Abstract
Although the mortality rates of gastric cancer (GC) are gradually declining, gastric cancer is still the fourth leading cause of cancer-related death worldwide. This may be due to the high rate of patients who are diagnosed with GC at advanced stages. However, in countries such as Japan with endoscopic screening systems, more than half of GCs are discovered at an early stage, enabling endoscopic resection (ER). Especially after the introduction of endoscopic submucosal dissection (ESD) in Japan around 2000, a high en bloc resection rate allowing pathological assessment of margin and depth has become possible. While ER is a diagnostic method of treatment and may not always be curative, it is widely accepted as standard treatment because it is less invasive than surgery and can provide an accurate diagnosis for deciding whether additional surgery is necessary. The curability of ER is currently assessed by the completeness of primary tumor removal and the possibility of lymph node metastasis. This review introduces methods, indications, and curability criteria for ER of EGC. Despite recent advances, several problems remain unsolved. This review will also outline the latest evidence concerning future issues.
1. Introduction
Gastric cancer (GC) is the fifth most common cancer and the fourth leading cause of cancer-related death worldwide [1]. Helicobacter pylori (H. pylori) infection is the leading risk factor for GC, and thus GC occurs mainly in Asia and Eastern Europe, where there is a high prevalence of H. pylori infection [1]. Other factors, including tobacco smoking, alcohol, and salted food consumption, are also known to be risk factors for GC but, compared to H. pylori infection, have a much more limited influence on GC prevalence [2,3,4]. In Japan and South Korea, which are two of the leading countries in incidence of GC, following China, GC screening programs on a national level have been implemented with radiographic (X-ray) examination or gastroscopy recommended at annual or biannual intervals [5]. These screening programs have been reported to increase the detection rate of early gastric cancer (EGC) and prevent GC-related mortality [6,7]. This, in turn, enables minimally invasive treatment with endoscopic resection (ER), which is now accepted as first-line management for most EGC. ER includes widely performed methods such as endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), with ESD being the method of treatment most recommended in current guidelines for EGC [8,9]. This review outlines the status and future prospects of endoscopic diagnosis and treatment for GC.
2. Endoscopic Screening and Diagnosis
2.1. Endoscopic Screening for Discovering Early Gastric Cancer
Despite the high prevalence and high cancer-related death rate of GC worldwide, there is no international standard for screening for GC, with the exception of Japan and South Korea. For example, national guidelines in Japan for GC screening recommend annual X-ray screening from the age of 40 years or biennial endoscopic screening from 50 years [5]. Though only an X-ray examination was conducted for screening for GC until 2016, endoscopic screening was added to GC screening due to the evidence of a lower rate of gastric cancer-related death and a higher detection rate of EGC in endoscopic screening in Japan than X-ray examination [10,11,12]. Similarly, Korean national guidelines for GC screening recommend endoscopic screening for subjects aged 40 years or over [13]. Recent reports on these screening programs have focused on the need for quality indicators during endoscopic screening, with previous reports suggesting that sufficient observation time is important for improving the detection of GC [14]. Although there may still be room for improvement in the quality and permeation of GC screening, these screening programs have already been shown to greatly reduce the death rate from GC [10,11,15].
2.2. Indications of Endoscopic Resection for Gastric Cancer
Whether a lesion is indicated for ER is determined by the risk of lymph node metastasis and whether it can be resected en bloc [8]. Initially, the Japanese gastric cancer treatment guidelines, first edition, which was the first guideline to determine the indication for ER of GC, defined the indication for ER as well-differentiated intramucosal cancers of less than 20 mm diameter without ulceration findings (UL0).
Thereafter, studies examining the long-term prognosis of ESD have accumulated, and currently, indications for ER are divided into absolute indications, expanded indications, and relative indications, which are defined as follows [16,17,18,19,20].
Absolute indications are defined as lesions with an estimated risk of lymph node metastasis of less than 1% and with equal long-term outcomes to surgical resection (Table 1) [8,21]. Expanded indications are defined as lesions with an estimated risk of lymph node metastasis of less than 1% but with poor evidence equal to surgical resection in terms of long-term outcomes. Relative indications are defined as lesions other than absolute indications or extended indications and may be indicated based on the possibility of curative endoscopic treatment due to the uncertainty of preoperative endoscopic diagnosis and the patient’s general condition.

Table 1.
Indications of endoscopic resection of gastric cancer *,**.
2.3. Assessment of Horizontal Extent and Depth Diagnosis of Gastric Cancer
Following the detection of GC, the selection of optimal treatment methods is required. Especially in cases of EGC, accurate endoscopic diagnosis of the horizontal extent and depth is essential for deciding indications for ER. While the primary diagnosis of EGC is performed with white light observation, which is available at most health care centers with endoscopy equipment, the efficacy of more detailed endoscopic examination at tertiary referral centers to determine indications for treatment has also been reported [8]. Magnifying endoscopy with narrow-band imaging and chromoendoscopy are two methods that have been reported to improve the accuracy of diagnosis of horizontal extent in EGC, with magnifying endoscopy having higher accuracy rates [22]. The most widely accepted algorithms for the differential diagnosis and assessment of the horizontal extent of EGC are based on the Magnifying Endoscopy Simple Diagnostic Algorithm for Gastric cancer (MESDA-G) [23]. In the MESDA-G algorithm, evaluation of vessels and surface patterns with magnifying endoscopy with NBI is performed. The existence of a demarcation line (DL), which is an abrupt change in these patterns, leads to a diagnosis of EGC, with accuracy rates of up to 97% [24]. However, a clear DL cannot always be distinguished in undifferentiated type cancer because the type infiltrates beneath the surface epithelium [25]. Therefore, biopsy samples from surrounding areas adjacent to the endoscopically detectable cancerous region are recommended in lesions of undifferentiated intramucosal cancers in order to accurately assess the horizontal extent of these lesions [22,25].
Depth diagnosis of EGC, especially differentiation of submucosal invasion of more than 500 µm (T1b2), is also essential in the decision-making process of whether to perform ER for EGC. Macroscopic endoscopic findings such as tumor size > 30 mm, remarkable redness, uneven surface, submucosal tumorlike margin elevation, and mucosal fold convergence are reported to be useful in the depth diagnosis of EGC. The accuracy of depth diagnosis using those findings is reported to be 82.5–96.9% [26,27,28]. While these findings are subjective and may be influenced by the endoscopists’ experience, similar accuracy rates have been reported in cases diagnosed by experts in various high-volume centers, demonstrating the clinical efficacy of this method.
Another method for the depth diagnosis of GC is endoscopic ultrasonography (EUS); however, at present, the efficacy of EUS is controversial [29]. In a retrospective study to compare the accuracy of EUS and macroscopic findings in conventional endoscopy for depth diagnosis of GC, conventional endoscopy was more useful for depth diagnosis than EUS; 73.7% vs. 67.4%, p < 0.001 [30]. Moreover, ulcer findings have been shown to reduce the diagnostic accuracy of EUS [31]. In addition, although EUS enables an objective assessment of the invasion depth of GC, this advantage is counterbalanced by the disadvantages of additional time and costs of performing EUS. Thus, because of the limited accuracy and additional burden of performing EUS, it is recommended that depth diagnosis be made by conventional endoscopy and EUS be used as a secondary method only for selective cases [8,21].
2.4. History of Endoscopic Resection for Gastric Cancer
The history of endoscopic resection for GC dates back to 1974 when Oguro et al. reported the first polypectomy treatment for EGC [32]. The introduction of polypectomy enabled ER of gastric lesions, which previously could only be resected through radical surgery, and was a turning point in the management of EGC. However, while polypectomy enabled ER of gastric polypoid lesions, technical difficulties for ER of flat lesions remained. This problem was greatly resolved when Tada et al. reported the first EMR treatment for EGC in 1983 [33]. However, even with the introduction of EMR, the size of lesions that could be resected en bloc was limited. The first ESD to treat EGC was first described in the 1990s [34,35,36], enabling en bloc resection of lesions larger than 2 cm. The en bloc resection rate, histologic complete resection rate, and curative resection rate in ESD have been reported to be significantly higher than EMR, with the local recurrence rate in ESD significantly lower than EMR [37,38]. As a result, the European Society of Gastrointestinal Endoscopy (ESGE) and Japanese guidelines recommend ESD as the first-line treatment of EGC [8,9,21].
2.5. Methods of Endoscopic Resection for Gastric Cancer
ESD (How to Perform)
ESD comprises several steps, as previously reported [34,35,36]. First, marking the border of the lesion is performed, with markings located approximately 5 mm outside the horizontal margin of the lesion. Close inspection of the lesion is required at this time, and the use of a magnifying endoscope is recommended when possible. This is followed by injection of a lifting solution such as sodium hyaluronate solution or saline around the lesion. Using an endoscopic dissection device, a mucosal incision should be performed around the markings as landmarks. Additional injection of a sodium hyaluronate solution or similar solution into the submucosa under the lesion should be performed as necessary to thicken the target submucosa in order to prevent intraoperative perforation. Submucosal tissue dissection beneath the lesion is performed with care to avoid tissue damage to the muscle layer and perform en bloc resection of the lesion. Thereafter, prophylactic hemostasis should be performed for visible vessels remaining in the resection area after ESD. (Figure 1).
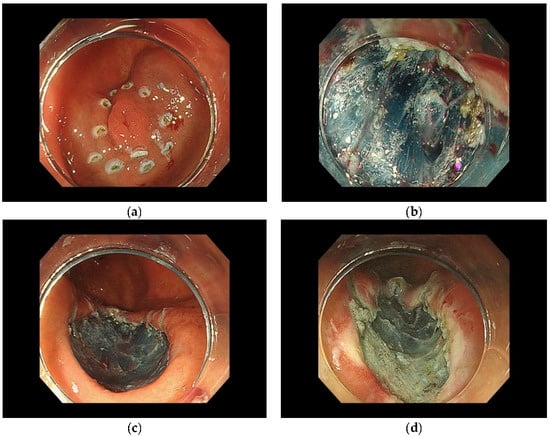
Figure 1.
Method of ESD. (a) Markings located outside the horizontal margin of the lesion. (b) Submucosal tissue dissection beneath the lesion. (c) Endoscopic complete resection. (d) The defect after coagulation of visible vessels remaining in the resection area.
2.6. Curability of Endoscopic Resection for Gastric Cancer
According to current guidelines, endoscopic curability is based on local factors and risk factors for lymph node metastasis. Endoscopic curability (eCura) is divided into eCuraA, B, and C (Table 2).

Table 2.
Curability of endoscopic resection of gastric cancer *,**.
2.6.1. eCuraA
eCuraA is defined as treatment results with equal or superior long-term outcomes compared to additional surgical resection. When the lesion is resected en bloc with tumor-negative margins, the following conditions, (1) UL0 predominantly well-differentiated intramucosal cancers regardless of size without lymphatic or venous invasion (Ly0, V0); (2) UL0 predominantly undifferentiated intramucosal cancers less than 20 mm in diameter (Ly0, V0); or (3) UL1 well-differentiated intramucosal cancers less than 30 mm in diameter (Ly0, V0), are considered eCuraA [8].
2.6.2. eCuraB
eCuraB is defined as treatment results for which sufficient evidence on long-term results has not been obtained, but curability can be expected. Specifically, predominantly differentiated adenocarcinoma with sm1 invasion, size ≤ 30 mm with Ly0, V0, HM0, and VM0 is considered eCuraB [8].
2.6.3. eCuraC
eCuraC indicates treatment results that do not fit into either eCuraA or eCuraB and may require additional treatment. Differentiated type lesions that do not meet the criteria for eCuraA or eCuraB only due to positive lateral margin or non-en bloc resection are classified as eCuraC-1, while others are classified as eCuraC-2. In eCuraC-1, surgery, ESD, diathermy, or follow-up may be considered, depending on the institution’s policy. eCuraC-2 is subject to additional surgical resection [8]. However, there are many patients in which surgery cannot be performed due to the patient’s condition, and a multicenter retrospective study in Japan showed that 905 of 1969 eCuraC patients (46%) were selected for follow-up [39]. Hatta et al. proposed a risk assessment for lymph node metastasis. When the tumor size was >30 mm, VM1, V1, and submucosal invasion of more than 500 µm were defined as 1 point each, and Ly1 was defined as 3 points, lymph node metastasis was observed in 2.5% of patients in the low-risk group (total 1–3 points), 6.7% of patients in the intermedia group (2–4 points), and 22.7% of patients in the high-risk group (5–7 points) [40]. Based on the risk of lymph node metastasis and the patient’s general condition, the patient, surgeon, and gastroenterologist discuss and decide whether to perform additional surgery.
3. Complication of ESD
The main complications of ESD for EGC are postoperative hemorrhage and perforation. A large-scale Japanese multicenter prospective study for 10,821 cases of ESD for EGC reported complication rates of 4.4%, 0.7%, 2.3%, and 0.4% for postoperative hemorrhage, postoperative hemorrhage requiring blood transfusion, intraoperative perforation, and delayed perforation, respectively [41]. While most of these complications can be managed conservatively, preparation of endoscopic devices for the management of complications should they occur and cooperation with surgeons in the case of severe adverse events is necessary [42]. For the introduction of ESD to hospitals with no previous experience, training under the supervision of an expert is recommended to prevent complications [42]. Under expert supervision, it is suggested that there is no significant difference in complication rates between trainees and supervisors, while it is reasonable for a trainee to experience 30 cases before they can perform ESD with sufficient resection speed [43,44,45].
3.1. Postoperative Hemorrhage
Oda et al. reported that all cases of postoperative hemorrhage occurred within 14 days after ESD and that postoperative hemorrhage from lower gastric lesions tended to occur earlier than bleeding from the upper stomach [46]. In a meta-analysis, postoperative hemorrhage was reported to occur at a rate of 5.1% (95% CI, 4.5–5.7%). Risk factors of postoperative hemorrhage include male sex, cardiovascular disease, anticoagulants, cirrhosis, chronic kidney disease, tumor size > 20 mm, resection size > 30 mm, localization in the lesser curvature, flat and depressed type, carcinoma histology, and ulceration [47]. As for the prevention of postoperative hemorrhage, in a prospective randomized controlled trial, a proton pump inhibitor (PPI) was shown to be superior to an H2-receptor antagonist in preventing postoperative hemorrhage. Moreover, the usefulness of coagulation of visible vessels remaining in the resection area after ESD has been reported. This method has been established as post-ESD coagulation (PEC), and Takizawa et al. reported that the postoperative hemorrhage rate was reduced from 7.4% to 3.2% by PEC in their analysis of 1083 lesions [48].
Although second-look endoscopy has been widely used in the past, randomized controlled trials and meta-analyses have shown no significant difference in postoperative hemorrhage rates, and current guidelines for ESD and EMR indicate that second-look endoscopy after ESD is not necessary [8,49,50].
Methods such as mucosal closure with a detachable snare and clips, with an over-the-scope clip, and with endoscopic hand-suturing have been reported as having promise for postoperative hemorrhage prevention [51,52,53].
3.2. Perforation
Procedure time, lesions in the upper stomach, and tumor size are known to be risk factors for perforation [54,55]. In a retrospective study, 115 of 117 patients with perforation during EMR for EGC were successfully treated with endoscopic endoclips [56]. Therefore, when the perforation is identified during the endoscopic procedure, endoscopic closure is first attempted. Of course, even now, emergency surgery may be required after gastric perforation, and although rare, peritoneal dissemination after gastric perforation during ESD for EGC may occur, requiring careful follow-up [57].
4. Future Perspectives
ESD is the first-line treatment for EGC in current guidelines [8,9,21]. However, there are some issues to be resolved, including topics such as screening for GC on a global level, implementation of ESD in Western countries with a low incidence of GC, endoscopic detection and diagnosis of EGC after H. pylori eradication, and optimizing indications of endoscopic treatment for the elderly.
Especially, there is a large difference in the incidence of GC in various regions, and this is one of the main factors prolonging the establishment of a screening program for GC and implementation of ESD in countries with a low incidence of GC. While many countries with a high prevalence of H. pylori infection could greatly benefit from a screening program for GC, issues such as cost, medical insurance, and other social and economic problems must be overcome to implement a screening program on a national or global level. As for the implementation of ESD in countries with a low incidence of GC, while it has been reported that ESD could be safely and usefully performed in Western countries, the establishment of training methods is also an issue [58]. ESGE guidelines recommend performing at least 10 ESD procedures under expert supervision and 25 ESD procedures per year to maintain ESD performance [42]. However, these goals may not be easily reached in countries with a low incidence of GC [59]. These issues are complex and may not be resolved in the near future.
Concerning endoscopic detection and diagnosis of EGC after H. pylori eradication, it is estimated that 10 million people in Japan received H. pylori eradication therapy between 2000 and 2016, and with the declining incidence of GC, the proportion of EGC after H. pylori eradication is increasing [60]. EGC after H. pylori eradication is generally characterized by smaller size, non-cardiac location, and microscopic depressed type [61,62]. However, the possibility that H. pylori eradication may impede early detection of GC has also been suggested, with a significantly higher prevalence of EGC with submucosal invasion after H. pylori eradication compared to the non-eradicated group [63]. For these reasons, regular endoscopy is important after H. pylori eradication, but establishing a useful method for detecting EGC after H. pylori eradication is an issue [64].
With the aging of the population, ESD is increasingly being performed for lesions which are diagnosed as a relative indication in elderly patients who are in poor condition and may not be able to tolerate surgical treatment. As mentioned above, endoscopic curability is assessed by risk factors for lymph node metastasis because lymph node metastasis of GC is extremely important regardless of the clinical stage [8,21,65]. However, the risk of acceptable lymph node metastasis may be different in patients in poor condition because of the high risk of complications from additional treatment and limited prognosis [66,67]. Therefore, the indication in the elderly is currently under investigation in multi-institution prospective trials (JCOG 1902). The main objective of study JCOG1902 is to determine whether follow-up is acceptable after ESD for EGC with a lymph node metastasis risk of 10% or less in patients aged 75 years or older in men and 80 years or older in women. This trial is expected to provide a basis for expanding the range of indications for early gastric cancer in the elderly.
5. Conclusions
This review described the present status and future perspective of endoscopic treatment of GC. It is important to understand the indications and curability for ER of EGC.
Author Contributions
H.H.: Conception and design; drafting of the article, Y.S.: Conception and design; critical revision of the article for important intellectual content, K.O.: Review and Editing, S.M.: Review and Editing, H.N.: Review and Editing, J.S.: Review and Editing, D.K.: Review and Editing, M.O.: Review and Editing, R.C.: Review and Editing, S.N.: Review and Editing, Y.M.: Review and Editing, H.M.: Review and Editing, D.O.: Review and Editing, S.Y.: Review and Editing, Y.T. (Yu Takahashi): Review and Editing, N.K.: Review and Editing, Y.T. (Yosuke Tsuji): Review and Editing, N.Y.: Review and Editing, M.F.: Final approval of the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Sawada, N.; Matsuda, T.; Iwasaki, M.; Sasazuki, S.; Shimazu, T.; Shibuya, K.; Tsugane, S. Attributable causes of cancer in Japan in 2005—Systematic assessment to estimate current burden of cancer attributable to known preventable risk factors in Japan. Ann. Oncol. 2012, 23, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2020, 150, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Takachi, R.; Inoue, M.; Shimazu, T.; Sasazuki, S.; Ishihara, J.; Sawada, N.; Yamaji, T.; Iwasaki, M.; Iso, H.; Tsubono, Y.; et al. Consumption of sodium and salted foods in relation to cancer and cardiovascular disease: The Japan Public Health Center-based Prospective Study. Am. J. Clin. Nutr. 2010, 91, 456–464. [Google Scholar] [CrossRef] [Green Version]
- Hamashima, C. Update version of the Japanese Guidelines for Gastric Cancer Screening. Jpn J. Clin. Oncol. 2018, 48, 673–683. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, S.; Ishikawa, S.; Yoshida, Y. Reduction of gastric cancer mortality by endoscopic and radiographic screening in an isolated island: A retrospective cohort study. Aust. J. Rural Health 2013, 21, 319–324. [Google Scholar] [CrossRef]
- Hamashima, C.; Ogoshi, K.; Okamoto, M.; Shabana, M.; Kishimoto, T.; Fukao, A. A community-based, case-control study evaluating mortality reduction from gastric cancer by endoscopic screening in Japan. PLoS ONE 2013, 8, e79088. [Google Scholar] [CrossRef] [Green Version]
- Ono, H.; Yao, K.; Fujishiro, M.; Oda, I.; Uedo, N.; Nimura, S.; Yahagi, N.; Iishi, H.; Oka, M.; Ajioka, Y.; et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2021, 33, 4–20. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Dinis-Ribeiro, M.; Ponchon, T.; Repici, A.; Vieth, M.; De Ceglie, A.; Amato, A.; Berr, F.; Bhandari, P.; Bialek, A.; et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015, 47, 829–854. [Google Scholar] [CrossRef] [Green Version]
- Hosokawa, O.; Miyanaga, T.; Kaizaki, Y.; Hattori, M.; Dohden, K.; Ohta, K.; Itou, Y.; Aoyagi, H. Decreased death from gastric cancer by endoscopic screening: Association with a population-based cancer registry. Scand. J. Gastroenterol. 2008, 43, 1112–1115. [Google Scholar] [CrossRef]
- Matsumoto, S.; Yamasaki, K.; Tsuji, K.; Shirahama, S. Results of mass endoscopic examination for gastric cancer in Kamigoto Hospital, Nagasaki Prefecture. World J. Gastroenterol. 2007, 13, 4316–4320. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.S.; Jun, J.K.; Park, E.C.; Park, S.; Jung, K.W.; Han, M.A.; Choi, I.J.; Lee, H.Y. Performance of different gastric cancer screening methods in Korea: A population-based study. PLoS ONE 2012, 7, e50041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.S.; Oh, D.K.; Han, M.A.; Lee, H.; Jun, J.K.; Choi, K.S.; Park, E.U. The Korean guideline for gastric cancer screening. J. Korean Med. Assoc. 2015, 58, 373–384. [Google Scholar]
- Kawamura, T.; Wada, H.; Sakiyama, N.; Ueda, Y.; Shirakawa, A.; Okada, Y.; Sanada, K.; Nakase, K.; Mandai, K.; Suzuki, A.; et al. Examination time as a quality indicator of screening upper gastrointestinal endoscopy for asymptomatic examinees. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2017, 29, 569–575. [Google Scholar] [CrossRef]
- Ryu, J.E.; Choi, E.; Lee, K.; Jun, J.K.; Suh, M.; Jung, K.W.; Choi, K.S. Trends in the Performance of the Korean National Cancer Screening Program for Gastric Cancer from 2007 to 2016. Cancer Res. Treat. 2021. [Google Scholar] [CrossRef]
- Gotoda, T.; Yanagisawa, A.; Sasako, M.; Ono, H.; Nakanishi, Y.; Shimoda, T.; Kato, Y. Incidence of lymph node metastasis from early gastric cancer: Estimation with a large number of cases at two large centers. Gastric Cancer 2000, 3, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Hirasawa, T.; Gotoda, T.; Miyata, S.; Kato, Y.; Shimoda, T.; Taniguchi, H.; Fujisaki, J.; Sano, T.; Yamaguchi, T. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 2009, 12, 148–152. [Google Scholar] [CrossRef] [Green Version]
- Ono, H.; Yao, K.; Fujishiro, M.; Oda, I.; Nimura, S.; Yahagi, N.; Iishi, H.; Oka, M.; Ajioka, Y.; Ichinose, M.; et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig. Endosc. 2016, 28, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Hasuike, N.; Ono, H.; Boku, N.; Mizusawa, J.; Takizawa, K.; Fukuda, H.; Oda, I.; Doyama, H.; Kaneko, K.; Hori, S.; et al. A non-randomized confirmatory trial of an expanded indication for endoscopic submucosal dissection for intestinal-type gastric cancer (cT1a): The Japan Clinical Oncology Group study (JCOG0607). Gastric Cancer 2018, 21, 114–123. [Google Scholar] [CrossRef] [Green Version]
- Takizawa, K.; Ono, H.; Hasuike, N.; Takashima, A.; Minashi, K.; Boku, N.; Kushima, R.; Katayama, H.; Ogawa, G.; Fukuda, H.; et al. A nonrandomized, single-arm confirmatory trial of expanded endoscopic submucosal dissection indication for undifferentiated early gastric cancer: Japan Clinical Oncology Group study (JCOG1009/1010). Gastric Cancer 2021, 24, 479–491. [Google Scholar] [CrossRef]
- Japamese Gastric Cancer Association; Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021, 24, 1–21. [CrossRef] [PubMed] [Green Version]
- Nagahama, T.; Yao, K.; Maki, S.; Yasaka, M.; Takaki, Y.; Matsui, T.; Tanabe, H.; Iwashita, A.; Ota, A. Usefulness of magnifying endoscopy with narrow-band imaging for determining the horizontal extent of early gastric cancer when there is an unclear margin by chromoendoscopy (with video). Gastrointest. Endosc. 2011, 74, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Muto, M.; Yao, K.; Kaise, M.; Kato, M.; Uedo, N.; Yagi, K.; Tajiri, H. Magnifying endoscopy simple diagnostic algorithm for early gastric cancer (MESDA-G). Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2016, 28, 379–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, K.; Anagnostopoulos, G.K.; Ragunath, K. Magnifying endoscopy for diagnosing and delineating early gastric cancer. Endoscopy 2009, 41, 462–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, K.; Doyama, H.; Gotoda, T.; Ishikawa, H.; Nagahama, T.; Yokoi, C.; Oda, I.; Machida, H.; Uchita, K.; Tabuchi, M. Diagnostic performance and limitations of magnifying narrow-band imaging in screening endoscopy of early gastric cancer: A prospective multicenter feasibility study. Gastric Cancer 2014, 17, 669–679. [Google Scholar] [CrossRef]
- Abe, S.; Oda, I.; Shimazu, T.; Kinjo, T.; Tada, K.; Sakamoto, T.; Kusano, C.; Gotoda, T. Depth-predicting score for differentiated early gastric cancer. Gastric Cancer 2011, 14, 35–40. [Google Scholar] [CrossRef]
- Tsujii, Y.; Kato, M.; Inoue, T.; Yoshii, S.; Nagai, K.; Fujinaga, T.; Maekawa, A.; Hayashi, Y.; Akasaka, T.; Shinzaki, S.; et al. Integrated diagnostic strategy for the invasion depth of early gastric cancer by conventional endoscopy and EUS. Gastrointest. Endosc. 2015, 82, 452–459. [Google Scholar] [CrossRef]
- Nagahama, T.; Yao, K.; Imamura, K.; Kojima, T.; Ohtsu, K.; Chuman, K.; Tanabe, H.; Yamaoka, R.; Iwashita, A. Diagnostic performance of conventional endoscopy in the identification of submucosal invasion by early gastric cancer: The “non-extension sign” as a simple diagnostic marker. Gastric Cancer 2017, 20, 304–313. [Google Scholar] [CrossRef] [Green Version]
- Yanai, H.; Noguchi, T.; Mizumachi, S.; Tokiyama, H.; Nakamura, H.; Tada, M.; Okita, K. A blind comparison of the effectiveness of endoscopic ultrasonography and endoscopy in staging early gastric cancer. Gut 1999, 44, 361–365. [Google Scholar] [CrossRef]
- Choi, J.; Kim, S.G.; Im, J.P.; Kim, J.S.; Jung, H.C.; Song, I.S. Comparison of endoscopic ultrasonography and conventional endoscopy for prediction of depth of tumor invasion in early gastric cancer. Endoscopy 2010, 42, 705–713. [Google Scholar] [CrossRef]
- Pei, Q.; Wang, L.; Pan, J.; Ling, T.; Lv, Y.; Zou, X. Endoscopic ultrasonography for staging depth of invasion in early gastric cancer: A meta-analysis. J. Gastroenterol. Hepatol. 2015, 30, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Oguro, Y.; Hirashima, T.; Tajiri, H.; Yoshida, S.; Yamaguchi, H.; Yoshimori, M.; Itabashi, M.; Hirota, T. Endoscopic treatment of early gastric cancer: Polypectomy and laser treatment. Jpn J. Clin. Oncol. 1984, 14, 271–282. [Google Scholar]
- Tada, M.; Murakami, A.; Karita, M.; Yanai, H.; Okita, K. Endoscopic resection of early gastric cancer. Endoscopy 1993, 25, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, K.; Yoshida, S. Recent advances in endoscopic mucosal resection for early gastric cancer. Gan Kagaku Ryoho 1998, 25, 476–483. [Google Scholar] [PubMed]
- Gotoda, T.; Kondo, H.; Ono, H.; Saito, Y.; Yamaguchi, H.; Saito, D.; Yokota, T. A new endoscopic mucosal resection procedure using an insulation-tipped electrosurgical knife for rectal flat lesions: Report of two cases. Gastrointest. Endosc. 1999, 50, 560–563. [Google Scholar] [CrossRef]
- Ono, H.; Kondo, H.; Gotoda, T.; Shirao, K.; Yamaguchi, H.; Saito, D.; Hosokawa, K.; Shimoda, T.; Yoshida, S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut 2001, 48, 225–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.M.; Cho, E.; Kang, H.Y.; Kim, J.M. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: A systematic review and metaanalysis. Surg. Endosc. 2011, 25, 2666–2677. [Google Scholar] [CrossRef]
- Lian, J.; Chen, S.; Zhang, Y.; Qiu, F. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest. Endosc. 2012, 76, 763–770. [Google Scholar] [CrossRef]
- Hatta, W.; Gotoda, T.; Oyama, T.; Kawata, N.; Takahashi, A.; Yoshifuku, Y.; Hoteya, S.; Nakamura, K.; Hirano, M.; Esaki, M.; et al. Is radical surgery necessary in all patients who do not meet the curative criteria for endoscopic submucosal dissection in early gastric cancer? A multi-center retrospective study in Japan. J. Gastroenterol. 2017, 52, 175–184. [Google Scholar] [CrossRef]
- Hatta, W.; Gotoda, T.; Oyama, T.; Kawata, N.; Takahashi, A.; Yoshifuku, Y.; Hoteya, S.; Nakagawa, M.; Hirano, M.; Esaki, M.; et al. A Scoring System to Stratify Curability after Endoscopic Submucosal Dissection for Early Gastric Cancer: “eCura system”. Am. J. Gastroenterol. 2017, 112, 874–881. [Google Scholar] [CrossRef]
- Suzuki, H.; Takizawa, K.; Hirasawa, T.; Takeuchi, Y.; Ishido, K.; Hoteya, S.; Yano, T.; Tanaka, S.; Endo, M.; Nakagawa, M.; et al. Short-term outcomes of multicenter prospective cohort study of gastric endoscopic resection: ‘Real-world evidence’ in Japan. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2019, 31, 30–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pimentel-Nunes, P.; Pioche, M.; Albéniz, E.; Berr, F.; Deprez, P.; Ebigbo, A.; Dewint, P.; Haji, A.; Panarese, A.; Weusten, B.; et al. Curriculum for endoscopic submucosal dissection training in Europe: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2019, 51, 980–992. [Google Scholar] [CrossRef] [Green Version]
- Kakushima, N.; Fujishiro, M.; Kodashima, S.; Muraki, Y.; Tateishi, A.; Omata, M. A learning curve for endoscopic submucosal dissection of gastric epithelial neoplasms. Endoscopy 2006, 38, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, Y.; Ohata, K.; Sekiguchi, M.; Ito, T.; Chiba, H.; Gunji, T.; Yamamichi, N.; Fujishiro, M.; Matsuhashi, N.; Koike, K. An effective training system for endoscopic submucosal dissection of gastric neoplasm. Endoscopy 2011, 43, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Kakushima, N.; Mori, K.; Igarashi, K.; Kawata, N.; Tanaka, M.; Takizawa, K.; Ito, S.; Imai, K.; Hotta, K.; et al. Learning curve and clinical outcome of gastric endoscopic submucosal dissection performed by trainee operators. Surg. Endosc. 2017, 31, 3614–3622. [Google Scholar] [CrossRef]
- Goto, O.; Fujishiro, M.; Kodashima, S.; Ono, S.; Niimi, K.; Hirano, K.; Yamamichi, N.; Koike, K. A second-look endoscopy after endoscopic submucosal dissection for gastric epithelial neoplasm may be unnecessary: A retrospective analysis of postendoscopic submucosal dissection bleeding. Gastrointest. Endosc. 2010, 71, 241–248. [Google Scholar] [CrossRef]
- Libânio, D.; Costa, M.N.; Pimentel-Nunes, P.; Dinis-Ribeiro, M. Risk factors for bleeding after gastric endoscopic submucosal dissection: A systematic review and meta-analysis. Gastrointest. Endosc. 2016, 84, 572–586. [Google Scholar] [CrossRef] [Green Version]
- Takizawa, K.; Oda, I.; Gotoda, T.; Yokoi, C.; Matsuda, T.; Saito, Y.; Saito, D.; Ono, H. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection--an analysis of risk factors. Endoscopy 2008, 40, 179–183. [Google Scholar] [CrossRef]
- Mochizuki, S.; Uedo, N.; Oda, I.; Kaneko, K.; Yamamoto, Y.; Yamashina, T.; Suzuki, H.; Kodashima, S.; Yano, T.; Yamamichi, N.; et al. Scheduled second-look endoscopy is not recommended after endoscopic submucosal dissection for gastric neoplasms (the SAFE trial): A multicentre prospective randomised controlled non-inferiority trial. Gut 2015, 64, 397–405. [Google Scholar] [CrossRef]
- Nishizawa, T.; Suzuki, H.; Kinoshita, S.; Goto, O.; Kanai, T.; Yahagi, N. Second-look endoscopy after endoscopic submucosal dissection for gastric neoplasms. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2015, 27, 279–284. [Google Scholar] [CrossRef]
- Lee, B.I.; Kim, B.W.; Kim, H.K.; Choi, H.; Ji, J.S.; Hwang, S.M.; Cho, Y.S.; Chae, H.S.; Choi, K.Y. Routine mucosal closure with a detachable snare and clips after endoscopic submucosal dissection for gastric epithelial neoplasms: A randomized controlled trial. Gut Liver 2011, 5, 454–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maekawa, S.; Nomura, R.; Murase, T.; Ann, Y.; Harada, M. Complete closure of artificial gastric ulcer after endoscopic submucosal dissection by combined use of a single over-the-scope clip and through-the-scope clips (with videos). Surg. Endosc. 2015, 29, 500–504. [Google Scholar] [CrossRef] [Green Version]
- Goto, O.; Oyama, T.; Ono, H.; Takahashi, A.; Fujishiro, M.; Saito, Y.; Abe, S.; Kaise, M.; Iwakiri, K.; Yahagi, N. Endoscopic hand-suturing is feasible, safe, and may reduce bleeding risk after gastric endoscopic submucosal dissection: A multicenter pilot study (with video). Gastrointest. Endosc. 2020, 91, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Ishihara, R.; Uedo, N.; Takeuchi, Y.; Nagai, K.; Matsui, F.; Kawada, N.; Yamashina, T.; Kanzaki, H.; Hanafusa, M.; et al. Factors predicting perforation during endoscopic submucosal dissection for gastric cancer. Gastrointest. Endosc. 2012, 75, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Mannen, K.; Tsunada, S.; Hara, M.; Yamaguchi, K.; Sakata, Y.; Fujise, T.; Noda, T.; Shimoda, R.; Sakata, H.; Ogata, S.; et al. Risk factors for complications of endoscopic submucosal dissection in gastric tumors: Analysis of 478 lesions. J. Gastroenterol. 2010, 45, 30–36. [Google Scholar] [CrossRef]
- Minami, S.; Gotoda, T.; Ono, H.; Oda, I.; Hamanaka, H. Complete endoscopic closure of gastric perforation induced by endoscopic resection of early gastric cancer using endoclips can prevent surgery (with video). Gastrointest. Endosc. 2006, 63, 596–601. [Google Scholar] [CrossRef]
- Hirao, M.; Yamada, T.; Michida, T.; Nishikawa, K.; Hamakawa, T.; Mita, E.; Mano, M.; Sekimoto, M. Peritoneal Seeding after Gastric Perforation during Endoscopic Submucosal Dissection for Gastric Cancer. Dig. Surg. 2018, 35, 457–460. [Google Scholar] [CrossRef]
- Fernández-Esparrach, G.; Marín-Gabriel, J.C.; de Tejada, A.H.; Albéniz, E.; Nogales, O.; Del Pozo-García, A.J.; Rosón, P.J.; Goicotxea, U.; Uchima, H.; Terán, A.; et al. Implementation of endoscopic submucosal dissection in a country with a low incidence of gastric cancer: Results from a prospective national registry. United Eur. Gastroenterol. J. 2021, 9, 718–726. [Google Scholar] [CrossRef]
- Ribeiro-Mourão, F.; Pimentel-Nunes, P.; Dinis-Ribeiro, M. Endoscopic submucosal dissection for gastric lesions: Results of an European inquiry. Endoscopy 2010, 42, 814–819. [Google Scholar] [CrossRef]
- Tsuda, M.; Asaka, M.; Kato, M.; Matsushima, R.; Fujimori, K.; Akino, K.; Kikuchi, S.; Lin, Y.; Sakamoto, N. Effect on Helicobacter pylori eradication therapy against gastric cancer in Japan. Helicobacter 2017, 22. [Google Scholar] [CrossRef] [Green Version]
- Kamada, T.; Hata, J.; Sugiu, K.; Kusunoki, H.; Ito, M.; Tanaka, S.; Inoue, K.; Kawamura, Y.; Chayama, K.; Haruma, K. Clinical features of gastric cancer discovered after successful eradication of Helicobacter pylori: Results from a 9-year prospective follow-up study in Japan. Aliment Pharmacol. Ther. 2005, 21, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Kato, M.; Takahashi, M.; Haneda, M.; Shinada, K.; Nishida, U.; Yoshida, T.; Sonoda, N.; Ono, S.; Nakagawa, M.; et al. Clinicopathological analysis of early-stage gastric cancers detected after successful eradication of Helicobacter pylori. Helicobacter 2011, 16, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Ito, M.; Boda, T.; Kotachi, T.; Kiso, M.; Masuda, K.; Kurihara, M.; Kuroki, K.; Yorita, N.; Nagasaki, N.; et al. Gastric Cancer with Submucosal Invasion after Successful Helicobacter pylori Eradication: A Propensity Score-Matched Analysis of Patients with Annual Patient Endoscopic Survey. Digestion 2019, 99, 59–65. [Google Scholar] [CrossRef]
- Ito, M.; Tanaka, S.; Chayama, K. Characteristics and Early Diagnosis of Gastric Cancer Discovered after Helicobacter pylori Eradication. Gut Liver 2021, 15, 338–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelc, Z.; Skórzewska, M.; Rawicz-Pruszyński, K.; Polkowski, W.P. Lymph Node Involvement in Advanced Gastric Cancer in the Era of Multimodal Treatment-Oncological and Surgical Perspective. Cancers 2021, 13, 2509. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, E.S.; Lee, Y.J.; Cho, K.B.; Park, K.S.; Jang, B.K.; Chung, W.J.; Hwang, J.S.; Ryu, S.W. Comparison of quality of life and worry of cancer recurrence between endoscopic and surgical treatment for early gastric cancer. Gastrointest. Endosc. 2015, 82, 299–307. [Google Scholar] [CrossRef]
- Nunobe, S.; Oda, I.; Ishikawa, T.; Akazawa, K.; Katai, H.; Isobe, Y.; Miyashiro, I.; Tsujitani, S.; Ono, H.; Tanabe, S.; et al. Surgical outcomes of elderly patients with Stage I gastric cancer from the nationwide registry of the Japanese Gastric Cancer Association. Gastric Cancer 2020, 23, 328–338. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).