Real-World Evidence of Systemic Therapy Sequencing on Overall Survival for Patients with Metastatic BRAF-Mutated Cutaneous Melanoma
Abstract
:1. Introduction
2. Methodology
Statistical Analysis
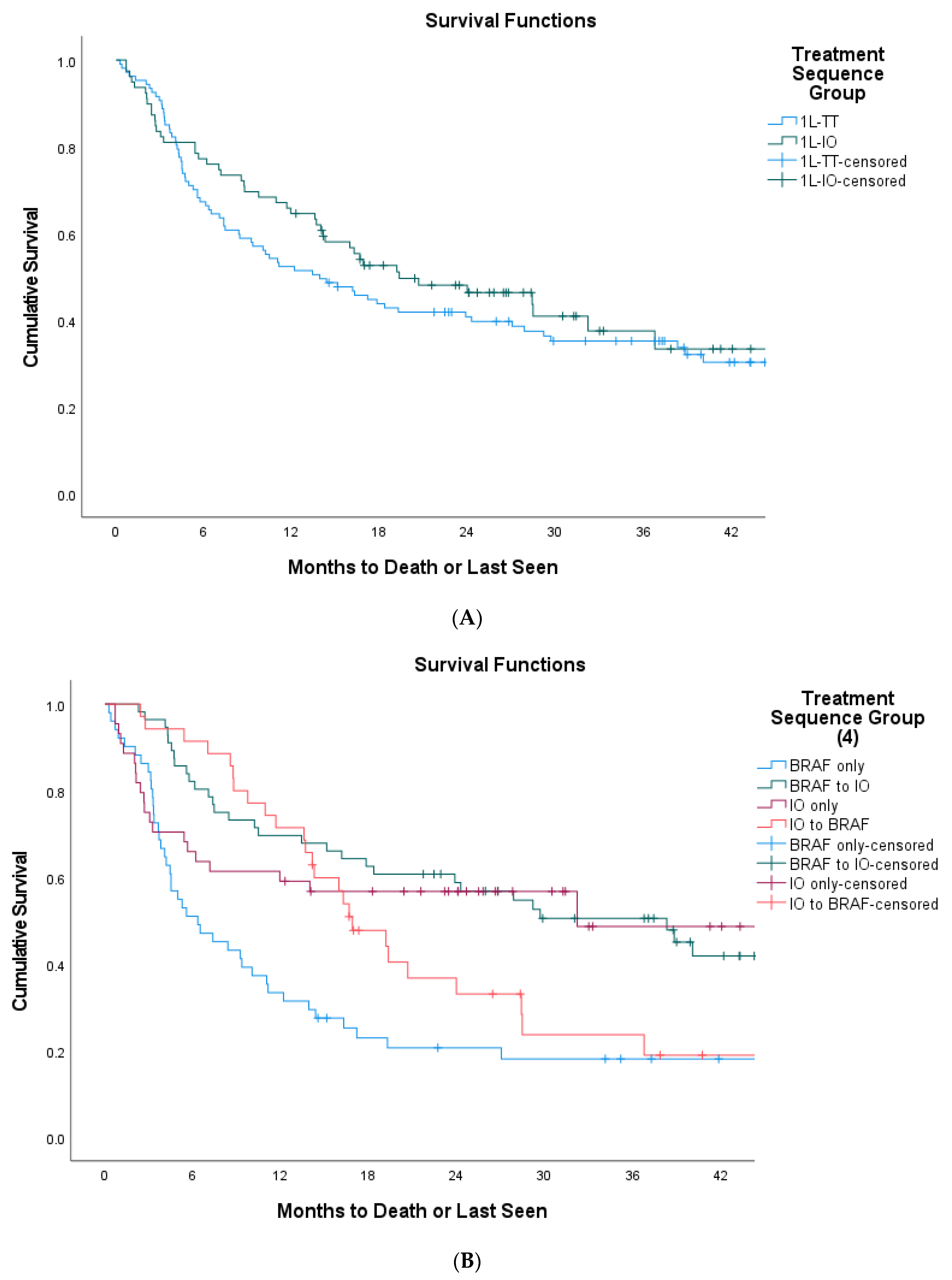
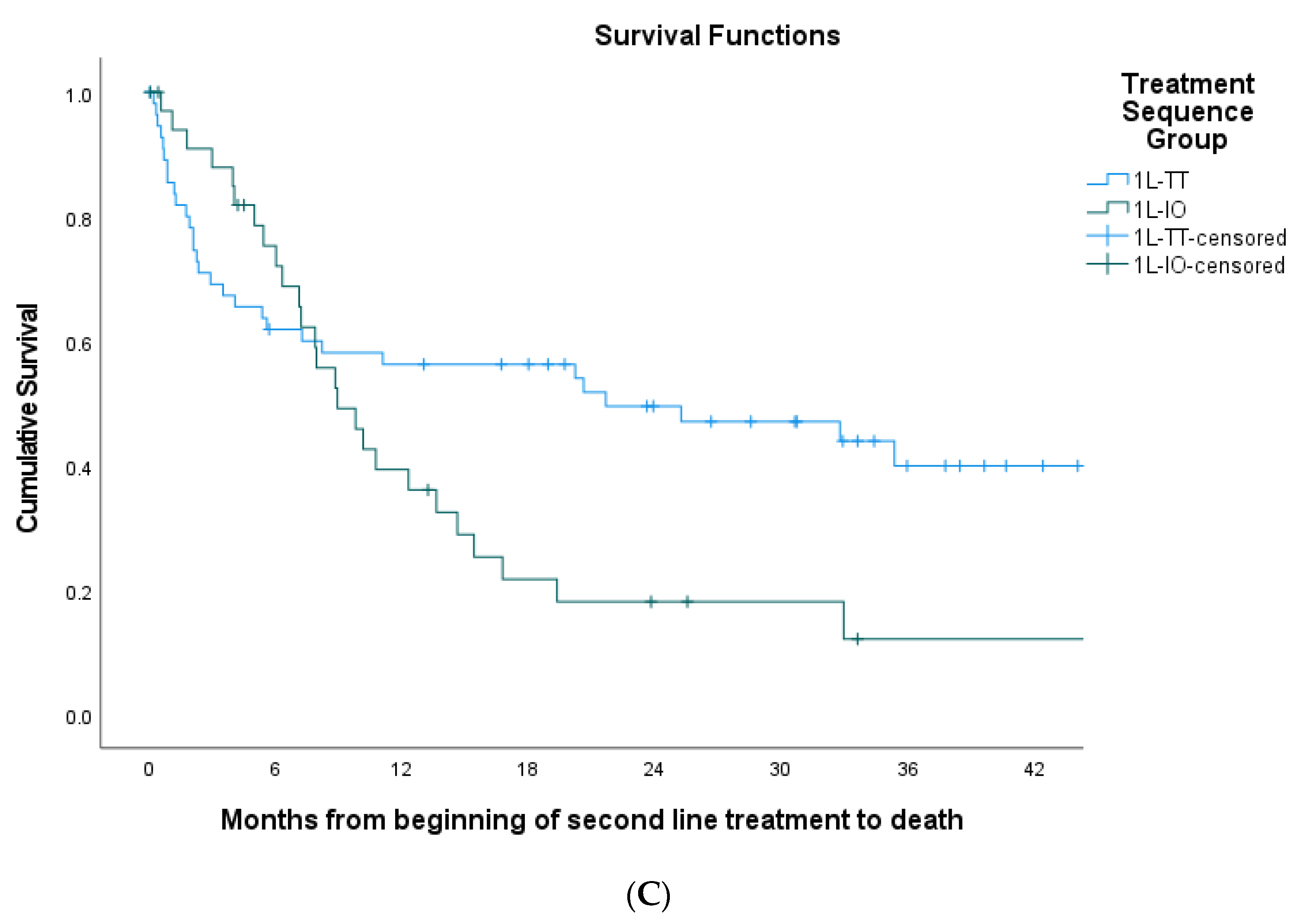
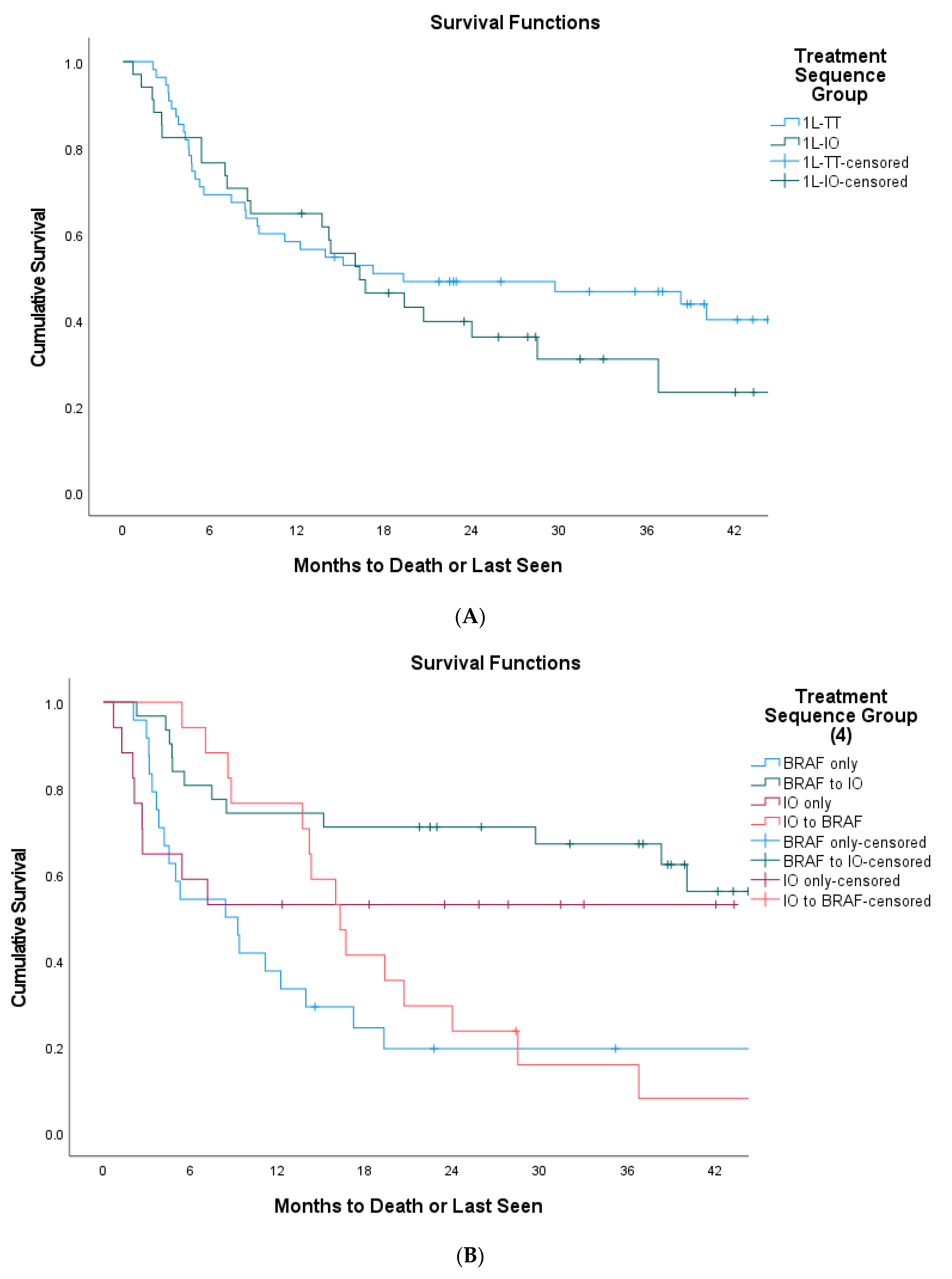
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Comprehensive Cancer Network. Cutaneous Melanoma (Version 3.2020). Available online: https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf (accessed on 6 July 2020).
- Seth, R.; Messersmith, H.; Kaur, V.; Kirkwood, J.M.; Kudchadkar, R.; McQuade, J.L.; Provenzano, A.; Swami, U.; Weber, J.; Alluri, K.C.; et al. Systemic Therapy for Melanoma: ASCO Guideline. J. Clin. Oncol. 2020, 38, 3947–3970. [Google Scholar] [CrossRef]
- Keilholz, U.; Ascierto, P.A.; Dummer, R.; Robert, C.; Lorigan, P.; van Akkooi, A.; Arance, A.; Blank, C.U.; Chiarion Sileni, V.; Donia, M.; et al. ESMO consensus conference recommendations on the management of metastatic melanoma: Under the auspices of the ESMO guidelines committee. Ann. Oncol. 2020, 31, 1435–1448. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion Sileni, V.; Schachter, J.; Garbe, C.; Bondarenko, I.; et al. Five-year outcomes with Dabrafenib plus Trametinib in metastatic melanoma. N. Eng. J. Med. 2019, 38, 626–636. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Dréno, B.; Larkin, J.; Ribas, A.; Liskay, G.; Maio, M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. 5-Year Outcomes with Cobimetinib plus Vemurafenib in BRAF V600 Mutation-Positive Advanced Melanoma: Extended Follow-Up of the coBRIM Study. Clin. Cancer Res. 2021, 27, 5225–5235. [Google Scholar] [CrossRef]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Encorafenib plus Binimetinib versus Vemurafenib or Encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): A multicenter, open-label, randomized phase 3 trial. Lancet Oncol. 2018, 19, 603–615. [Google Scholar] [CrossRef] [Green Version]
- Robert, C.; Ribas, A.; Schachter, J.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.M.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicenter, randomized, controlled, phase 3 study. Lancet Oncol. 2019, 20, 1239–1251. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Eng. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [Green Version]
- Ugurel, S.; Rohmel, J.; Ascierto, P.A.; Flaherty, K.T.; Grob, J.J.; Hauschild, A.; Larkin, J.; Long, G.V.; Lorigan, P.; McArthur, G.A.; et al. Survival of patients with advanced metastastic melanoma: The impact of novel therapies—Update 2017. Eur. J. Cancer 2017, 83, 247–257. [Google Scholar] [CrossRef] [Green Version]
- Nathan, P.; Dummer, P.; Long, G.V.; Ascierto, P.A.; Tawbi, H.A.; Robert, C.; Rutkowski, P.; Leonov, O.; Dutriaux, C.; Mandala, M.; et al. Spartalizumab plus dabrafenib and trametinib in patients with previously untreated BRAF V600-mutant unresectable or metastatic melanoma: Results from the randomized part 3 of the phase III COMBI-I trial. ESMO Virtual Congr. 2020, 31, S1172. [Google Scholar] [CrossRef]
- Ferrucci, P.F.; Di Giacomo, A.M.; Del Vecchio, M.; Atkinson, V.; Schmidt, H.; Schachter, J.; Queirolo, P.; Long, G.V.; Stephens, R.; Svane, I.M.; et al. Keynote-022 part 3: A randomized, double-blind, phase 2 study of pembrolizumab, dabrafenib, and trametinib in BRAF-mutant melanoma. J. Immunother. Cancer 2020, 8, e001806. [Google Scholar] [CrossRef]
- Gutzmer, R.; Stroyakovskiy, D.; Gogas, H.; Robert, C.; Lewis, K.; Protsenko, S.; Pereira, R.P.; Eigentler, T.; Rutkowski, P.; Demidov, L.; et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFv600 mutation-positive melanoma (IMspire150): Primary analysis of the randomized, double-blind, placebo-controlled, phase 3 trial. Lancet 2020, 395, 1835–1844. [Google Scholar] [CrossRef]
- Schummer, P.; Schilling, B.; Gesierich, A. Long-term outcomes in BRAF-mutated melanoma treated with combined targeted therapy or immune checkpoint blockade: Are we approaching a true cure? Am. J. Clin. Dermatol. 2020, 21, 493–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ascierto, P.A.; Mandala, M.; Ferrucci, P.F.; Rutkowski, P.; Guidoboni, M.; Arance Fernandez, A.M.; Ferraresi, V.; Maiello, E.; Guida, M.; Del Vecchio, M.; et al. LBA45 First report of efficacy and safety from the phase II study SECOMBIT (Sequential COMBo Immuno and Targeted therapy study). Ann. Oncol. 2020, 31 (Suppl. 4), S1173–S1174. [Google Scholar] [CrossRef]
- Moser, J.G.; Chen, D.; Hu-Lieskovan, S.; Grossmann, K.F.; Patel, S.; Colonna, S.V.; Ying, J.; Hyngstrom, J.R. Real-world survival of patients with advanced BRAF V600 mutated melanoma treated with front-line BRAF/MEK inhibitors, anti-PD-1 antibodies, or nivolumab/ipilimumab. Cancer Med. 2019, 8, 7637–7643. [Google Scholar] [CrossRef] [Green Version]
- Schilling, B.; Martens, A.; Geukes Foppen, M.H.; Gebhardt, C.; Hassel, J.C.; Rozeman, E.A.; Gesierich, A.; Gutzmer, R.; Kahler, K.C.; Livingstone, E.; et al. First-line therapy-stratified survival in BRAF-mutant melanoma: A retrospective multicenter analysis. Cancer Immunol. Immunother. 2019, 68, 765–772. [Google Scholar] [CrossRef]
- Czarnecka, A.M.; Teterycz, P.; Mariuk-Jarema, A.; Lugowska, I.; Rogala, P.; Dudzisz-Sledz, M.; Switaj, T.; Rutkowski, P. Treatment sequencing and clinical outcomes in BRAF-positive and BRAF-negative unresectable and metastatic melanoma patients treated with new systemic therapies in routine practice. Target. Oncol. 2019, 14, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Whitman, E.D.; Liu, F.X.; Cao, X.; Diede, S.J.; Haiderall, A.; Abernethy, A.P. Treatment patterns and outcomes for patients with advanced melanoma in US oncology clinical practices. Future Oncol. 2018, 15, 459–471. [Google Scholar] [CrossRef]
- Pavlick, A.C.; Zhao, R.; Lee, C.-H.; Ritchings, C.; Rao, S. First-line immunotherapy versus targeted therapy in patients with BRAF-mutant advanced melanoma: A real-world analysis. Future Oncol. 2021, 17, 689–699. [Google Scholar] [CrossRef]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Eng. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [Green Version]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gumus, M.; Mazieres, J.; Hermes, B.; Senler, F.C.; Csoszi, T.; Fulop, A.; et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N. Eng. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodriguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Manuel, D.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Eng. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.J.; Gonzalez, R.; Lewis, K.D.; Hamid, O.; Infante, J.R.; Patel, M.R.; Hodi, F.S.; Wallin, J.; Pitcher, B.; Cha, E.; et al. Atezolizumab (A) + cobimetinib (C) + vemurafenib (V) in BRAF-V600-mutant metastatic melanoma (mel): Updated safety and clinical activity. J. Clin. Oncol. 2017, 35, 3063. [Google Scholar] [CrossRef]
- Ribas, A.; Hodi, F.S.; Lawrence, D.; Atkinson, V.; Agarwal, S.; Carlino, M.S.; Fisher, R.; Long, G.V.; Miller, W.H.; Huang, Y.; et al. 1216OKEYNOTE-022 update: Phase 1 study of first-line pembrolizumab (pembro) plus dabrafenib (D) and trametinib (T) for BRAFmutant advanced melanoma. Ann. Oncol. 2017, 28, V428–V448. Available online: https://oncologypro.esmo.org/meeting-resources/esmo-2017-congress/KEYNOTE-022-update-phase-1-study-of-first-line-pembrolizumab-pembro-plus-dabrafenib-D-and-trametinib-T-for-BRAF-mutant-advanced-melanoma (accessed on 6 July 2020). [CrossRef]
- Ascierto, P.A.; Ferrucci, P.F.; Stephens, R.; Del Vecchio, M.; Atkinson, V.; Schmidt, H.; Schachter, J.; Queirolo, P.; Long, G.V.; Di Giacomo, A.M.; et al. KEYNOTE-022 Part 3: Phase 2 randomized study of 1L dabrafenib (D) and trametinib (T) plus pembrolizumab (pembro) or placebo (PBO) for BRAF-mutant advanced melanoma. Ann. Oncol. 2018, 29, 442–466. [Google Scholar] [CrossRef]
- Ribas, A.; Butler, M.; Lutzky, J.; Lawrence, D.P.; Robert, C.; Linette, W.M.P.; Ascierto, P.A.; Kuzel, T.; Algazi, A.P.; Postow, M.A.; et al. Phase I study combining anti-PD-L1 (MEDI4736) with BRAF (dabrafenib) and/or MEK (trametinib) inhibitors in advanced melanoma. J. Clin. Oncol. 2015, 33, 3003. [Google Scholar] [CrossRef]
- Amin, A.; Lawson, D.H.; Salama, A.K.; Koon, H.B.; Guthrie, T., Jr.; Thomas, S.S.; O’Day, S.J.; Shaheen, M.F.; Zhang, B.; Francis, S.; et al. Phase II study of vemurafenib followed by ipilimumab in patients with previously untreated BRAFmutated metastatic melanoma. J. Immunother. Cancer 2016, 4, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tawbi, H.A.H.; Amaria, R.N.; Glitza, I.C.; Milton, D.; Hwu, W.J.; Patel, S.P.; Wong, M.K.K.; Yee, C.; Woodman, S.E.; McQuade, J.L.; et al. Safety and preliminary activity data from a single center phase II study of triplet combination of nivolumab (N) with dabrafenib (D) and trametinib (T) [trident] in patients (Pts) with BRAF-mutated metastatic melanoma (MM). J. Clin. Oncol. 2018, 36, 9560. [Google Scholar] [CrossRef]
- Leichsenring, J.; Stögbauer, F.; Volckmar, A.-L.; Buchhalter, I.; Oliveira, C.; Kirchner, M.; Frohling, S.; Hassel, J.; Enk, A.; Schirmacher, P.; et al. Genetic profiling of melanoma in routine diagnostics: Assay performance and molecular characteristics in a consecutive series of 274 cases. Pathology 2018, 50, 703–710. [Google Scholar] [CrossRef] [Green Version]
- Heinzerling, L.; Kühnapfel, S.; Meckbach, D.; Baiter, M.; Kaempgen, E.; Keikavoussi, P.; Schuler, G.; Agaimy, A.; Bauer, J.; Hartmann, A.; et al. Rare BRAF mutations in melanoma patients: Implications for molecular testing in clinical practice. Br. J. Cancer 2013, 108, 2164–2171. [Google Scholar] [CrossRef] [Green Version]
- Menzer, C.; Menzies, A.M.; Carlino, M.S.; Reijers, I.; Groen, E.J.; Eigentler, T.; de Groot, J.W.B.; van der Veldt, A.A.M.; Johnson, D.B.; Meiss, F.; et al. Targeted therapy in advanced melanoma with rare BRAF mutations. J. Clin. Oncol. 2019, 37, 3142–3151. [Google Scholar] [CrossRef] [Green Version]

| Total | 1L-IO Group | 1L-TT Group | p-Value | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Age | ||||
| <65 | 93 (50) | 43 (54) | 50 (47) | 0.374 |
| ≥65 | 93 (50) | 36 (46) | 57 (53) | |
| Gender | ||||
| Male | 124 (67) | 55 (70) | 69 (54) | 0.53 |
| Female | 62 (33) | 24 (30) | 38 (36) | |
| ECOG | ||||
| 0–1 | 81 (44) | 40 (91) | 41 (72) | 0.023 |
| 2 and above | 20 (11) | 4 (9) | 16 (28) | |
| Missing | 85 (45) | - | - | |
| LDH | ||||
| ≥Median (280) | 72 (39) | 33 (52) | 39 (48) | 0.739 |
| <Median | 74 (40) | 31 (48) | 43 (52) | |
| Missing | 40 (21) | - | ||
| Cancer Stage | ||||
| Unresectable Stage III | 9 (5) | 4 (5) | 5 (5) | 1 |
| Metastatic | 177 (95) | 75 (95) | 102 (95) | |
| Number of Metastatic Sites | ||||
| >2 | 88 (47) | 42 (53) | 46 (43) | 0.184 |
| ≤2 | 98 (53) | 37 (47) | 61 (57) | |
| Baseline Brain Metastasis | ||||
| Yes | 46 (25) | 17 (22) | 29 (27) | 0.397 |
| No | 140 (75) | 62 (78) | 78 (73) | |
| BRAF-Mutant Types | ||||
| V600E/K | 89 (48) | 35 (44) | 54 (51) | 0.326 |
| V600D | 5 (3) | 1 (1) | 4 (4) | |
| Unknown Subtypes | 92 (49) | 44 (55) | 48 (45) | |
| Received Palliative RT | ||||
| Yes | 108 (58) | 46 (58) | 62 (58) | 1 |
| No | 78 (42) | 33 (42) | 45 (42) | |
| Received Palliative Surgery | ||||
| Yes | 11 (6) | 6 (8) | 5 (5) | 0.532 |
| No | 175 (94) | 73 (92) | 102 (95) | |
| First Line Regimen | ||||
| Anti-PD1 | 56 | 56 (71) | - | N/A |
| Anti-PD1 + CTLA-4 | 23 | 23 (29) | - | |
| BRAF + MEKi | 100 | - | 100 (93) | |
| BRAFi | 7 | - | 7 (7) |
| 1L-IO | 1L-TT | p-Value | |
|---|---|---|---|
| n (%) | n (%) | ||
| Received 2L Therapy | |||
| Yes | 35 (44) | 56 (52) | 0.302 |
| No | 44 (56) | 51 (48) | |
| Reason for 1L Treatment Discontinuation | |||
| Progression | 29 (37) | 50 (47) | N/A |
| Toxicity | 17 (21) | 13 (12) | |
| Treatment Completion | 18 (23) | N/A | |
| Unknown | 15 (19) | 43 (41) | |
| Progressed on 1L and Died before 2L Therapy | |||
| Yes | 13 (45) | 37 (74) | 0.015 |
| No | 16 (55) | 13 (26) | |
| 2L Therapy | |||
| Anti-PD1 | 0 (0) | 41 (73) | N/A |
| Anti-PD1 + Anti-CTLA4 | 0 (0) | 15 (27) | |
| BRAFi + MEKi | 35 (100) | 0 (0) | |
| Reason for 2L Treatment Discontinuation | |||
| Progression | 22 (62) | 29 (52) | N/A |
| Toxicity | 3 (9) | 4 (7) | |
| Treatment Completion | 7 (20) | N/A | |
| Unknown | 3 (9) | 23 (41) | |
| Time on 1L Therapy | |||
| Median (Months) | 5.3 | 4.2 | 0.791 |
| 25th and 75th Percentile (Months) | 1.4, 12.3 | 2.6, 7.6 | |
| Time on 2L Therapy | |||
| Median (Months) | 5.3 | 4.9 | 0.716 |
| 25th and 75th Percentile (Months) | 2.3, 10.1 | 0.88, 18.8 | |
| Time from 1L Therapy Initiation Date to 2L Therapy Initiation Date | |||
| Median (Months) | 5.5 | 6.2 | 0.338 |
| 25th and 75th Percentile (Months) | 1.9, 11.7 | 3.6, 11.9 | |
| Time from 2L therapy Initiation Date to Death or Last Follow Up | |||
| Median (Months) | 7.9 | 17.3 | 0.245 |
| 25th and 75th Percentile (Months) | 4.2, 14.6 | 2.1, 32.9 |
| Total Population (n = 186) | |||
|---|---|---|---|
| Overall Survival | |||
| HR | 95% CI | p-Value | |
| Number of Metastatic Sites > 2 | 2.195 | 1.302–3.699 | 0.003 |
| Baseline Brain Metastasis | 1.833 | 1.073–3.130 | 0.027 |
| Baseline ECOG ≥ 2 | 3.957 | 2.226–7.034 | <0.001 |
| Sequencing Group (1L-TT as Reference) | 0.838 | 0.502–1.400 | 0.500 |
| BRAF V600E/K Mutant Population Only (n = 89) | |||
| Overall Survival | |||
| HR | 95% CI | p-Value | |
| Number of Metastatic Sites > 2 | 1.812 | 0.884–3.710 | 0.104 |
| Baseline Brain Metastasis | 1.903 | 0.944–3.836 | 0.072 |
| Baseline ECOG ≥ 2 | 4.098 | 1.918–8.755 | <0.001 |
| Sequencing Group (1L-TT as Reference) | 0.777 | 0.412–1.467 | 0.437 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kartolo, A.; Deluce, J.; Hopman, W.M.; Liu, L.; Baetz, T.; Ernst, S.; Lenehan, J.G. Real-World Evidence of Systemic Therapy Sequencing on Overall Survival for Patients with Metastatic BRAF-Mutated Cutaneous Melanoma. Curr. Oncol. 2022, 29, 1501-1513. https://doi.org/10.3390/curroncol29030126
Kartolo A, Deluce J, Hopman WM, Liu L, Baetz T, Ernst S, Lenehan JG. Real-World Evidence of Systemic Therapy Sequencing on Overall Survival for Patients with Metastatic BRAF-Mutated Cutaneous Melanoma. Current Oncology. 2022; 29(3):1501-1513. https://doi.org/10.3390/curroncol29030126
Chicago/Turabian StyleKartolo, Adi, Jasna Deluce, Wilma M. Hopman, Linda Liu, Tara Baetz, Scott Ernst, and John G. Lenehan. 2022. "Real-World Evidence of Systemic Therapy Sequencing on Overall Survival for Patients with Metastatic BRAF-Mutated Cutaneous Melanoma" Current Oncology 29, no. 3: 1501-1513. https://doi.org/10.3390/curroncol29030126
APA StyleKartolo, A., Deluce, J., Hopman, W. M., Liu, L., Baetz, T., Ernst, S., & Lenehan, J. G. (2022). Real-World Evidence of Systemic Therapy Sequencing on Overall Survival for Patients with Metastatic BRAF-Mutated Cutaneous Melanoma. Current Oncology, 29(3), 1501-1513. https://doi.org/10.3390/curroncol29030126





