Health Technology Assessment Process for Oncology Drugs: Impact of CADTH Changes on Public Payer Reimbursement Recommendations
Abstract
1. Introduction
2. Materials and Methods
3. Results
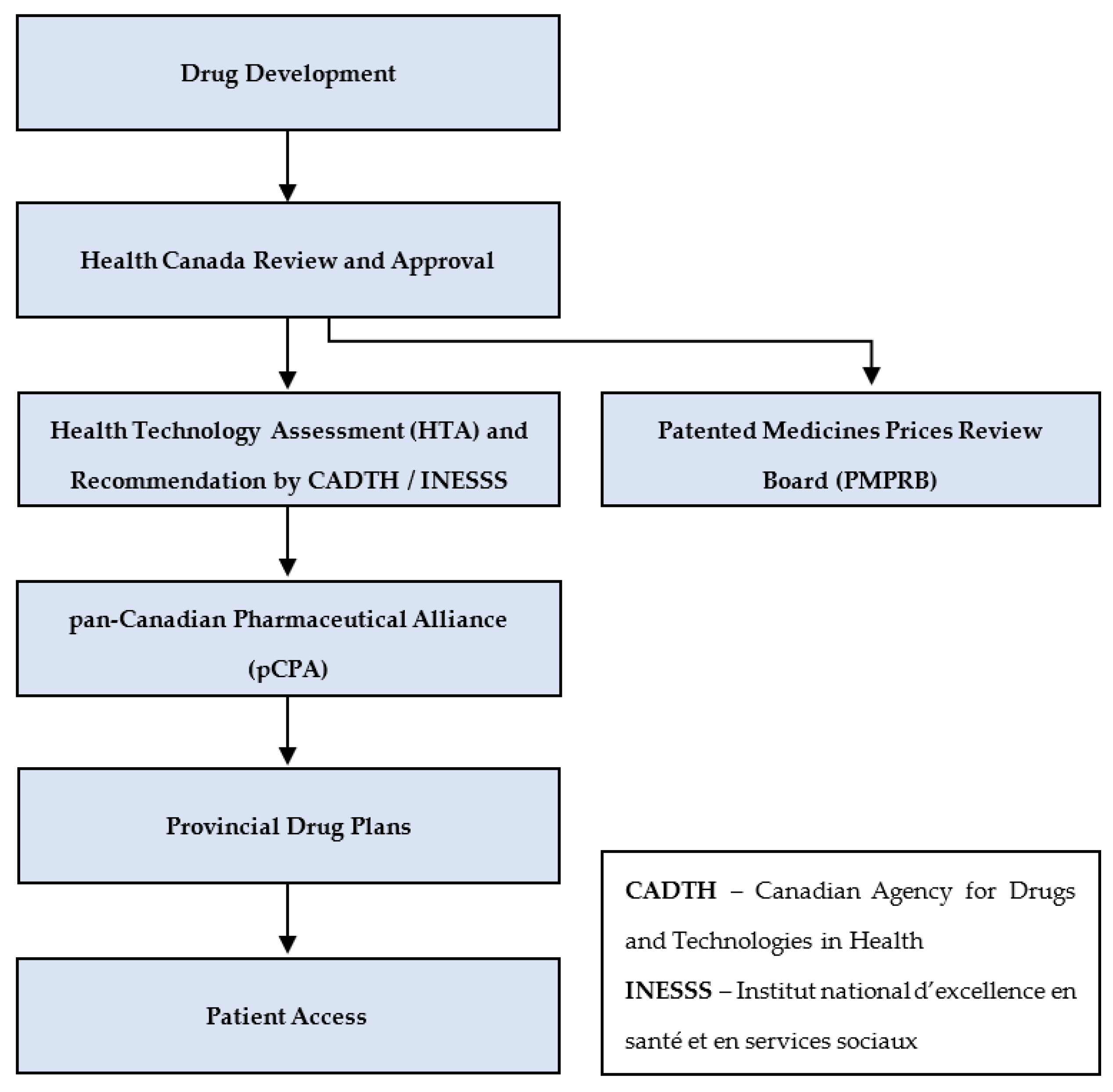
3.1. From Drug Development to Public Access—An Overview
3.1.1. Federal Government Processes
3.1.2. Pan-Canadian Processes
3.1.3. Provinces and Territories
3.2. Fundamentals of Health Technology Assessment in Public Drug Access
3.2.1. The Science of QALYs: Is a QALY Just a Number?
3.2.2. Are QALYs the Same across Disease Areas?
3.2.3. How Much to Pay for a QALY?
3.3. History of Oncology HTA in Canada
- The need for a distinct process separate from CADTH to address oncology-specific needs;
- The need for highly credible clinical oncology expertise deeply and transparently integrated within all parts of the process;
- The need for patient inclusion in the process;
- The need for recommendations that focus on patient as well as payer needs, with a clear and transparent rationale for those recommendations [38].
3.4. Analysis of CADTH’s Evaluative Process
Reduction in Cost per QALY Threshold
“CADTH does its works both independently but in response to what’s being asked of us. Canada has not set a cost effectiveness threshold and so there isn’t common agreement across Canada on what those should be so the 50 QALY, the $50,000 QALY has been selected for consistency across all CADTH products and conditions and it allows our decision makers in the absence of any gradiated (sic) or alternate numbers to be able to compare across different interventions and drugs. However it’s important to also note that we do look at the impact of other potential price reductions as part of our work and those are clearly reported in the documents so although that’s the one that gets the most visibility, all of the other price thresholds are also reflected on in our report. And I think for us the high price reductions if they are sitting in the 80 to 90 percent for the most part are a signal to the pCPA and to jurisdictions that these are products that may have greater complexity, higher uncertainty and may, may result and signal more work to be able to negotiate. As we said, always evolving this place and continuing to work but that sort of is the purpose right now is in the absence of Canada agreeing on any other thresholds, we‘re using the one as sort of a consistent place to be able for people to make judgment but certainly there is more in the reports and so for people to take a look at that as well”.
4. Discussion
4.1. Potential Implications of the Threshold Reduction
“The CADTH reanalysis results indicated that enzalutamide plus ADT was not cost-effective at a willingness-to-pay threshold of $50,000 per quality-adjusted life-year (QALY), with an incremental cost-effectiveness ratio of $294,805 per QALY at the current price. Based on current list prices, at a willingness-to-pay threshold of $50,000 per QALY, a price reduction of approximately 75% is required”.[47]
“The ICER for venetoclax plus azacitidine is $125,580 per QALY gained when compared to LDAC. A 100% reduction in the price of venetoclax would still not achieve an ICER of $50,000 per QALY compared to LDAC. Azacitidine is more costly than LDAC and would also need to be reduced in price to reach this threshold”.[48]
“If testing is required to determine eligibility based on NTRK status, then there is no price at which larotrectinib could be considered cost-effective at a $50,000 per QALY threshold. If the cost of testing to determine eligibility based on NTRK status is excluded from the total treatment cost, then larotrectinib would require a price reduction of greater than 90% to be considered cost-effective at a $50,000 per QALY threshold”.[49]
“The ICER for enfortumab vedotin is $506,439 when compared with taxanes. A price reduction of 93% would be required for enfortumab vedotin to be able to achieve an ICER of $50,000 per QALY compared to a taxane”.[50]
“In the EGP’s best-case estimate, the incremental cost of cemiplimab was $176,966 and the incremental benefit gain was 1.48 LYs and 1.06 QALYs over a 30-year life-time horizon, for an estimated ICUR of $166,221 per QALY. An upper bound of $223,828 per QALY was achieved with cemiplimab being administered until treatment progression (no capping at 22 or 24 months). The cost of cemiplimab was the main cost driver; and most of the QALY gained (70%) was accrued in the post-progression period and in the extrapolated phase of the model. The deterministic sequential analysis showed that for a willingness-to-pay below $52,539 per QALY, BSC would be the preferred treatment option. For a willingness-to-pay between $52,539 and $161,278 per QALY, chemotherapy would be the preferred option, and that cemiplimab would be the preferred option for a willingness-to-pay above $161,278 per QALY. The price reduction scenarios showed that a 40% price reduction would be needed to bring the ICUR around $100,000 per QALY while an 80% price reduction would be required to bring the ICUR around $50,000 per QALY”.[51]
4.2. Cost per QALY Thresholds for Decision Making in Comparator Countries
5. Conclusions—Recommendations for CADTH
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marchildon, G.P.; Allin, S.; Merkur, S. Canada: Health System Review; World Health Organization, Regional Office for Europe: Copenhagen, Denmark; Copenhagen PP: Copenhagen, Denmark; Volume 22, ISBN 1817-6119.
- Leeson, H. Constitutional Jurisdiction over Health and Health Care Services in Canada. In The Governance of Health Care in Canada; University of Toronto Press: Toronto, ON, Canada, 2004; pp. 50–82. ISBN 144268139X. [Google Scholar]
- Canada Health Act. Available online: https://laws-lois.justice.gc.ca/eng/acts/c-6/page-1.html (accessed on 23 January 2022).
- Government of Canada. The Constitution Acts, 1867 to 1982. Available online: https://laws-lois.justice.gc.ca/eng/const/page-3.html#h-17 (accessed on 26 January 2022).
- CADTH. Reimbursement Review Reports. Available online: https://www.cadth.ca/reimbursement-review-reports (accessed on 23 January 2022).
- Strategy Institute Presents. 20th Annual Market Access Virtual Summit. Available online: https://www.marketaccesscanada.ca/wp-content/uploads/2021/09/MA_Brochure_2021_V4.pdf (accessed on 26 January 2022).
- Strategy Institute. Home Page. Available online: https://www.strategyinstitute.com/ (accessed on 26 January 2022).
- Canada.ca. Notice of Compliance—Drug Products. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/notice-compliance.html (accessed on 27 January 2022).
- Canada.ca. Guidance Document: Notice of Compliance with Conditions (NOC/c). Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/guidance-documents/notice-compliance-conditions.html (accessed on 10 September 2020).
- Canada.ca. Guidance for Industry—Priority Review of Drug Submissions. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/guidance-documents/priority-review/drug-submissions.html (accessed on 10 September 2020).
- Canada.ca. Agile Licensing for Drugs: Regulatory Innovation for Health Products. Available online: https://www.canada.ca/en/health-canada/corporate/about-health-canada/activities-responsibilities/strategies-initiatives/health-products-food-regulatory-modernization/agile-licensing-drugs.html (accessed on 24 January 2022).
- Canada.ca. Project Orbis: Faster Access to Promising Cancer Treatments. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/international-activities/project-orbis.html (accessed on 24 January 2022).
- Compendium of Policies, Guidelines and Procedures—Updated February 2017. Available online: http://www.pmprb-cepmb.gc.ca/view.asp?ccid=492 (accessed on 27 January 2022).
- Government of Canada. Moving Forward on Implementing National Pharmacare. Available online: https://www.budget.gc.ca/2019/docs/themes/pharmacare-assurance-medicaments-en.html (accessed on 27 January 2022).
- CADTH. About CADTH. Available online: https://www.cadth.ca/about-cadth (accessed on 27 January 2022).
- CADTH. CADTH Reimbursement Reviews. Available online: https://www.cadth.ca/cadth-reimbursement-reviews (accessed on 27 January 2022).
- PCPA. The Negotiation Process. Available online: https://www.pcpacanada.ca/negotiation-process (accessed on 27 January 2022).
- Pan-Canadian Pharmaceutical Alliance PCPA Brand Process Guidelines. Available online: http://www.canadaspremiers.ca/wp-content/uploads/2018/11/pCPA_Brand_Process_Guidelines.pdf (accessed on 10 September 2020).
- Chen, Y. Health Technology Assessment and Economic Evaluation: Is It Applicable for the Traditional Medicine? Integr. Med. Res. 2022, 11, 100756. [Google Scholar] [CrossRef] [PubMed]
- Fanshel, S.; Bush, J.W. A Health-Status Index and Its Application to Health-Services Outcomes. Oper. Res. 1970, 18, 1021–1066. [Google Scholar] [CrossRef]
- Torrance, G.W.; Thomas, W.H.; Sackett, D.L. A Utility Maximization Model for Evaluation of Health Care Programs. Health Serv. Res. 1972, 7, 118–133. [Google Scholar] [PubMed]
- Weinstein, M.C.; Stason, W.B. Foundations of Cost-Effectiveness Analysis for Health and Medical Practices. N. Engl. J. Med. 1977, 296, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.C.; Torrance, G.; McGuire, A. QALYs: The Basics. Value Health 2009, 12, S5–S9. [Google Scholar] [CrossRef] [PubMed]
- Prieto, L.; Sacristán, J.A. Problems and Solutions in Calculating Quality-Adjusted Life Years (QALYs). Health Qual. Life Outcomes 2003, 1, 80. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, S.J.; Ali, S. Health Outcomes in Economic Evaluation: The QALY and Utilities. Br. Med. Bull. 2010, 96, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Devlin, N.J.; Lorgelly, P.K. QALYs as a Measure of Value in Cancer. J. Cancer Policy 2017, 11, 19–25. [Google Scholar] [CrossRef]
- Nord, E. Cost-Value Analysis in Health Care: Making Sense out of QALYs; Cambridge University Press: Cambridge, UK, 1999; ISBN 9780511609145. [Google Scholar]
- Duru, G.; Auray, J.P.; Béresniak, A.; Lamure, M.; Paine, A.; Nicoloyannis, N. Limitations of the Methods Used for Calculating Quality-Adjusted Life-Year Values. Pharmacoeconomics 2002, 20, 463–473. [Google Scholar] [CrossRef] [PubMed]
- The Conference Board of Canada. A Tool or a Rule? The Use of HTA in Drug Pricing in Canada—Policy Roundtable Highlights; The Conference Board of Canada: Ottawa, ON, Canada, 2019. [Google Scholar]
- Kernick, D.P. Introduction to Health Economics for the Medical Practitioner. Postgrad. Med. J. 2003, 79, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Canadian Agency for Drugs and Technologies in Health. Guidelines for the Economic Evaluation of Health Technologies: Canada, 4th ed.; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2017. [Google Scholar]
- Weinstein, M.C. A QALY Is a QALY Is a QALY—Or Is It? J. Health Econ. 1988, 7, 289–290. [Google Scholar] [CrossRef]
- YHEC—York Health Economics Consortium. Incremental Cost-Effectiveness Ratio (ICER). Available online: https://yhec.co.uk/glossary/incremental-cost-effectiveness-ratio-icer/ (accessed on 25 January 2022).
- Rawlins, M. Chapter 5—Health Technology Assessment. In Clinical Pharmacology, 11th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2012; ISBN 9780702040849. [Google Scholar]
- Pearson, S.D. The ICER Value Framework: Integrating Cost Effectiveness and Affordability in the Assessment of Health Care Value. Value Health 2018, 21, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, A.; Chabot, I.; Glennie, J. Evolution of Health Technology Assessment: Best Practices of the Pan-Canadian Oncology Drug Review. ClinicoEcon. Outcomes Res. 2015, 7, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Government of Saskatchewan. Provinces Introduce National Interim Process to Review and Evaluate Cancer Drugs. Available online: https://www.saskatchewan.ca/government/news-and-media/2007/february/22/provinces-introduce-national-interim-process-to-review-and-evaluate-cancer-drugs (accessed on 27 January 2022).
- Parliament of Canada Testimony: House of Commons Standing Committee on Health. 30 April 2007. Available online: https://www.ourcommons.ca/DocumentViewer/en/39-1/HESA/meeting-51/evidence (accessed on 27 January 2022).
- PDCI Market Access. Effective April 1 2014: PCODR Is Transferring to CADTH. Available online: https://www.pdci.ca/effective-april-1-2014-pcodr-is-transferring-to-cadth/ (accessed on 27 January 2022).
- PCODR Expert Review Committee Deliberative Framework. Available online: https://cadth.ca/sites/default/files/pcodr/The pCODR Expert Review Committee %28pERC%29/pcodr_perc_deliberative_frame.pdf (accessed on 25 May 2021).
- CADTH. CADTH Announces Aligned Drug Reimbursement Review Process. Available online: https://www.cadth.ca/news/cadth-announces-aligned-drug-reimbursement-review-process (accessed on 27 January 2022).
- Griffiths, E.A.; Vadlamudi, N.K. CADTH’s $50,000 Cost-Effectiveness Threshold: Fact or Fiction? Value Health 2016, 19, A488–A489. [Google Scholar] [CrossRef]
- Skedgel, C.; Wranik, D.; Hu, M. The Relative Importance of Clinical, Economic, Patient Values and Feasibility Criteria in Cancer Drug Reimbursement in Canada: A Revealed Preferences Analysis of Recommendations of the Pan-Canadian Oncology Drug Review 2011–2017. Pharmacoeconomics 2018, 36, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.R.; Mai, H.; Trudeau, M.E.; Mittmann, N.; Chiasson, K.; Chan, K.K.W.; Cheung, M.C. Reimbursement Recommendations for Cancer Drugs Supported by Phase II Evidence in Canada. Curr. Oncol. 2020, 27, e495–e500. [Google Scholar] [CrossRef] [PubMed]
- CADTH. CADTH Pharmaceutical Reviews Update—Issue 16. Available online: https://www.cadth.ca/cadth-pharmaceutical-reviews-update-issue-16 (accessed on 27 January 2022).
- Innovative Medicines Canada. Available online: http://innovativemedicines.ca/resource/explaining-public-reimbursement-delays-new-medicines-canadian-patients/ (accessed on 27 January 2022).
- Enzalutamide (Xtandi) MCSPC—PERC Final Recommendation. Available online: https://www.cadth.ca/enzalutamide-xtandi-metastatic-castration-sensitive-prostate-cancer-details (accessed on 27 January 2022).
- CADTH Reimbursement Recommendation—Venetoclax (Venclexta). Available online: https://cadth.ca/sites/default/files/DRR/2021/PC0238 Venclexta (AZA)—Final Rec.pdf (accessed on 27 January 2022).
- CADTH Reimbursement Recommendation—Larotrectinib (Vitrakvi). Available online: https://www.cadth.ca/sites/default/files/DRR/2021/PC0221 Vitrakvi—CADTH Final Rec KG_NA_Corrected-meta.pdf (accessed on 27 January 2022).
- CADTH Reimbursement Recommendation—Enfortumab Vedotin (Padcev). Available online: https://www.cadth.ca/sites/default/files/DRR/2022/PC0251REC-Padcev Final-meta.pdf (accessed on 27 January 2022).
- Cemiplimab (Libtayo) CSCC—PERC Final Recommendation. Available online: https://www.cadth.ca/sites/default/files/pcodr/Reviews2020/10187CemiplimabCSCC_fnRec_REDACT_EarlyConv_22Jan2020_final.pdf (accessed on 27 January 2022).
- Institute for Clinical and Economic Review 2020–2023 Value Assessment Framework. Available online: https://icer-review.org/wp-content/uploads/2019/05/ICER_2020_2023_VAF_013120-4.pdf (accessed on 27 January 2022).
- Schurer, M.; Matthijsse, S.M.; Vossen, C.Y.; van Keep, M.; Horscroft, J.; Chapman, A.-M.; Akehurst, R.L. Varying Willingness to Pay Based on Severity of Illness: Impact on Health Technology Assessment Outcomes of Inpatient and Outpatient Drug Therapies in The Netherlands. Value Health 2022, 25, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Tandvårds-Och Läkemedelsförmånsverket. Slutrapport—Ska TLV Genomföra Hälsoekonomiska Bedömningar Av Läkemedel Inom Slutenvården; Tandvårds-Och Läkemedelsförmånsverket: Stockholm, Sweden, 2009. [Google Scholar]
- Svensson, M.; Nilsson, F.O.L.; Arnberg, K. Reimbursement Decisions for Pharmaceuticals in Sweden: The Impact of Disease Severity and Cost Effectiveness. Pharmacoeconomics 2015, 33, 1229–1236. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binder, L.; Ghadban, M.; Sit, C.; Barnard, K. Health Technology Assessment Process for Oncology Drugs: Impact of CADTH Changes on Public Payer Reimbursement Recommendations. Curr. Oncol. 2022, 29, 1514-1526. https://doi.org/10.3390/curroncol29030127
Binder L, Ghadban M, Sit C, Barnard K. Health Technology Assessment Process for Oncology Drugs: Impact of CADTH Changes on Public Payer Reimbursement Recommendations. Current Oncology. 2022; 29(3):1514-1526. https://doi.org/10.3390/curroncol29030127
Chicago/Turabian StyleBinder, Louise, Majd Ghadban, Christina Sit, and Kathleen Barnard. 2022. "Health Technology Assessment Process for Oncology Drugs: Impact of CADTH Changes on Public Payer Reimbursement Recommendations" Current Oncology 29, no. 3: 1514-1526. https://doi.org/10.3390/curroncol29030127
APA StyleBinder, L., Ghadban, M., Sit, C., & Barnard, K. (2022). Health Technology Assessment Process for Oncology Drugs: Impact of CADTH Changes on Public Payer Reimbursement Recommendations. Current Oncology, 29(3), 1514-1526. https://doi.org/10.3390/curroncol29030127



