The Serious Illness Care Program in Oncology: Evidence, Real-World Implementation and Ongoing Barriers
Abstract
:1. Why Are Serious Illness Conversations Needed?
2. What Are the Components of the Serious Illness Care Program?
- Set up the conversation
- Assess understanding and preferences
- Share prognosis
- Explore key topics (i.e., goals, fears and worries, sources of strength)
- Close the conversation
- Document your conversation
- Communicate with key clinicians
- Population identification
- Training and coaching program
- Triggering
- Pre-visit letter
- Serious illness conversation guide
- Electronic medical record module documentation
- Family guide
- Implementation roadmap and system change resources
3. Evidence for the Serious Illness Care Program in Oncology
4. Examples of Real-World Implementation of SICP in the Oncology Setting
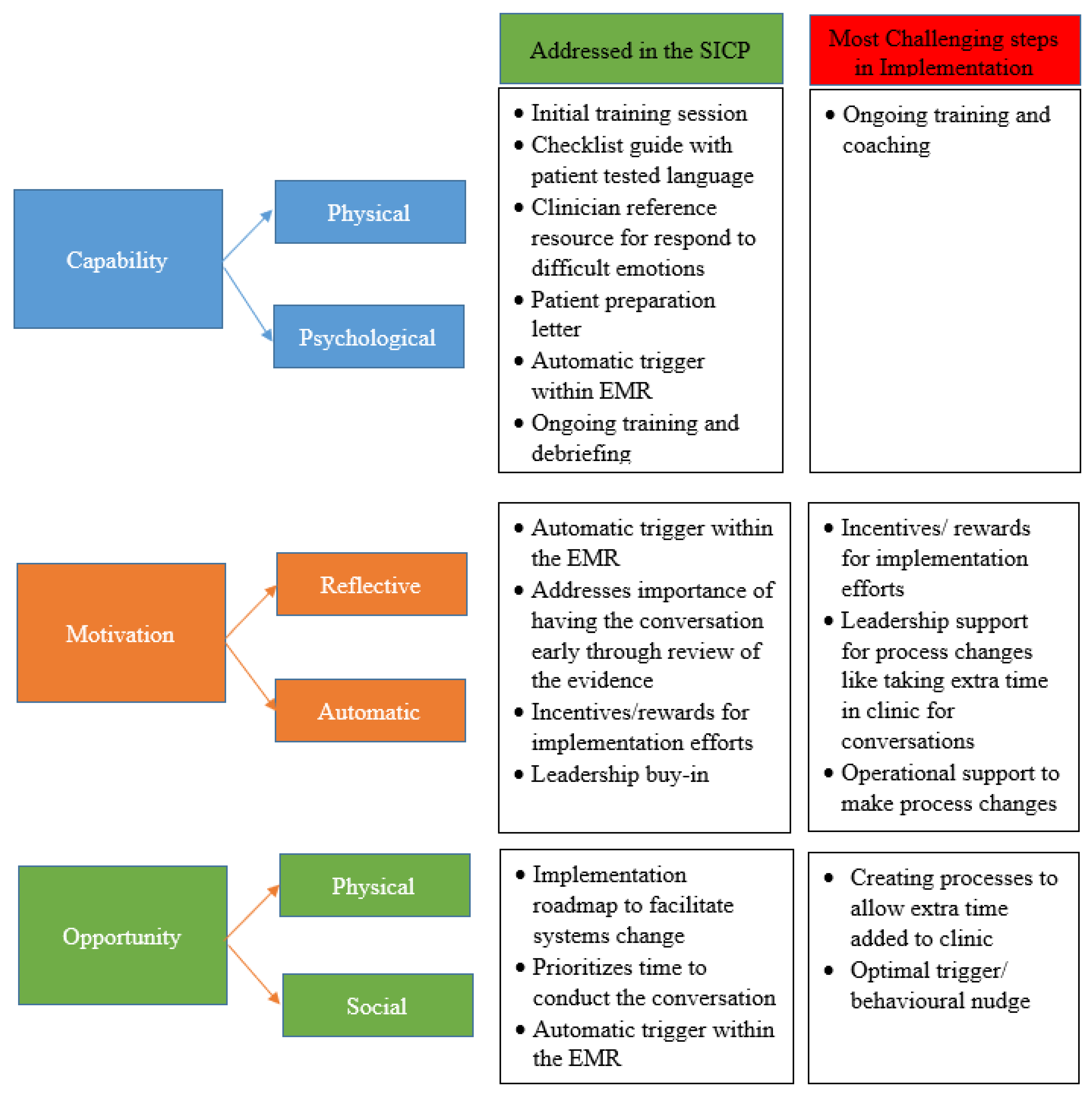
5. Challenges and Barriers to Real-World Implementation
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhi, W.I.; Smith, T.J. Early integration of palliative care into oncology: Evidence, challenges and barriers. Ann. Palliat Med. 2015, 4, 122–131. [Google Scholar] [PubMed]
- Temel, J.S.; Greer, J.A.; Muzikansky, A.; Gallagher, E.R.; Admane, S.; Jackson, V.A.; Dahlin, C.M.; Blinderman, C.D.; Jacobsen, J.; Pirl, W.F.; et al. Early Palliative Care for Patients with Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2010, 363, 733–742. [Google Scholar] [CrossRef] [Green Version]
- Temel, J.S.; Greer, J.A.; El-Jawahri, A.; Pirl, W.F.; Park, E.R.; Jackson, V.A.; Back, A.L.; Kamdar, M.; Jacobsen, J.; Chittenden, E.H.; et al. Effects of Early Integrated Palliative Care in Patients with Lung and GI Cancer: A Randomized Clinical Trial. J. Clin. Oncol. 2017, 35, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Bakitas, M.; Lyons, K.D.; Hegel, M.T.; Balan, S.; Brokaw, F.C.; Seville, J.; Hull, J.G.; Li, Z.; Tosteson, T.D.; Byock, I.R.; et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: The Project ENABLE II randomized controlled trial. JAMA 2009, 302, 741–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakitas, M.A.; Tosteson, T.D.; Li, Z.; Lyons, K.; Hull, J.G.; Li, Z.; Dionne-Odom, J.N.; Frost, J.; Dragnev, K.H.; Hegel, M.T.; et al. Early Versus Delayed Initiation of Concurrent Palliative Oncology Care: Patient Outcomes in the ENABLE III Randomized Controlled Trial. J. Clin. Oncol. 2015, 33, 1438–1445. [Google Scholar] [CrossRef]
- Zimmermann, C.; Swami, N.; Krzyzanowska, M.; Hannon, B.; Leighl, N.; Oza, A.; Moore, M.; Rydall, A.; Rodin, G.; Tannock, I.; et al. Early palliative care for patients with advanced cancer: A cluster-randomised controlled trial. Lancet 2014, 383, 1721–1730. [Google Scholar] [CrossRef]
- Touzel, M.; Shadd, J. Content Validity of a Conceptual Model of a Palliative Approach. J. Palliat. Med. 2018, 21, 1627–1635. [Google Scholar] [CrossRef]
- Kelley, A.S. Defining “serious illness”. J. Palliat. Med. 2014, 17, 985. [Google Scholar] [CrossRef]
- Mack, J.W.; Weeks, J.C.; Wright, A.A.; Block, S.D.; Prigerson, H.G. End-of-Life Discussions, Goal Attainment, and Distress at the End of Life: Predictors and Outcomes of Receipt of Care Consistent with Preferences. J. Clin. Oncol. 2010, 28, 1203–1208. [Google Scholar] [CrossRef] [Green Version]
- Wright, A.A.; Zhang, B.; Ray, A.; Mack, J.W.; Trice, E.; Balboni, T.; Prigerson, H.G. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 2008, 300, 1665–1673. [Google Scholar] [CrossRef] [Green Version]
- Detering, K.M.; Hancock, A.D.; Reade, M.; Silvester, W. The impact of advance care planning on end of life care in elderly patients: Randomised controlled trial. BMJ 2010, 340, c1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Wright, A.A.; Huskamp, H.A.; Nilsson, M.E.; Maciejewski, M.L.; Earle, C.C.; Prigerson, H.G. Health care costs in the last week of life: Associations with end-of-life conversations. Arch. Intern. Med. 2009, 169, 480–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mack, J.W.; Cronin, A.; Taback, N.; Huskamp, H.A.; Keating, N.L.; Malin, J.L.; Weeks, J.C. End-of-life care discussions among patients with advanced cancer: A cohort study. Ann. Intern. Med. 2012, 156, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Heyland, D.K.; Allan, E.D.; Rocker, G.; Dodek, P.; Pichora, D.; Gafni, A.; Canadian Researchers at the End-of-Life Network (CARENET). Discussing prognosis with patients and their families near the end of life: Impact on satisfaction with end-of-life care. Open Med. 2009, 3, e101–e110. [Google Scholar]
- Bernacki, R.E.; Block, S.D. American College of Physicians High Value Care Task F. Communication about serious illness care goals: A review and synthesis of best practices. JAMA Intern. Med. 2014, 174, 1994–2003. [Google Scholar] [CrossRef]
- Bernacki, R.; Hutchings, M.; Vick, J.; Smith, G.; Paladino, J.; Lipsitz, S.; Block, S.D. Development of the Serious Illness Care Program: A randomised controlled trial of a palliative care communication intervention. BMJ Open 2015, 5, e009032. [Google Scholar] [CrossRef] [Green Version]
- Paladino, J.; Brannen, E.; Benotti, E.; Henrich, N.; Ritchie, C.; Sanders, J.; Lakin, J.R. Implementing Serious Illness Communication Processes in Primary Care: A Qualitative Study. Am. J. Hosp. Palliat. Med. 2021, 38, 459–466. [Google Scholar] [CrossRef]
- Lakin, J.R.; Benotti, E.; Paladino, J.; Henrich, N.; Sanders, J. Interprofessional Work in Serious Illness Communication in Primary Care: A Qualitative Study. J. Palliat. Med. 2019, 22, 751–763. [Google Scholar] [CrossRef]
- Bernacki, R.; Paladino, J.; Neville, B.A.; Hutchings, M.; Kavanagh, J.; Geerse, O.P.; Block, S.D. Effect of the Serious Illness Care Program in Outpatient Oncology: A Cluster Randomized Clinical Trial. JAMA Intern. Med. 2019, 179, 751–759. [Google Scholar] [CrossRef]
- Paladino, J.; Bernacki, R.; Neville, B.A.; Kavanagh, J.; Miranda, S.P.; Palmor, M.; Block, S.D. Evaluating an Intervention to Improve Communication Between Oncology Clinicians and Patients with Life-Limiting Cancer: A Cluster Randomized Clinical Trial of the Serious Illness Care Program. JAMA Oncol. 2019, 5, 801–809. [Google Scholar] [CrossRef]
- Geerse, O.P.; Lamas, D.J.; Bernacki, R.E.; Sanders, J.J.; Paladino, J.; Berendsen, A.J.; Hiltermann, T.J.; Lindvall, C.; Fromme, E.K.; Block, S.D. Adherence and Concordance between Serious Illness Care Planning Conversations and Oncology Clinician Documentation among Patients with Advanced Cancer. J. Palliat. Med. 2021, 24, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Geerse, O.; Lamas, D.J.; Sanders, J.J.; Paladino, J.; Kavanagh, J.; Henrich, N.J.; Berendsen, A.J.; Hiltermann, T.J.; Fromme, E.K.; Bernacki, R.E.; et al. A Qualitative Study of Serious Illness Conversations in Patients with Advanced Cancer. J. Palliat. Med. 2019, 22, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.; Lupichuk, S.; Tan, A.; Sinnarajah, A.; Simon, J. Real World Implementation of the Serious Illness Care Program in Cancer Care: Results of a Quality Improvement Initiative. J. Palliat. Med. 2021, 24, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Wixon-Genack, J.; Kavanagh, J.; Sanders, J.J.; Paladino, J.; O’Connor, N.R. Serious Illness Conversations with Outpatient Oncology Clinicians: Understanding the Patient Experience. JCO Oncol. Pr. 2020, 16, e1507–e1515. [Google Scholar] [CrossRef]
- Lagrotteria, A.; Swinton, M.; Simon, J.; King, S.; Boryski, G.; Ma, I.W.Y.; Dunne, F.; Singh, J.; Bernacki, R.E.; You, J.J. Clinicians’ Perspectives After Implementation of the Serious Illness Care Program: A Qualitative Study. JAMA Netw. Open 2021, 4, e2121517. [Google Scholar] [CrossRef]
- Ma, C.; Riehm, L.E.; Bernacki, R.; Paladino, J.; You, J.J. Quality of clinicians’ conversations with patients and families before and after implementation of the Serious Illness Care Program in a hospital setting: A retrospective chart review study. CMAJ Open 2020, 8, E448–E454. [Google Scholar] [CrossRef]
- Ariadne Labs. Serious Illness Care. Available online: https://www.ariadnelabs.org/serious-illness-care/ (accessed on 28 August 2021).
- Paladino, J.; Koritsanszky, L.; Neal, B.J.; Lakin, J.R.; Kavanagh, J.; Lipsitz, S.; Fromme, E.K.; Sanders, J.; Benjamin, E.; Block, S.; et al. Effect of the Serious Illness Care Program on Health Care Utilization at the End of Life for Patients with Cancer. J. Palliat. Med. 2020, 23, 1365–1369. [Google Scholar] [CrossRef]
- Earle, C.C.; Landrum, M.B.; Souza, J.M.; Neville, B.A.; Weeks, J.C.; Ayanian, J.Z. Agressiveness of cancer near the end of the life: Is it a quality care issue. J. Clin. Oncol. 2008, 26, 3860–3866. [Google Scholar] [CrossRef]
- Lakin, J.R.; Neal, B.J.; Maloney, F.L.; Paladino, J.; Vogeli, C.; Tumblin, J.; Vienneau, M.; Fromme, E.; Cunningham, R.; Block, S.D.; et al. A systematic intervention to improve serious illness communication in primary care: Effect on expenses at the end of life. Healthcare 2020, 8, 100431. [Google Scholar] [CrossRef]
- Brinkman-Stoppelenburg, A.; Rietjens, J.A.; Van Der Heide, A. The effects of advance care planning on end-of-life care: A systematic review. Palliat. Med. 2014, 28, 1000–1025. [Google Scholar] [CrossRef]
- Michie, S.; Van Stralen, M.M.; West, R. The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implement. Sci. 2011, 6, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manz, C.R.; Chen, J.; Liu, M.; Chivers, C.; Regli, S.H.; Braun, J.; Draugelis, M.; Hanson, C.W.; Shulman, L.N.; Schuchter, L.M.; et al. Validation of a Machine Learning Algorithm to Predict 180-Day Mortality for Outpatients with Cancer. JAMA Oncol. 2020, 6, 1723. [Google Scholar] [CrossRef] [PubMed]
- Bertsimas, D.; Dunn, J.; Pawlowski, C.; Silberholz, J.; Weinstein, A.; Zhuo, Y.D.; Chen, E.; ElFiky, A.A. Applied Informatics Decision Support Tool for Mortality Predictions in Patients With Cancer. JCO Clin. Cancer Inform. 2018, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.B.; Kakad, M.; Bates, D.W. Integrating predictive analytics into high-value care: The dawn of precision delivery. JAMA 2016, 315, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Manz, C.R.; Parikh, R.B.; Small, D.S.; Evans, C.N.; Chivers, C.; Regli, S.H.; Patel, M.S. Effect of integrating machine learning mortality estimates with behavioral nudges to clinicians on serious illness conversations among patients with cancer: A stepped-wedge cluster randomized clinical trial. JAMA Oncol. 2020, 6, e204759. [Google Scholar] [CrossRef]
- Beddard-Huber, E.; Gaspard, G.; Yue, K. Adaptations to the Serious Illness Conversation Guide to Be More Culturally Safe. Int. J. Indig. Health 2021, 16, 38–53. [Google Scholar] [CrossRef]
- BC Center for Palliative Care. Implementation of the Serious Illness Conversation Program: Lessons Learned from a Canadian Health System. Available online: https://bc-cpc.ca/wp-content/uploads/2021/05/BCCPC-Implementation-of-the-Serious-Illness-Conversation-Program.pdf. (accessed on 22 January 2022).
- Powell, D.E.; Carraccio, C. Toward Competency-Based Medical Education. N. Engl. J. Med. 2018, 378, 3–5. [Google Scholar] [CrossRef]
- Ariadne Labs. Driving Equity in Serious Illness Care: A Convening. Available online: https://www.ariadnelabs.org/wp-content/uploads/2021/12/Driving-Equity-in-Serious-Illness-Care-_-2021-Convening-Report-FINAL.pdf (accessed on 5 December 2021).
| Author(s) | Type of Analysis | Participants/Sample | Primary Outcome (s) | Secondary Outcome (s) | Results |
|---|---|---|---|---|---|
| Bernacki et al. [22] | Primary analysis | n = 278 patients (134 intervention, 144 control) n = 91 clinicians (48 intervention, 43 control) | Goal concordant care and peacefulness at the end of life | Therapeutic alliance, anxiety, depression, survival |
|
| Paladino et al. [23] | Secondary analysis | n = 278 patients n = 91 clinicians | N/A | Documentation of at least 1 serious illness conversation before death, timing of the initial conversation before death, quality of the conversations, accessibility in the EMR |
|
| Author(s) | Type of Analysis | Participants/Sample | Primary Outcome (s) | Secondary Outcome (s) | Results |
| Geerse et al. [24] | Secondary analysis | 25 Audio recordings of 16 clinicians who conducted the serious illness conversation | Concordance between written documentation and recorded audiotape conversations, adherence to the Serious Illness Conversation Guide questions | N/A |
|
| Geerse et al. [25] | Secondary analysis | 25 audio recorded serious illness conversations | Qualitative analysis to describe content of the conversation | N/A |
|
| Paladino et al. [26] | Secondary analysis | n = 157 patients who died within 2 years of enrollment of the study (74 intervention, 83 control) | Mean number of aggressive indicators using National Quality Forum-endorsed indicators of aggressiveness at the end of life | Chemotherapy in last 14 days, ≥2 hospitalization or ED visits in last 30 days, ≥1 ICU stay in last 30 days, no hospice use or <3 days, death in acute care hospital |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karim, S.; Levine, O.; Simon, J. The Serious Illness Care Program in Oncology: Evidence, Real-World Implementation and Ongoing Barriers. Curr. Oncol. 2022, 29, 1527-1536. https://doi.org/10.3390/curroncol29030128
Karim S, Levine O, Simon J. The Serious Illness Care Program in Oncology: Evidence, Real-World Implementation and Ongoing Barriers. Current Oncology. 2022; 29(3):1527-1536. https://doi.org/10.3390/curroncol29030128
Chicago/Turabian StyleKarim, Safiya, Oren Levine, and Jessica Simon. 2022. "The Serious Illness Care Program in Oncology: Evidence, Real-World Implementation and Ongoing Barriers" Current Oncology 29, no. 3: 1527-1536. https://doi.org/10.3390/curroncol29030128
APA StyleKarim, S., Levine, O., & Simon, J. (2022). The Serious Illness Care Program in Oncology: Evidence, Real-World Implementation and Ongoing Barriers. Current Oncology, 29(3), 1527-1536. https://doi.org/10.3390/curroncol29030128





