Repeated Multimodality Ablative Therapies for Oligorecurrent Pulmonary Metastatic Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Treatment Procedures and Follow-Up
2.3. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
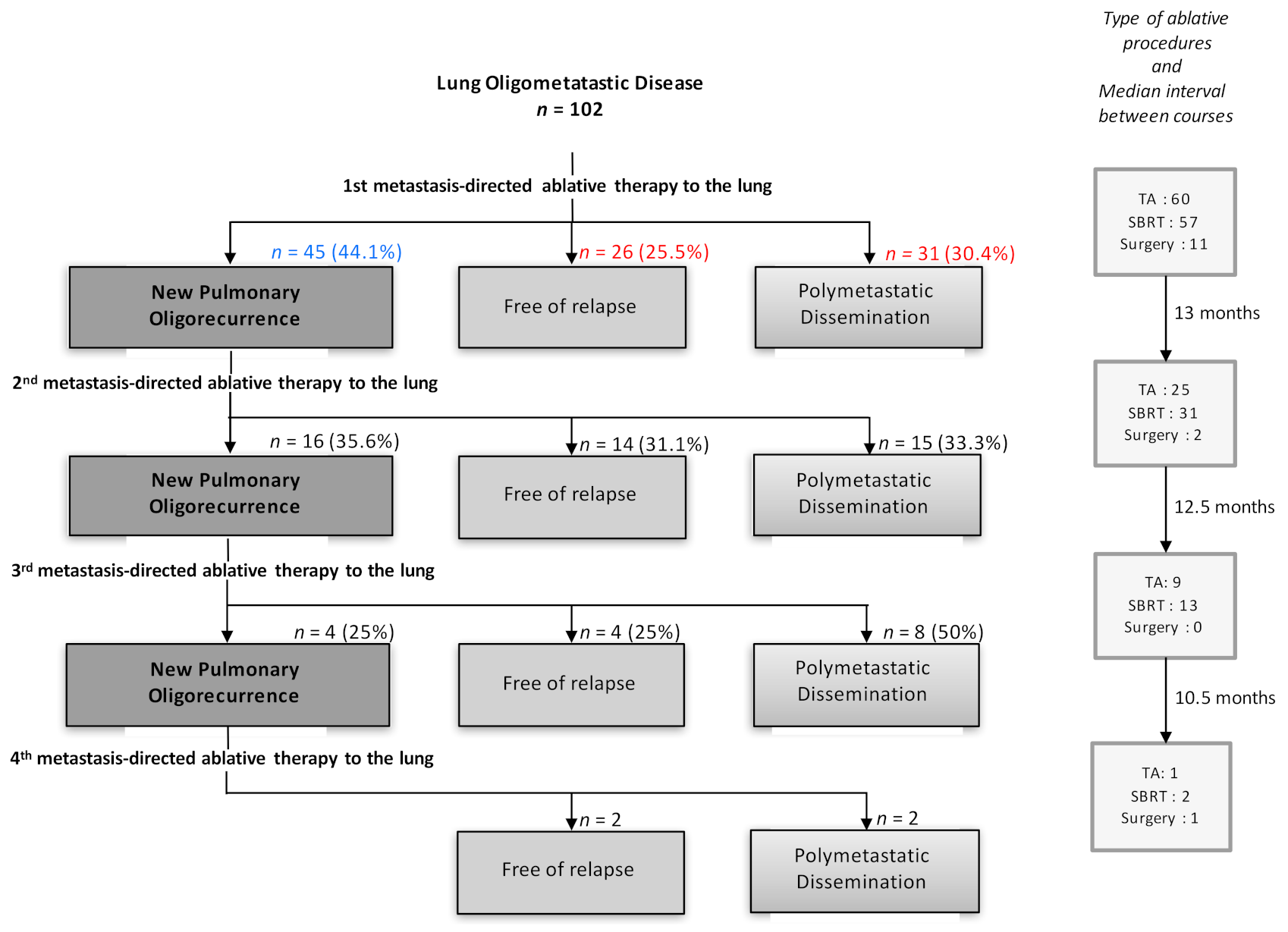
3.2. Treatment Characteristics
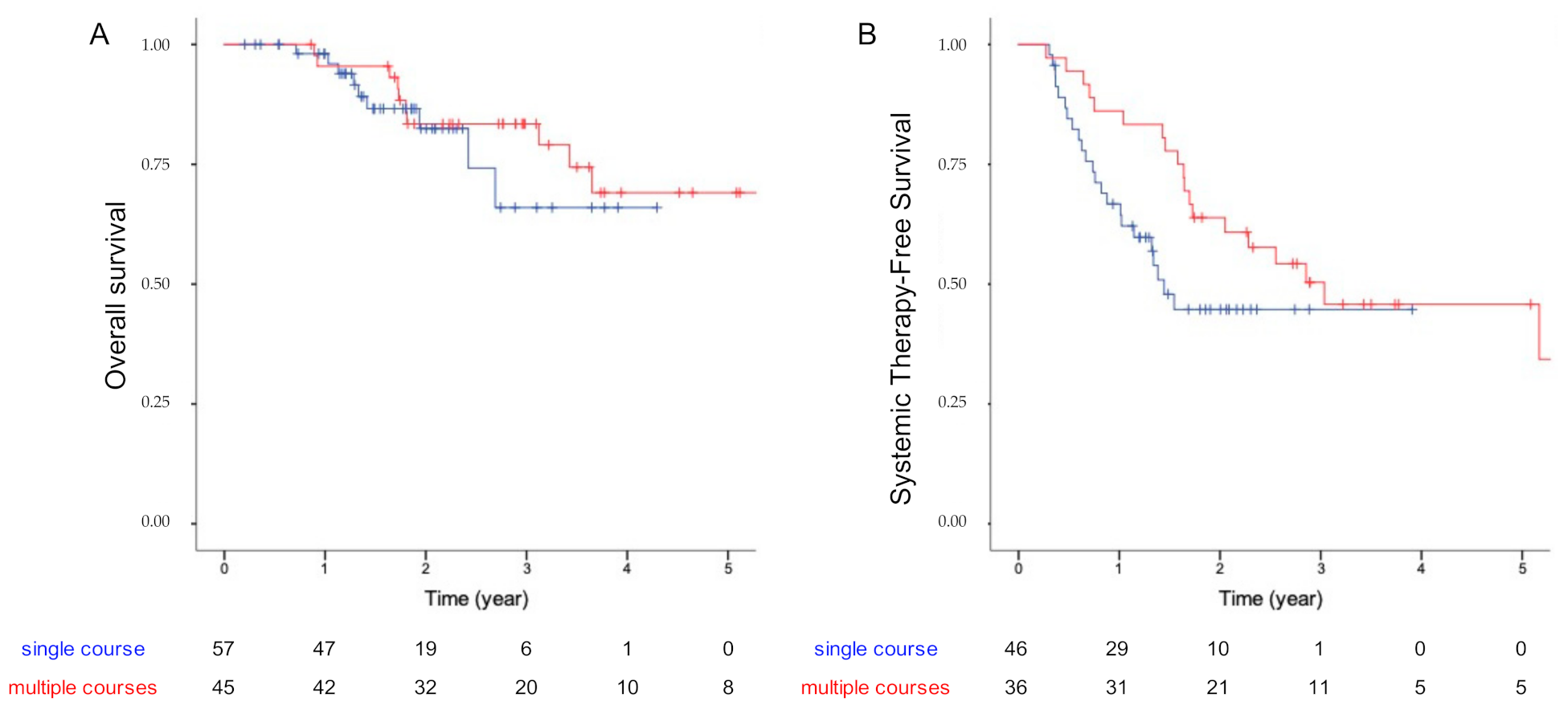
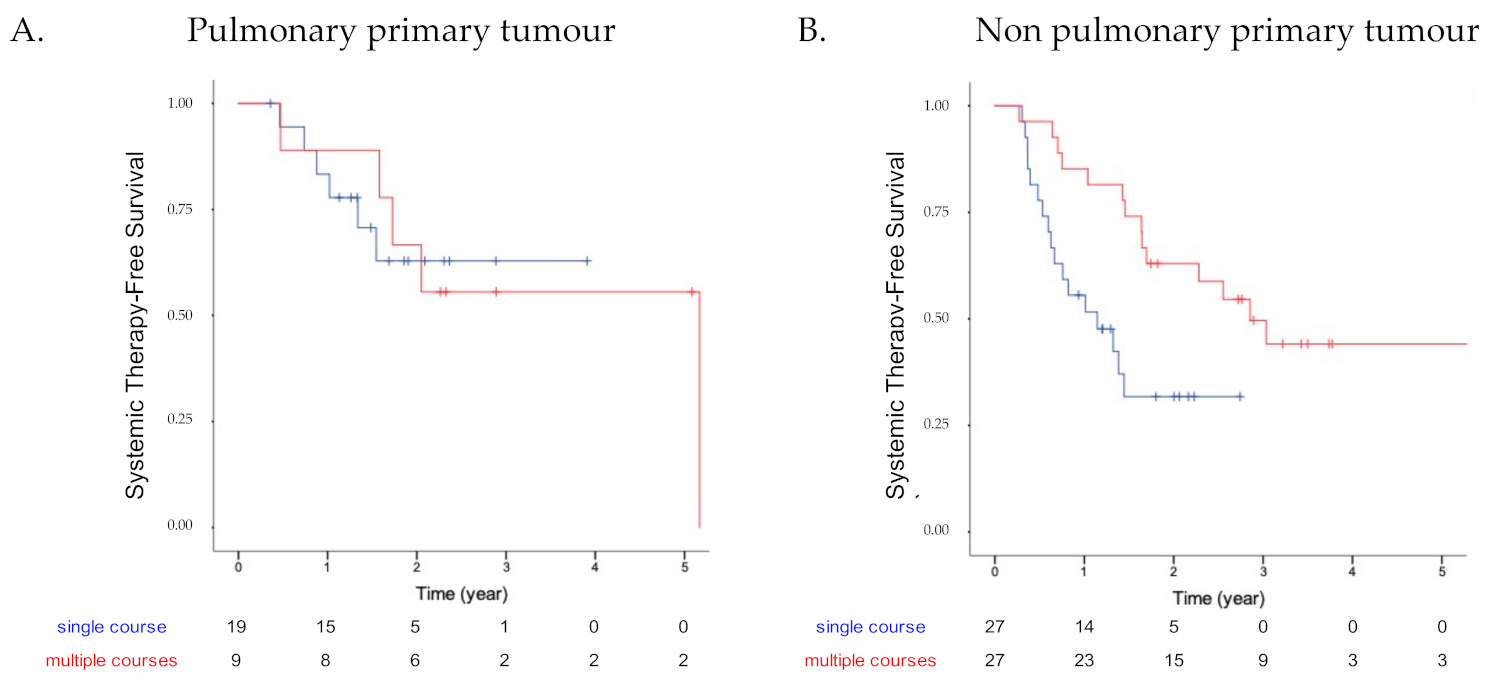
3.3. Patients’ Outcome
3.4. Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Hellman, S. Karnofsky Memorial Lecture. Natural History Of Small Breast Cancers. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1994, 12, 2229–2234. [Google Scholar] [CrossRef] [PubMed]
- Guckenberger, M.; Lievens, Y.; Bouma, A.B.; Collette, L.; Dekker, A.; Desouza, N.M.; Dingemans, A.-M.C.; Fournier, B.; Hurkmans, C.; Lecouvet, F.E.; et al. Characterisation And Classification Of Oligometastatic Disease: A European Society For Radiotherapy And Oncology And European Organisation For Research And Treatment Of Cancer Consensus Recommendation. Lancet Oncol. 2020, 21, E18–E28. [Google Scholar] [CrossRef] [Green Version]
- Battaglia, A.; De Meerleer, G.; Tosco, L.; Moris, L.; Van Den Broeck, T.; Devos, G.; Everaerts, W.; Joniau, S. Novel Insights Into The Management Of Oligometastatic Prostate Cancer: A Comprehensive Review. Eur. Urol. Oncol. 2019, 2, 174–188. [Google Scholar] [CrossRef]
- Al-Shafa, F.; Arifin, A.J.; Rodrigues, G.B.; Palma, D.A.; Louie, A.V. A Review Of Ongoing Trials Of Stereotactic Ablative Radiotherapy For Oligometastatic Cancers: Where Will The Evidence Lead? Front. Oncol. 2019, 9, 543. [Google Scholar] [CrossRef]
- Palma, D.A.; Salama, J.K.; Lo, S.S.; Senan, S.; Treasure, T.; Govindan, R.; Weichselbaum, R. The Oligometastatic State-Separating Truth From Wishful Thinking. Nat. Rev. Clin. Oncol. 2014, 11, 549–557. [Google Scholar] [CrossRef]
- Lewis, S.L.; Porceddu, S.; Nakamura, N.; Palma, D.A.; Lo, S.S.; Hoskin, P.; Moghanaki, D.; Chmura, S.J.; Salama, J.K. Definitive Stereotactic Body Radiotherapy (Sbrt) For Extracranial Oligometastases: An International Survey Of >1000 Radiation Oncologists. Am. J. Clin. Oncol. 2017, 40, 418–422. [Google Scholar] [CrossRef]
- Pan, H.; Simpson, D.R.; Mell, L.K.; Mundt, A.J.; Lawson, J.D. A Survey Of Stereotactic Body Radiotherapy Use In The United States. Cancer 2011, 117, 4566–4572. [Google Scholar] [CrossRef] [Green Version]
- Siva, S.; Macmanus, M.; Ball, D. Stereotactic Radiotherapy For Pulmonary Oligometastases: A Systematic Review. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2010, 5, 1091–1099. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.M.; Choi, E.K.; Lee, S.-W.; Yi, B.Y.; Ahn, S.D.; Shin, S.S.; Park, H.J.; Kim, S.S.; Park, J.-H.; Song, S.Y.; et al. Clinical Results Of Stereotactic Body Frame Based Fractionated Radiation Therapy For Primary Or Metastatic Thoracic Tumors. Acta Oncol. Stockh. Swed. 2006, 45, 1108–1114. [Google Scholar] [CrossRef] [Green Version]
- Milano, M.T.; Katz, A.W.; Muhs, A.G.; Philip, A.; Buchholz, D.J.; Schell, M.C.; Okunieff, P. A Prospective Pilot Study Of Curative-Intent Stereotactic Body Radiation Therapy In Patients With 5 Or Fewer Oligometastatic Lesions. Cancer 2008, 112, 650–658. [Google Scholar] [CrossRef]
- Rusthoven, K.E.; Kavanagh, B.D.; Burri, S.H.; Chen, C.; Cardenes, H.; Chidel, M.A.; Pugh, T.J.; Kane, M.; Gaspar, L.E.; Schefter, T.E. Multi-Institutional Phase I/Ii Trial Of Stereotactic Body Radiation Therapy For Lung Metastases. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 1579–1584. [Google Scholar] [CrossRef]
- Norihisa, Y.; Nagata, Y.; Takayama, K.; Matsuo, Y.; Sakamoto, T.; Sakamoto, M.; Mizowaki, T.; Yano, S.; Hiraoka, M. Stereotactic Body Radiotherapy For Oligometastatic Lung Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 398–403. [Google Scholar] [CrossRef]
- Navarria, P.; Ascolese, A.M.; Tomatis, S.; Cozzi, L.; De Rose, F.; Mancosu, P.; Alongi, F.; Clerici, E.; Lobefalo, F.; Tozzi, A.; et al. Stereotactic Body Radiotherapy (Sbrt) In Lung Oligometastatic Patients: Role Of Local Treatments. Radiat. Oncol. Lond. Engl. 2014, 9, 91. [Google Scholar] [CrossRef] [Green Version]
- De Baère, T.; Aupérin, A.; Deschamps, F.; Chevallier, P.; Gaubert, Y.; Boige, V.; Fonck, M.; Escudier, B.; Palussiére, J. Radiofrequency Ablation Is A Valid Treatment Option For Lung Metastases: Experience In 566 Patients With 1037 Metastases. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 987–991. [Google Scholar] [CrossRef]
- Chua, T.C.; Sarkar, A.; Saxena, A.; Glenn, D.; Zhao, J.; Morris, D.L. Long-Term Outcome Of Image-Guided Percutaneous Radiofrequency Ablation Of Lung Metastases: An Open-Labeled Prospective Trial Of 148 Patients. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2010, 21, 2017–2022. [Google Scholar] [CrossRef]
- Smith, S.L.; Jennings, P.E. Lung Radiofrequency And Microwave Ablation: A Review Of Indications, Techniques And Post-Procedural Imaging Appearances. Br. J. Radiol. 2015, 88, 20140598. [Google Scholar] [CrossRef] [Green Version]
- Vogl, T.J.; Naguib, N.N.N.; Gruber-Rouh, T.; Koitka, K.; Lehnert, T.; Nour-Eldin, N.-E.A. Microwave Ablation Therapy: Clinical Utility In Treatment Of Pulmonary Metastases. Radiology 2011, 261, 643–651. [Google Scholar] [CrossRef]
- Simon, C.J.; Dupuy, D.E.; Dipetrillo, T.A.; Safran, H.P.; Grieco, C.A.; Ng, T.; Mayo-Smith, W.W. Pulmonary Radiofrequency Ablation: Long-Term Safety And Efficacy In 153 Patients. Radiology 2007, 243, 268–275. [Google Scholar] [CrossRef]
- Lencioni, R.; Crocetti, L.; Cioni, R.; Suh, R.; Glenn, D.; Regge, D.; Helmberger, T.; Gillams, A.R.; Frilling, A.; Ambrogi, M.; et al. Response To Radiofrequency Ablation Of Pulmonary Tumours: A Prospective, Intention-To-Treat, Multicentre Clinical Trial (The Rapture Study). Lancet Oncol. 2008, 9, 621–628. [Google Scholar] [CrossRef]
- Chang, J.Y.; Senan, S.; Paul, M.A.; Mehran, R.J.; Louie, A.V.; Balter, P.; Groen, H.J.M.; Mcrae, S.E.; Widder, J.; Feng, L.; et al. Stereotactic Ablative Radiotherapy Versus Lobectomy For Operable Stage I Non-Small-Cell Lung Cancer: A Pooled Analysis Of Two Randomised Trials. Lancet Oncol. 2015, 16, 630–637. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.Y.; Mehran, R.J.; Feng, L.; Verma, V.; Liao, Z.; Welsh, J.W.; Lin, S.H.; O’reilly, M.S.; Jeter, M.D.; Balter, P.A.; et al. Stereotactic Ablative Radiotherapy For Operable Stage I Non-Small-Cell Lung Cancer (Revised Stars): Long-Term Results Of A Single-Arm, Prospective Trial With Prespecified Comparison To Surgery. Lancet Oncol. 2021, 22, 1448–1457. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.J.; Park, I.K.; Kim, Y.T.; Kang, C.H. Stereotactic Ablative Radiotherapy Versus Surgery In Older Patients With Stage I Lung Cancer. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2021, 60, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Molla, M.; Fernandez-Plana, J.; Albiol, S.; Fondevila, C.; Vollmer, I.; Cases, C.; Garcia-Criado, A.; Capdevila, J.; Conill, C.; Fundora, Y.; et al. Limited Liver Or Lung Colorectal Cancer Metastases. Systemic Treatment, Surgery, Ablation Or Sbrt. J. Clin. Med. 2021, 10, 2131. [Google Scholar] [CrossRef]
- Milano, M.T.; Katz, A.W.; Zhang, H.; Okunieff, P. Oligometastases Treated With Stereotactic Body Radiotherapy: Long-Term Follow-Up Of Prospective Study. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 878–886. [Google Scholar] [CrossRef]
- Widder, J.; Klinkenberg, T.J.; Ubbels, J.F.; Wiegman, E.M.; Groen, H.J.M.; Langendijk, J.A. Pulmonary Oligometastases: Metastasectomy Or Stereotactic Ablative Radiotherapy? Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2013, 107, 409–413. [Google Scholar] [CrossRef]
- Milano, M.T.; Katz, A.W.; Okunieff, P. Patterns Of Recurrence After Curative-Intent Radiation For Oligometastases Confined To One Organ. Am. J. Clin. Oncol. 2010, 33, 157–163. [Google Scholar] [CrossRef]
- Annede, P.; Darreon, J.; Benkemouche, A.; Valdenaire, S.; Tyran, M.; Kaeppelin, B.; Macagno, A.; Barrou, J.; Cagetti, L.V.; Favrel, V.; et al. Flattening Filter Free Vs. Flattened Beams For Lung Stereotactic Body Radiation Therapy. Anticancer Res. 2017, 37, 5133–5139. [Google Scholar] [CrossRef]
- Klement, R.J.; Hoerner-Rieber, J.; Adebahr, S.; Andratschke, N.; Blanck, O.; Boda-Heggemann, J.; Duma, M.; Eble, M.J.; Eich, H.C.; Flentje, M.; et al. Stereotactic Body Radiotherapy (Sbrt) For Multiple Pulmonary Oligometastases: Analysis Of Number And Timing Of Repeat Sbrt As Impact Factors On Treatment Safety And Efficacy. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2018, 127, 246–252. [Google Scholar] [CrossRef]
- Mazzola, R.; Fersino, S.; Ferrera, G.; Targher, G.; Figlia, V.; Triggiani, L.; Pasinetti, N.; Lo Casto, A.; Ruggieri, R.; Magrini, S.M.; et al. Stereotactic Body Radiotherapy For Lung Oligometastases Impacts On Systemic Treatment-Free Survival: A Cohort Study. Med. Oncol. Northwood Lond. Engl. 2018, 35, 121. [Google Scholar] [CrossRef]
- Merino Lara, T.; Helou, J.; Poon, I.; Sahgal, A.; Chung, H.T.; Chu, W.; Soliman, H.; Ung, Y.; Verma, S.; Cheema, P.; et al. Multisite Stereotactic Body Radiotherapy For Metastatic Non-Small-Cell Lung Cancer: Delaying The Need To Start Or Change Systemic Therapy? Lung Cancer 2018, 124, 219–226. [Google Scholar] [CrossRef]
- Palma, D.A.; Haasbeek, C.J.A.; Rodrigues, G.B.; Dahele, M.; Lock, M.; Yaremko, B.; Olson, R.; Liu, M.; Panarotto, J.; Griffioen, G.H.M.J.; et al. Stereotactic Ablative Radiotherapy For Comprehensive Treatment Of Oligometastatic Tumors (Sabr-Comet): Study Protocol For A Randomized Phase Ii Trial. BMC Cancer 2012, 12, 305. [Google Scholar] [CrossRef] [Green Version]
- Palma, D.A.; Olson, R.A.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.A.; Lock, M.I.; Rodrigues, G.; Yaremko, B.P.; et al. Stereotactic Ablative Radiation Therapy For The Comprehensive Treatment Of Oligometastatic Tumors (Sabr-Comet): Results Of A Randomized Trial. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, S3–S4. [Google Scholar] [CrossRef]
| Variable | All Patients (n = 102) | Single Course (n = 57) | Multiple Courses (n = 45) | p-Value |
|---|---|---|---|---|
| Age (years) median and IQR | 64.3 (56.57–72.40) | 64.2 (54.90–72.89) | 64.1 (58.97–72.01) | 0.79 |
| Baseline WHO status | 0.54 | |||
| 0 | 46 (45%) | 23 (40%) | 23 (51%) | |
| 1 | 49 (48%) | 30 (53%) | 19 (42%) | |
| 2 | 7 (7%) | 4 (7%) | 3 (7%) | |
| >2 | 0 (0%) | 0 (0%) | 0 (0%) | |
| Cardiorespiratory History | 26 (25%) | 20 (35%) | 6 (13%) | 0.02 |
| Primary Cancer | 0.03 | |||
| Bronchopulmonary | 36 (35%) | 26 (46%) | 10 (22%) | |
| Colorectal | 18 (18%) | 7 (12%) | 11 (24%) | |
| Renal | 13 (13%) | 4 (7%) | 9 (20%) | |
| Sarcoma | 15 (15%) | 10 (18%) | 5 (11%) | |
| Other | 20 (20%) | 10 (18%) | 10 (22%) | |
| Metastatic sites initially involved | 0.19 | |||
| 1 | 71 (70%) | 42 (74%) | 29 (64.44%) | |
| 2 | 29 (28%) | 15 (26%) | 14 (31.11%) | |
| 3 | 2 (2%) | 0 (0%) | 2 (4.44%) | |
| Non-pulmonary focal treatment | 29 (28%) | 14 (25%) | 15 (33%) | 0.35 |
| Brain | 8 (8%) | 4 (7%) | 4 (9%) | |
| Liver | 16 (16%) | 8 (14%) | 8 (18%) | |
| Other | 5 (5%) | 2 (4%) | 3 (7%) | |
| Time to metastases | 0.08 | |||
| Synchronous | 33 (32%) | 23 (40%) | 10 (22%) | |
| Metachronous | 69 (68%) | 34 (60%) | 35 (78%) | |
| Systemic therapy before ablative treatment | 47 (46%) | 29 (51%) | 18 (40%) | 0.28 |
| Chemotherapy | 26 (55%) | 15 (52%) | 11 (61%) | |
| Immunotherapy | 2 (4%) | 2 (7%) | ||
| Targeted therapy | 5 (11%) | 4 (14%) | 1 (6%) | |
| NA | 14 (30%) | 8 (27%) | 6 (33%) |
| SBRT (n = 103) | |
|---|---|
| Metastasis diameter (mm) (median and IQR) | 14.5 (11–23) |
| Lung Topography | |
| Central | 48 (47%) |
| Peripheral | 55 (53%) |
| Treatment Parameters | |
| Dose to PTV (Gy) (median and IQR) | 45.50 (40–48) |
| Fractionation (min–max) | 6 (4–8) |
| BED (median and IQR) | 71.25 (59.5–72) |
| Ipsilateral mean lung dose (Gy) (median and IQR) | 6.57 (4.25–9.51) |
| Ipsilateral lung V20 (%) (median and IQR) | 12.53 (7.25–16.96) |
| Ipsilateral lung V5 (%) (median and IQR) | 34.58 (21.83–45.69) |
| TA (n = 95) | |
| Metastasis diameter (mm) (median and IQR) | 12 (10–15) |
| Lung Topography | |
| Central | 16 (17%) |
| Peripheral | 79 (83%) |
| Techniques | |
| Radiofrequency | 78 |
| Microwave | 4 |
| Cryotherapy | 13 |
| Average length of hospitalization (day) | 2.62 |
| SURGERY (n = 14) | |
| Techniques | |
| Wedge | 6 |
| Lobectomy | 5 |
| NA | 3 |
| Univariate Analysis | |||
|---|---|---|---|
| Factors | HR | 95%-CI | p-Value |
| Age | 1.03 | 0.98–1.08 | 0.22 |
| WHO status > 1 | 3.23 | 1.03–10.13 | 0.04 |
| Cardiorespiratory History | 1.95 | 0.73–5.24 | 0.19 |
| Number of metastatic sites initially involved | 1.45 | 0.70–3.10 | 0.32 |
| Time to metastasis (synchronous ref.) | 1.31 | 0.49–3.50 | 0.59 |
| Primary Cancer (Bronchopulmonary ref.) | |||
| Colorectal | 1.79 | 0.45–7.26 | 0.55 |
| Renal | 1.38 | 0.30–6.30 | 0.55 |
| Sarcoma | 2.85 | 0.76–10.69 | 0.55 |
| Systemic therapy before ablative treatment | 0.99 | 0.35–2.79 | 0.98 |
| Interval from tumor diagnosis to treatment | 0.99 | 0.86–1.15 | 0.96 |
| Local relapse | 0.53 | 0.12–2.33 | 0.37 |
| Lung oligorecurrence | 0.92 | 0.35–2.40 | 0.86 |
| Multimetastatic relapse | 5.81 | 1.33–25.28 | 0.0078 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macagno, A.; de Nonneville, A.; Annede, P.; Piana, G.; Pougnet, I.; Daidj, N.; Moureau-Zabotto, L.; Darreon, J.; Padovani, L.; Bertucci, F.; et al. Repeated Multimodality Ablative Therapies for Oligorecurrent Pulmonary Metastatic Disease. Curr. Oncol. 2022, 29, 1683-1694. https://doi.org/10.3390/curroncol29030140
Macagno A, de Nonneville A, Annede P, Piana G, Pougnet I, Daidj N, Moureau-Zabotto L, Darreon J, Padovani L, Bertucci F, et al. Repeated Multimodality Ablative Therapies for Oligorecurrent Pulmonary Metastatic Disease. Current Oncology. 2022; 29(3):1683-1694. https://doi.org/10.3390/curroncol29030140
Chicago/Turabian StyleMacagno, Alban, Alexandre de Nonneville, Pierre Annede, Gilles Piana, Isabelle Pougnet, Nassima Daidj, Laurence Moureau-Zabotto, Julien Darreon, Laetitia Padovani, Francois Bertucci, and et al. 2022. "Repeated Multimodality Ablative Therapies for Oligorecurrent Pulmonary Metastatic Disease" Current Oncology 29, no. 3: 1683-1694. https://doi.org/10.3390/curroncol29030140
APA StyleMacagno, A., de Nonneville, A., Annede, P., Piana, G., Pougnet, I., Daidj, N., Moureau-Zabotto, L., Darreon, J., Padovani, L., Bertucci, F., & Salem, N. (2022). Repeated Multimodality Ablative Therapies for Oligorecurrent Pulmonary Metastatic Disease. Current Oncology, 29(3), 1683-1694. https://doi.org/10.3390/curroncol29030140





