) | 21 (0.9%) | ||
| IIA | 490 (60.3%) | 490 (21.8%) | ||
| IIB | 301 (37.1%) | 301 (13.4%) | ||
| III or IIINOS | 34 (4.6%) | 34 (1.5%) | ||
| IIIA | 436 (59.6%) | 436 (19.4%) | ||
| IIIB | 108 (14.8%) | 108 (4.8%) | ||
| IIIC | 154 (21.0%) | 154 (6.9%) | ||
| Median tumor size, mm (IQR) | 13.0 (10.0, 17.0) | 28.0 (22.0, 35.0) | 52.0 (30.0, 70.0) | 25.0 (15.0, 41.0) |
| Patients missing tumor size data | 233 (33.2%) | 282 (34.7%) | 267 (36.5%) | 782 (34.8%) |
| Laterality of original diagnosis | ||||
| Right | 363 (51.8%) | 390 (48.0%) | 360 (49.2%) | 1113 (49.6%) |
| Left | 337 (48.1%) | 427 (52.6%) | 377 (51.5%) | 1141 (50.8%) |
| Tumor morphology | ||||
| Ductal carcinoma | 418 (59.6%) | 446 (54.9%) | 360 (49.2%) | 1224 (54.5%) |
| Lobular carcinoma | 22 (3.1%) | 45 (5.5%) | 62 (8.5%) | 129 (5.7%) |
| Mixed carcinoma | 36–40 | 34–38 | 51 (7.0%) | 127 (5.7%) |
| Sarcoma | 0 | 0 | <6 | <6 |
| Other | <6 | <6 | <6 | 4–8 |
| Invasive cancer, missing morphology | 220 (31.4%) | 282 (34.7%) | 254 (34.7%) | 756 (33.7%) |
| Tumor estrogen receptor | ||||
| Borderline or positive | 403 (57.5%) | 405 (49.9%) | 332 (45.4%) | 1140 (50.8%) |
| Negative | 63 (9.0%) | 121 (14.9%) | 132 (18.0%) | 316 (14.1%) |
| Missing 1 | 235 (33.5%) | 286 (35.2%) | 268 (36.6%) | 789 (35.1%) |
| Tumor progesterone receptor | ||||
| Borderline or positive | 367 (52.4%) | 365 (45.0%) | 286 (39.1%) | 1018 (45.3%) |
| Negative | 99 (14.1%) | 161 (19.8%) | 176 (24.0%) | 436 (19.4%) |
| Missing 1 | 235 (33.5%) | 286 (35.2%) | 270 (36.9%) | 791 (35.2%) |
| Tumor human epidermal growth factor receptor 2 (HER2) status | ||||
| Negative or equivocal | 379 (54.1%) | 407 (50.1%) | 341 (46.6%) | 1127 (50.2%) |
| Positive | 43 (6.1%) | 77 (9.5%) | 86 (11.7%) | 206 (9.2%) |
| Missing 1 | 279 (39.8%) | 328 (40.4%) | 305 (41.7%) | 912 (40.6%) |
| (A) | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Stage, N (%) | ||||||
| Stage I N = 701 | Stage II N = 812 | Stage III N = 732 | Total N = 2245 | ||||
| Manual review classifications 1 | |||||||
| Patients with SBCEs | 27 (3.9%) | 83 (10.2%) | 182 (24.9%) | 292 (13.0%) | |||
| Patients with probable contralateral second primary breast cancers 2 | <6 | 5–10 | 11 (1.5%) | 22 (1.0%) | |||
| Algorithm SBCE classifications | |||||||
| Patients with SBCEs | 48 (6.8%) | 107 (13.2%) | 216 (29.5%) | 371 (16.5%) | |||
| Patients with likely contralateral second primary breast cancers 2 | 7 (1.0%) | 11 (1.4%) | 22 (3.0%) | 40 (1.8%) | |||
| Algorithm classifications by data type (criterion) | |||||||
| Cause of death data | |||||||
| Patients | <6 | 28–32 | 68 (9.3%) | 101 (4.5%) | |||
| Procedure and diagnosis data | |||||||
| Patients with SBCEs | 36 (5.1%) | 71 (8.7%) | 134 (18.3%) | 241 (10.7%) | |||
| Events | 37 | 79 | 159 | 275 | |||
| Contralateral events | 6 | 7 | 13 | 26 | |||
| Systemic treatment data | |||||||
| Patients with SBCEs | 9 (1.3%) | 42 (5.2%) | 88 (12.0%) | 139 (6.2%) | |||
| Events | 9 | 42 | 93 | 144 | |||
| Radiation therapy data | |||||||
| Patients with SBCEs | 20 (2.9%) | 47 (5.8%) | 89 (12.2%) | 156 (6.9%) | |||
| Events | 25 | 61 | 112 | 198 | |||
| Contralateral events | <6 | 5–9 | 13 | 23 | |||
| Manual record review location | |||||||
| Juravinski Cancer Centre | 433 (61.8%) | 474 (58.4%) | 416 (56.8%) | 1323 (58.9%) | |||
| Odette Cancer Centre | 268 (38.2%) | 338 (41.6%) | 316 (43.2%) | 922 (41.1%) | |||
| (B) | |||||||
| Algorithm Classifications (N) | Manual Record Review (N) | Total | |||||
| No SBCE | SBCE 1 | ||||||
| No SBCE | 1831 | 43 | 1874 | ||||
| SBCE | 122 | 249 | 371 | ||||
| Total | 1953 | 292 | 2245 | ||||
| (C) | |||||||
| N | Agreement Statistic % (95% Confidence Interval) | ||||||
| Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | Accuracy | Kappa 3 | Prevalence-Adjusted Bias-Adjusted Kappa 3 | |
| 2245 | 85.3 (80.7–89.1) | 93.8 (92.6–94.8) | 67.1 (62.1–71.9) | 97.7 (96.9–98.3) | 92.7 (91.5–93.7) | 70.9 (66.7–75.0) | 85.3 (83.0–87.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holloway, C.M.B.; Shabestari, O.; Eberg, M.; Forster, K.; Murray, P.; Green, B.; Esensoy, A.V.; Eisen, A.; Sussman, J. Identifying Breast Cancer Recurrence in Administrative Data: Algorithm Development and Validation. Curr. Oncol. 2022, 29, 5338-5367. https://doi.org/10.3390/curroncol29080424
Holloway CMB, Shabestari O, Eberg M, Forster K, Murray P, Green B, Esensoy AV, Eisen A, Sussman J. Identifying Breast Cancer Recurrence in Administrative Data: Algorithm Development and Validation. Current Oncology. 2022; 29(8):5338-5367. https://doi.org/10.3390/curroncol29080424
Chicago/Turabian StyleHolloway, Claire M. B., Omid Shabestari, Maria Eberg, Katharina Forster, Paula Murray, Bo Green, Ali Vahit Esensoy, Andrea Eisen, and Jonathan Sussman. 2022. "Identifying Breast Cancer Recurrence in Administrative Data: Algorithm Development and Validation" Current Oncology 29, no. 8: 5338-5367. https://doi.org/10.3390/curroncol29080424
APA StyleHolloway, C. M. B., Shabestari, O., Eberg, M., Forster, K., Murray, P., Green, B., Esensoy, A. V., Eisen, A., & Sussman, J. (2022). Identifying Breast Cancer Recurrence in Administrative Data: Algorithm Development and Validation. Current Oncology, 29(8), 5338-5367. https://doi.org/10.3390/curroncol29080424





