Innovations in the Management of Vaginal Cancer
Abstract
1. Introduction
1.1. Epidemiology and Risk Factors
1.2. Anatomy
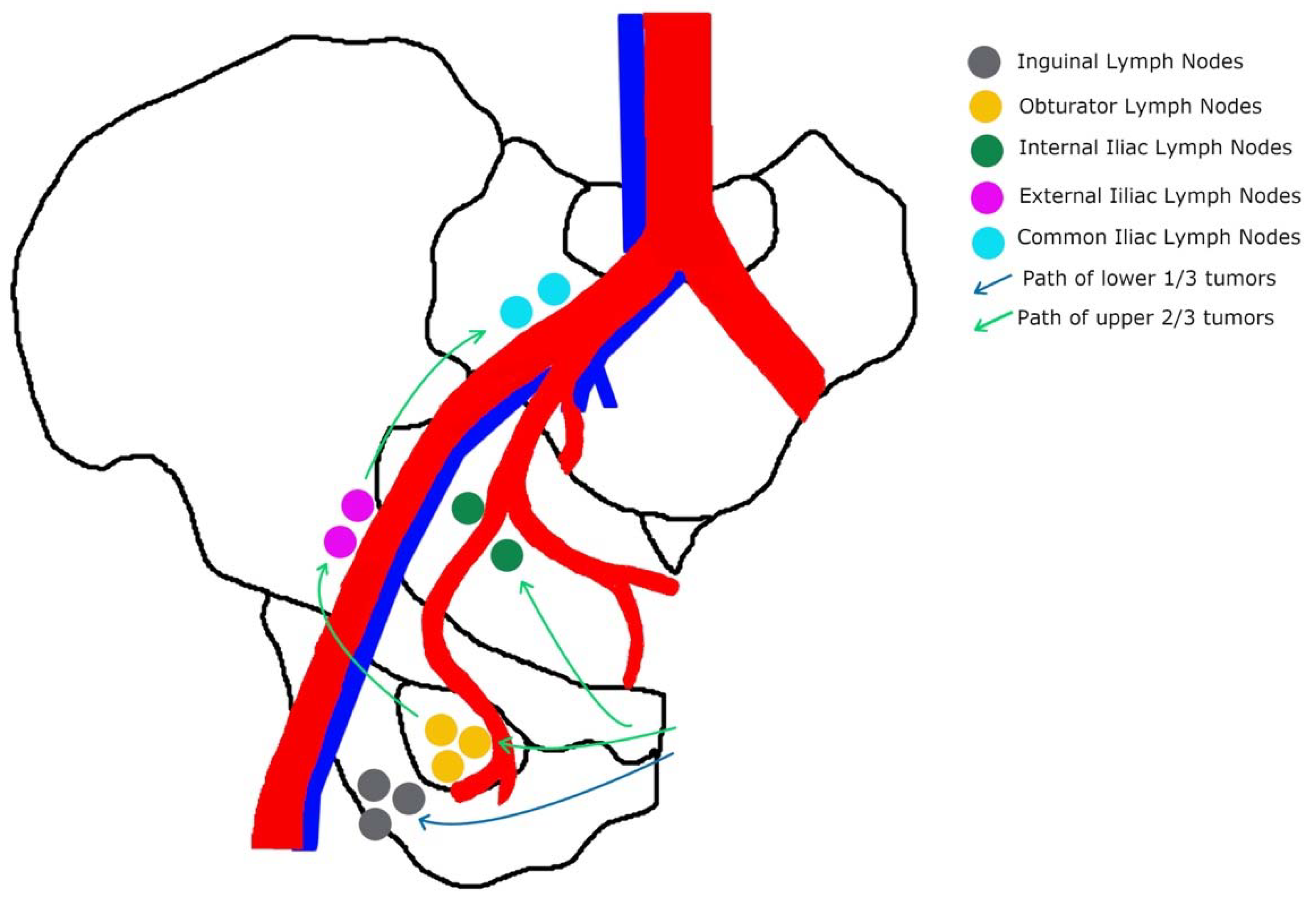
1.3. Prognostic Factors
1.4. Diagnosis and Staging
2. Materials and Methods
3. Advances in Surgical Management
4. Advances in Radiation Therapy
5. Advances in Chemoradiation Therapy
6. Advances in Systemic Treatments
6.1. Chemotherapy
6.2. Immunotherapy
7. Special Circumstances
7.1. Vaginal Melanoma
7.2. Vaginal Botryoid Rhabdomyosarcoma
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adams, T.S.; Rogers, L.J.; Cuello, M.A. Cancer of the vagina: 2021 update. Int. J. Gynecol. Obstet. 2021, 155, 19–27. [Google Scholar] [CrossRef]
- Vaginal Cancer Statistics. Canadian Cancer Society. 2022. Available online: https://cancer.ca/en/cancer-information/cancer-types/vaginal/statistics (accessed on 4 March 2022).
- Di Donato, V.; Bellati, F.; Fischetti, M.; Plotti, F.; Perniola, G.; Panici, P.B. Vaginal cancer. Crit. Rev. Oncol. Hematol. 2012, 81, 286–295. [Google Scholar] [CrossRef]
- Hiniker, S.M.; Roux, A.; Murphy, J.D.; Harris, J.P.; Tran, P.T.; Kapp, D.S.; Kidd, E.A. Primary squamous cell carcinoma of the vagina: Prognostic factors, treatment patterns, and outcomes. Gynecol. Oncol. 2013, 131, 380–385. [Google Scholar] [CrossRef]
- The American Cancer Society Medical and Editorial Content Team. Key Statistics for Vaginal Cancer. Available online: https://www.cancer.org/cancer/vaginal-cancer/about/key-statistics.html#written_by (accessed on 12 March 2022).
- Geng, Z.; Zhang, Q.; Jia, P.; Miao, J.; Lin, Q. Severe vaginal bleeding due to vaginal metastasis from renal cell carcinoma with inferior vena cava tumor thrombus. Medicine 2022, 101, e28586. [Google Scholar] [CrossRef]
- Bellati, F.; Palaia, I.; Gasparri, M.L.; Musella, A.; Panici, P.B. First case of isolated vaginal metastasis from breast cancer treated by surgery. BMC Cancer 2012, 12, 479. [Google Scholar] [CrossRef]
- Jahnke, A.; Domke, R.; Makovitzky, J.; Nizze, H.; Briese, V. Vaginal metastasis of lung cancer: A case report. Anticancer Res. 2005, 25, 1645–1648. [Google Scholar]
- Jhingran, A. Updates in the treatment of vaginal cancer. Int. J. Gynecol. Cancer 2022, 32, 344–351. [Google Scholar] [CrossRef]
- Saito, T.; Tabata, T.; Ikushima, H.; Yanai, H.; Tashiro, H.; Niikura, H.; Minaguchi, T.; Muramatsu, T.; Baba, T.; Yamagami, W.; et al. Japan Society of Gynecologic Oncology guidelines 2015 for the treatment of vulvar cancer and vaginal cancer. Int. J. Clin. Oncol. 2018, 23, 201–234. [Google Scholar] [CrossRef]
- Gadducci, A.; Fabrini, M.G.; Lanfredini, N.; Sergiampietri, C. Squamous cell carcinoma of the vagina: Natural history, treatment modalities and prognostic factors. Crit. Rev. Oncol. Hematol. 2015, 93, 211–224. [Google Scholar] [CrossRef]
- Zhou, W.; Yue, Y.; Pei, D. Survival benefit of vaginectomy compared to local tumor excision in women with FIGO stage I and II primary vaginal carcinoma: A SEER study. Arch. Gynecol. Obstet. 2020, 302, 1429–1439. [Google Scholar] [CrossRef]
- Montemorano, L.; Vetter, M.H.; Blumenfeld, M.; O’Malley, D.M. Positive sentinel lymph node in a patient with clinical stage I vaginal cancer. Gynecol. Oncol. Rep. 2020, 33, 100599. [Google Scholar] [CrossRef]
- Frumovitz, M.; Gayed, I.W.; Jhingran, A.; Euscher, E.D.; Coleman, R.L.; Ramirez, P.T.; Levenback, C.F. Lymphatic mapping and sentinel lymph node detection in women with vaginal cancer. Gynecol. Oncol. 2008, 108, 478–481. [Google Scholar] [CrossRef]
- Yin, D.; Wang, N.; Zhang, S.; Huo, N.; Xiao, Q.; Ling, O.; Lu, Y.; Wei, H. Radical hysterectomy and vaginectomy with sigmoid vaginoplasty for stage I vaginal carcinoma. Int. J. Gynecol. Obstet. 2013, 122, 132–135. [Google Scholar] [CrossRef]
- Smink, M.; Hermans, R.H.; Schoot, D.B.; Luyer, M.; Pijnenborg, J.M. First Report of Transvaginal Endoscopic Microsurgery in a Patient with Squamous Cell Carcinoma of the Vagina. J. Laparoendosc. Adv. Surg. Tech. 2013, 23, 154–157. [Google Scholar] [CrossRef]
- Jiang, H.; Qu, L.; Liu, X.; Hua, K.; Xu, H.; Guo, S.-W. A Comparison of Laparoscopic and Abdominal Radical Parametrectomy for Cervical or Vaginal Apex Carcinoma and Stage II Endometrial Cancer After Hysterectomy. JSLS 2013, 17, 249–262. [Google Scholar] [CrossRef]
- Mabuchi, Y.; Yahata, T.; Kobayashi, A.; Tanizaki, Y.; Minami, S.; Ino, K. Vaginal carcinoma in a young woman who underwent fertility-sparing treatment involving chemotherapy and conservative surgery. J. Obstet. Gynaecol. Res. 2015, 41, 989–992. [Google Scholar] [CrossRef]
- Fang, B.-R.; Ameet, H.; Li, X.-F.; Lu, Q.; Wang, X.-C.; Zeng, A.; Qiao, Q. Pedicled thinned deep inferior epigastric artery perforator flap for perineal reconstruction: A preliminary report. J. Plast. Reconstr. Aesthetic Surg. 2011, 64, 1627–1634. [Google Scholar] [CrossRef]
- Tapisiz, O.L.; Gungor, T.; Demiralp, C.O.; Demirseren, M.E.; Yalcin, H.; Mollamahmutoglu, L. Vertical rectus abdominis myocutaneous flap for vaginal reconstruction after radical pelvic surgery for Stage II vaginal carcinoma. Eur. J. Gynaecol. Oncol. 2011, 32, 216–217. [Google Scholar]
- Höckel, M.; Horn, L.-C.; Einenkel, J. (Laterally) Extended Endopelvic Resection: Surgical treatment of locally advanced and recurrent cancer of the uterine cervix and vagina based on ontogenetic anatomy. Gynecol. Oncol. 2012, 127, 297–302. [Google Scholar] [CrossRef]
- Windhofer, C.; Papp, C.; Staudach, A.; Michlits, W. Local Fasciocutaneous Infragluteal (FCI) Flap for Vulvar and Vaginal Reconstruction: A New Technique in Cancer Surgery. Int. J. Gynecol. Cancer 2012, 22, 132–138. [Google Scholar] [CrossRef]
- Hashimoto, I.; Abe, Y.; Nakanishi, H. The internal pudendal artery perforator flap: Free-style pedicle perforator flaps for vulva, vagina, and buttock reconstruction. Plast. Reconstr. Surg. 2014, 133, 924–933. [Google Scholar] [CrossRef]
- Liedl, B.; Khoder, W.Y.; Ruhdorfer-Metz, B.; Stief, C.G.; Waidelich, R. Outcomes of a bladder preservation technique in female patients undergoing pelvic exenteration surgery for advanced gynaecological tumours. Int. Urogynecol. J. 2014, 25, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Zhao, W.; Chen, G.; Zhang, A.; Sun, F.; Hu, W.; Ling, B. Comparison of laparoscopic peritoneal vaginoplasty and sigmoid colon vaginoplasty performed during radical surgery for primary vaginal carcinoma. World J. Surg. Oncol. 2014, 12, 302. [Google Scholar] [CrossRef][Green Version]
- Smith, H.O.; Genesen, M.C.; Runowicz, C.D.; Goldberg, G.L. The Rectus Abdominis Myocutaneous Flap Modifications, Complications, and Sexual Function. Cancer 1998, 83, 510–520. [Google Scholar] [CrossRef]
- Fowler, J.M. Incorporating pelvic/vaginal reconstruction into radical pelvic surgery. Gynecol. Oncol. 2009, 115, 154–163. [Google Scholar] [CrossRef]
- Kaartinen, I.S.; Vuento, M.H.; Hyöty, M.K.; Kallio, J.; Kuokkanen, H.O. Reconstruction of the pelvic floor and the vagina after total pelvic exenteration using the transverse musculocutaneous gracilis flap. J. Plast. Reconstr. Aesthetic Surg. 2015, 68, 93–97. [Google Scholar] [CrossRef]
- Guerri, S.; Perrone, A.M.; Buwenge, M.; Ferioli, M.; Macchia, G.; Tagliaferri, L.; Ferrandina, G.; Galuppi, A.; Andrulli, A.D.; Frakulli, R.; et al. Definitive Radiotherapy in Invasive Vaginal Carcinoma: A Systematic Review. Oncologist 2019, 24, 132–141. [Google Scholar] [CrossRef]
- Yang, J.; Delara, R.; Magrina, J.; Magtibay, P.; Langstraat, C.; Dinh, T.; Karlin, N.; Vora, S.A.; Butler, K. Management and outcomes of primary vaginal Cancer. Gynecol. Oncol. 2020, 159, 456–463. [Google Scholar] [CrossRef]
- Lian, J.; Dundas, G.; Carlone, M.; Ghosh, S.; Pearcey, R. Twenty-year review of radiotherapy for vaginal cancer: An institutional experience. Gynecol. Oncol. 2008, 111, 298–306. [Google Scholar] [CrossRef]
- Frank, S.J.; Jhingran, A.; Levenback, C.; Eifel, P.J. Definitive radiation therapy for squamous cell carcinoma of the vagina. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 138–147. [Google Scholar] [CrossRef]
- Schmid, M.P.; Fokdal, L.; Westerveld, H.; Chargari, C.; Rohl, L.; Morice, P.; Nesvacil, N.; Mazeron, R.; Haie-Meder, C.; Pötter, R.; et al. Recommendations from gynaecological (GYN) GEC-ESTRO working group—ACROP: Target concept for image guided adaptive brachytherapy in primary vaginal cancer. Radiother. Oncol. 2020, 145, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Greenwalt, J.C.; Amdur, R.J.; Morris, C.G.; Morgan, L.S.; Castagno, J.; Markham, M.; Rich, S.S.; Yeung, A.R. Outcomes of Definitive Radiation Therapy for Primary Vaginal Carcinoma. Am. J. Clin. Oncol. 2015, 38, 583–587. [Google Scholar] [CrossRef]
- Rajagopalan, M.S.; Xu, K.M.; Lin, J.F.; Sukumvanich, P.; Krivak, T.C.; Beriwal, S. Adoption and impact of concurrent chemoradiation therapy for vaginal cancer: A National Cancer Data Base (NCDB) study. Gynecol. Oncol. 2014, 135, 495–502. [Google Scholar] [CrossRef]
- Ghia, A.J.; Gonzalez, V.; Tward, J.; Stroup, A.M.; Pappas, L.; Gaffney, D.K. Primary Vaginal Cancer and Chemoradiotherapy: A Patterns-of-Care Analysis. Int. J. Gynecol. Cancer 2011, 21, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, D.T.; Viswanathan, A.N. Concurrent Chemoradiation for Vaginal Cancer. PLoS ONE 2013, 8, e65048. [Google Scholar] [CrossRef] [PubMed]
- Samant, R.; Lau, B.; Choan, E.; Le, T.; Tam, T. Primary Vaginal Cancer Treated with Concurrent Chemoradiation Using Cis-Platinum. Int. J. Radiat. Oncol. 2007, 69, 746–750. [Google Scholar] [CrossRef]
- Nashiro, T.; Yagi, C.; Hirakawa, M.; Inamine, M.; Nagai, Y.; Sakumoto, K.; Tamaki, W.; Ogawa, K.; Toita, T.; Aoki, Y. Concurrent chemoradiation for locally advanced squamous cell carcinoma of the vagina: Case series and literature review. Int. J. Clin. Oncol. 2008, 13, 335–339. [Google Scholar] [CrossRef]
- Diao, Y.; Jiao, J.; Song, K.; Wang, L.; Lv, T.; Dai, S.; Yao, Q. Effects of neoadjuvant chemotherapy on patients with primary vaginal squamous cell carcinoma. Mol. Clin. Oncol. 2017, 7, 395–398. [Google Scholar] [CrossRef][Green Version]
- Lv, L.; Sun, Y.; Liu, H.; Lou, J.; Peng, Z. Neoadjuvant chemotherapy followed by radical surgery and reconstruction of the vagina in a patient with stage II primary vaginal squamous carcinoma. J. Obstet. Gynaecol. Res. 2010, 36, 1245–1248. [Google Scholar] [CrossRef]
- Thigpen, J.; Blessing, J.A.; Homesley, H.D.; Berek, J.S.; Creasman, W.T. Phase II trial of cisplatin in advanced or recurrent cancer of the vagina: A gynecologic oncology group study. Gynecol. Oncol. 1986, 23, 101–104. [Google Scholar] [CrossRef]
- How, J.A.; Jazaeri, A.A.; Soliman, P.T.; Fleming, N.D.; Gong, J.; Piha-Paul, S.A.; Janku, F.; Stephen, B.; Naing, A. Pembrolizumab in vaginal and vulvar squamous cell carcinoma: A case series from a phase II basket trial. Sci. Rep. 2021, 11, 3667. [Google Scholar] [CrossRef] [PubMed]
- Naumann, R.W.; Hollebecque, A.; Meyer, T.; Devlin, M.-J.; Oaknin, A.; Kerger, J.; López-Picazo, J.M.; Machiels, J.-P.; Delord, J.-P.; Evans, T.R.J.; et al. Safety and Efficacy of Nivolumab Monotherapy in Recurrent or Metastatic Cervical, Vaginal, or Vulvar Carcinoma: Results from the Phase I/II CheckMate 358 Trial. J. Clin. Oncol. 2019, 37, 2825–2834. [Google Scholar] [CrossRef]
- Umesaki, N.; Kawamura, N.; Tsujimura, A.; Ichimura, T.; Tanaka, T.; Ogita, S. Stage II vaginal cancer responding to chemotherapy with irinotecan and cisplatin: A case report. Oncol. Rep. 1999, 6, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Panici, P.B.; Bellati, F.; Plotti, F.; Di Donato, V.; Antonilli, M.; Perniola, G.; Manci, N.; Muzii, L.; Angioli, R. Neoadjuvant chemotherapy followed by radical surgery in patients affected by vaginal carcinoma. Gynecol. Oncol. 2008, 111, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Vaysse, C.; Pautier, P.; Filleron, T.; Maisongrosse, V.; Rodier, J.-F.; Lavoue, V.; Reyal, F.; Thomas, L.; de la Fouchardiere, A.; Delannes, M. A large retrospective multicenter study of vaginal melanomas. Melanoma Res. 2013, 23, 138–146. [Google Scholar] [CrossRef]
- Todo, Y.; Okamoto, K.; Suzuki, Y.; Minobe, S.; Kato, H. Radicality of initial surgery for primary malignant melanoma of the vagina. Melanoma Res. 2016, 26, 173–180. [Google Scholar] [CrossRef]
- Geisler, J.P.; Look, K.Y.; Moore, D.A.; Sutton, G.P. Pelvic Exenteration for Malignant Melanomas of the Vagina or Urethra with over 3 mm of Invasion. Gynecol. Oncol. 1995, 59, 338–341. [Google Scholar] [CrossRef]
- Sezen, D.; Patel, R.R.; Tang, C.; Onstad, M.; Nagarajan, P.; Patel, S.P.; Welsh, J.W.; Lin, L.L. Immunotherapy combined with high- and low-dose radiation to all sites leads to complete clearance of disease in a patient with metastatic vaginal melanoma. Gynecol. Oncol. 2021, 161, 645–652. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Larkin, J.; Sosman, J.A.; Lebbé, C.; Brady, B.; Neyns, B.; Schmidt, H.; Hassel, J.C.; Hodi, F.S.; Lorigan, P.; et al. Efficacy and Safety of Nivolumab Alone or in Combination with Ipilimumab in Patients with Mucosal Melanoma: A Pooled Analysis. J. Clin. Oncol. 2017, 35, 226–235. [Google Scholar] [CrossRef]
- Michlitsch, J.G.; Romao, R.L.; Gleason, J.M.; Braga, L.H.; Allen, L.; Gupta, A.; Lorenzo, A.J. Local control for vaginal botryoid rhabdomyosarcoma with pre-rectal transperineal surgical resection and autologous buccal graft vaginal replacement: A novel, minimally invasive, radiation-sparing approach. J. Pediatr. Surg. 2018, 53, 1374–1380. [Google Scholar] [CrossRef]
- Xie, W.; Shen, K.; Yang, J.; Cao, D.; Yu, M.; Wang, Y. Conservative management of primary vaginal endodermal sinus tumor and rhabdomyosarcoma. Oncotarget 2017, 8, 63453–63460. [Google Scholar] [CrossRef] [PubMed]
| Series/Report | Chemotherapy Agents | Stage | Notes |
|---|---|---|---|
| Diao (2017) | Irinotecan Cisplatin | Stage I, n = 2 Stage II, n = 1 |
|
| Mabuchi (2015) | Irinotecan Nedaplatin | Stage I, n = 1 |
|
| Lv (2010) | Bleomycin Cisplatin | Stage II, n = 1 |
|
| Benedetti (2008) | Paclitaxel Cisplatin | Stage II, n = 11 |
|
| Umesaki (1999) | Irinotecan Cisplatin | Stage II, n = 1 |
|
| Thigpen (1986) | Cisplatin | Stage IV or recurrent, n = 16 |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulkarni, A.; Dogra, N.; Zigras, T. Innovations in the Management of Vaginal Cancer. Curr. Oncol. 2022, 29, 3082-3092. https://doi.org/10.3390/curroncol29050250
Kulkarni A, Dogra N, Zigras T. Innovations in the Management of Vaginal Cancer. Current Oncology. 2022; 29(5):3082-3092. https://doi.org/10.3390/curroncol29050250
Chicago/Turabian StyleKulkarni, Anjali, Nupur Dogra, and Tiffany Zigras. 2022. "Innovations in the Management of Vaginal Cancer" Current Oncology 29, no. 5: 3082-3092. https://doi.org/10.3390/curroncol29050250
APA StyleKulkarni, A., Dogra, N., & Zigras, T. (2022). Innovations in the Management of Vaginal Cancer. Current Oncology, 29(5), 3082-3092. https://doi.org/10.3390/curroncol29050250




