Is It Time to Commit to a Process to Re-Evaluate Oncology Drugs? A Descriptive Analysis of Systemic Therapies for Solid Tumour Indications Reviewed in Canada from 2017 to 2021
Abstract
:1. Introduction
2. Methods
3. Findings
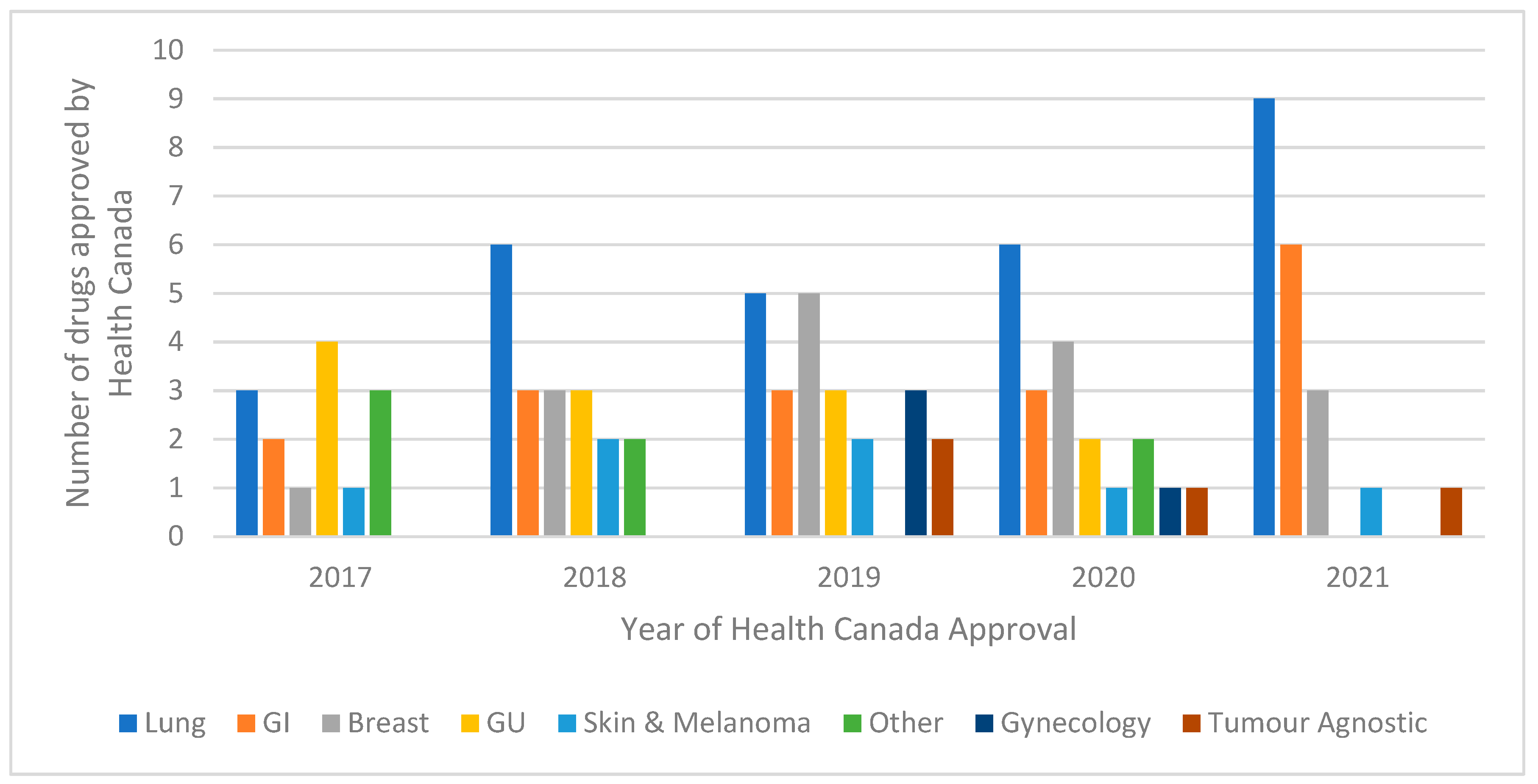
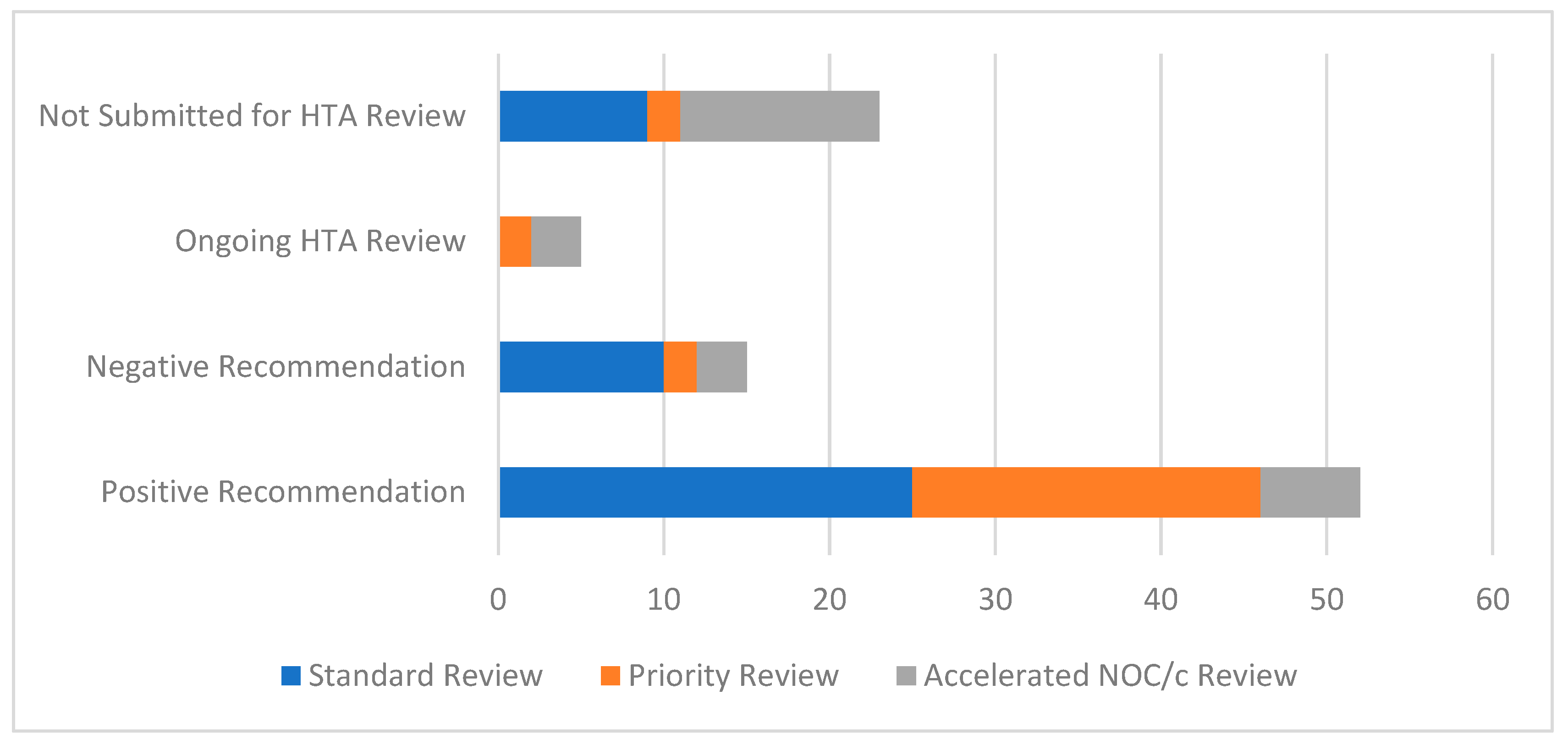
3.1. Overview of Systemic Therapies Approved by Health Canada
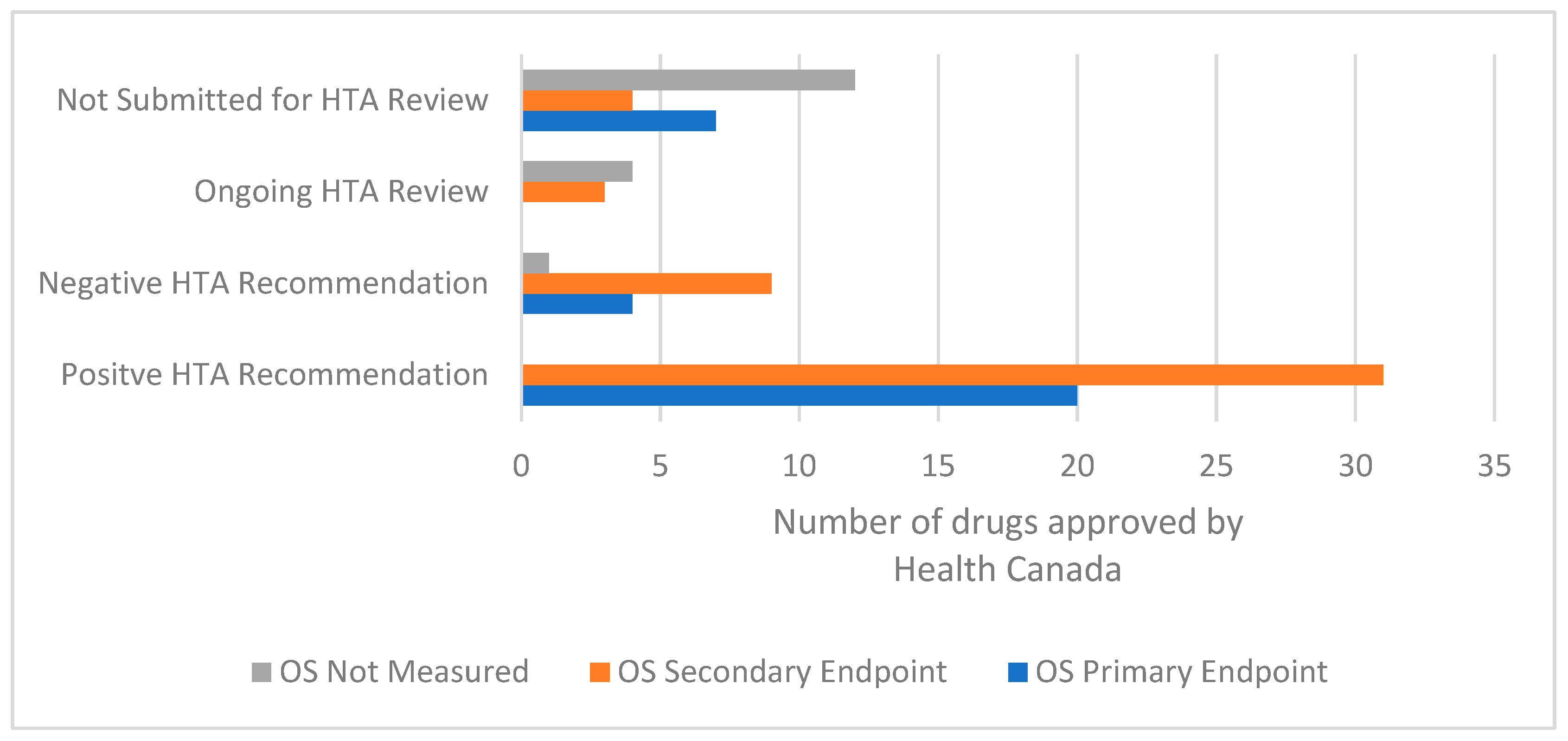
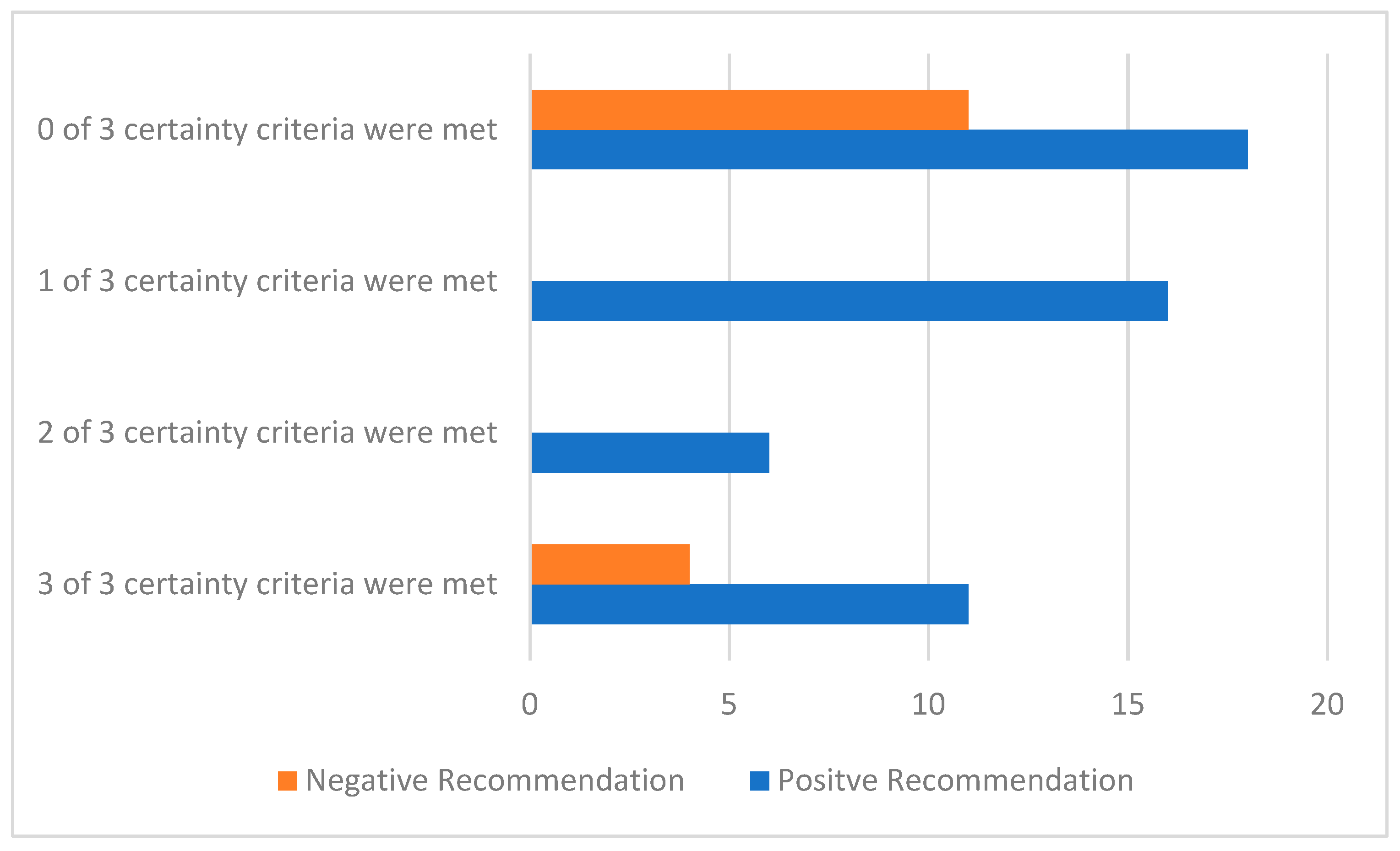
3.2. Uncertainty in the Evidence and the Time of the HTA Recommendation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García-Mochón, L.; Balbino, J.E.; Lima, A.O.D.L.; Martinez, A.C.; Ruiz, E.M.; Velasco, R.P. HTA and decision-making processes in Central, Eastern and South Eastern Europe: Results from a survey. Health Policy 2019, 123, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Barnieh, L.; Manns, B.; Harris, A.; Blom, M.; Donaldson, C.; Klarenbach, S.; Husereau, D.; Lorenzetti, D.; Clement, F. A Synthesis of Drug Reimbursement Decision-Making Processes in Organisation for Economic Co-operation and Development Countries. Value Health 2014, 17, 98–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Health Canada. Guidance for Industry—Priority Review of Drug Submissions. 2005. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/guidance-documents/priority-review/drug-submissions.html (accessed on 10 December 2021).
- Health Canada. Guidance Document: Notice of Compliance with Conditions (NOC/c). 2005. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/guidance-documents/notice-compliance-conditions.html (accessed on 10 December 2021).
- Hanna, T.P.; King, W.D.; Thibodeau, S.; Jalink, M.; Paulin, G.A.; Harvey-Jones, E.; O’Sullivan, D.E.; Booth, C.M.; Sullivan, R.; Aggarwal, A. Mortality due to cancer treatment delay: Systematic review and meta-analysis. BMJ 2020, 371, m4087. [Google Scholar] [CrossRef] [PubMed]
- Gotfrit, J.; Shin, J.J.; Mallick, R.; Stewart, D.J.; Wheatley-Price, P. Potential Life-Years Lost: The Impact of the Cancer Drug Regulatory and Funding Process in Canada. Oncologist 2019, 25, e130–e137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CADTH 2018–2021 Strategic Plan: Transforming How We Manage Health Technologies. Available online: https://www.cadth.ca/sites/default/files/corporate/planning_documents/CADTH_2018_2021_Strategic_Plan_Overview.pdf (accessed on 10 December 2021).
- CanREValue about Us—Canadian Centre for Applied Research in Cancer Control. 2018. Available online: https://cc-arcc.ca/canrevalue-about/ (accessed on 10 December 2021).
- Driscoll, J.; Rixe, O. Overall Survival: Still the Gold Standard: Why overall survival remains the definitive end point in cancer clinical trials. Cancer J. 2009, 15, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Fallowfield, L.; Fleissig, A. The value of progression-free survival to patients with advanced-stage cancer. Nat. Rev. Clin. Oncol. 2011, 9, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Saad, E.D.; Buyse, M. Statistical controversies in clinical research: End points other than overall survival are vital for regulatory approval of anticancer agents. Ann. Oncol. 2016, 27, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Health Canada. Drug and Health Product Submissions Under Review (SUR): New Drug Submissions Completed. 2021. Available online: https://www.canada.ca/en/health-canada/services/drug-health-product-review-approval/submissions-under-review/new-drug-submissions-completed.html (accessed on 10 December 2021).
- Reimbursement Review Reports. Available online: https://www.cadth.ca/reimbursement-review-reports (accessed on 10 December 2021).
- Cherny, N.I.; Dafni, U.; Bogaerts, J.; Latino, N.J.; Pentheroudakis, G.; Douillard, J.-Y.; Tabernero, J.; Zielinski, C.; Piccart, M.J.; de Vries, E.G.E. ESMO-Magnitude of Clinical Benefit Scale version 1.1. Ann. Oncol. 2017, 28, 2340–2366. [Google Scholar] [CrossRef] [PubMed]
- Cherny, N.I.; de Vries, E.; Dafni, U.; Garrett-Mayer, E.; McKernin, S.E.; Piccart, M.; Latino, N.J.; Douillard, J.-Y.; Schnipper, L.E.; Somerfield, M.R.; et al. Comparative Assessment of Clinical Benefit Using the ESMO-Magnitude of Clinical Benefit Scale Version 1.1 and the ASCO Value Framework Net Health Benefit Score. J. Clin. Oncol. 2019, 37, 336–349. [Google Scholar] [CrossRef] [PubMed]
- European Society of Medical Oncology. ESMO-Magnitude of Clinical Benefit Scale (MCBS) Scorecards. Available online: https://www.esmo.org/guidelines/esmo-mcbs/esmo-mcbs-scorecards?filterType=agent (accessed on 10 December 2021).
- pCODR Expert Review Committee Recommendation for Lenvatinib for RCC, 2019. Available online: https://cadth.ca/sites/default/files/pcodr/Reviews2019/10140LenvatinibRCC_FnRec_approvedbyChair_Post_04Jan2019_final.pdf (accessed on 10 December 2021).
- Bayoumi, A.; Laupacis, A. Outdated criteria for drug plan reimbursement obstruct evidence-based care. Can. Med. Assoc. J. 2021, 193, E1573–E1574. [Google Scholar] [CrossRef] [PubMed]
- Department of Health and Social Care. Extent and Causes of International Variations in Drug Usage. 2010. Available online: https://www.gov.uk/government/publications/extent-and-causes-of-international-variations-in-drug-usage (accessed on 10 December 2021).
- England NHS. NHS England » Cancer Drugs Fund. Available online: https://www.england.nhs.uk/cancer/cdf/ (accessed on 10 December 2021).
- Pham, F.-V.; Jacquet, E.; Monard, A.; Brunel, L.; Blay, J.-Y.; Albin, N. Added therapeutic benefit regarding ESMO-MCBS and French health technology assessment of drugs granted early access program. Ann. Oncol. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Makady, A.; van Veelen, A.; de Boer, A.; Hillege, H.; Klungel, O.; Goettsch, W. Implementing managed entry agreements in practice: The Dutch reality check. Health Policy 2019, 123, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Siviero, P.D. Managed Entry Agreements as a Way to Implement Outcomes of Assessment and Enable Patient Access to Innovation. 2012. Available online: https://www.aifa.gov.it/sites/default/files/03-23-2012_siviero_p__dia_copenaghen_2012_1_0.pdf (accessed on 10 December 2021).
- Beaver, J.A.; Howie, L.J.; Pelosof, L.; Kim, T.; Liu, J.; Goldberg, K.B.; Sridhara, R.; Blumenthal, G.M.; Farrell, A.T.; Keegan, P.; et al. A 25-Year Experience of US Food and Drug Administration Accelerated Approval of Malignant Hematology and Oncology Drugs and Biologics. JAMA Oncol. 2018, 4, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.; Macaulay, R. PCN265 characterisation of managed access agreements under the nice cancer drugs fund. Value Health 2020, 23, S69. [Google Scholar] [CrossRef]
- Cancer Drugs Fund|Technology Appraisal Guidance|NICE Guidance|Our Programmes|What We Do|about|NICE. Available online: https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-technology-appraisal-guidance/cancer-drugs-fund (accessed on 10 December 2021).
- BC Cancer Registry. Available online: http://www.bccancer.bc.ca/health-professionals/professional-resources/bc-cancer-registry (accessed on 10 December 2021).
- Alberta Health Services. Alberta Cancer Registry. Available online: https://www.albertahealthservices.ca/cancer/Page17367.aspx (accessed on 10 December 2021).
- Evidence Building Program (EBP). 2016. Available online: https://www.cancercareontario.ca/en/Funding/Evidence_Building_Program (accessed on 10 December 2021).
- English Summary. Tisagenlecleucel for the Treatment of Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia. Available online: https://www.inesss.qc.ca/fileadmin/doc/INESSS/Rapports/Therapies_cellulaires/INESSS_Kymriah_LLA_EnglishSummary.pdf (accessed on 10 December 2021).
- Vreman, R.A.; Bloem, L.T.; Van Oirschot, S.; Hoekman, J.; Van Der Elst, M.E.; Leufkens, H.G.; Klungel, O.H.; Goettsch, W.G.; Mantel-Teeuwisse, A.K. The Role of Regulator-Imposed Post-Approval Studies in Health Technology Assessments for Conditionally Approved Drugs. Int. J. Health Policy Manag. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- pCODR Expert Review Committee Recommendation for Venetoclax for CLL, 2018. Available online: https://cadth.ca/sites/default/files/pcodr/pcodr_venetoclax_venclexta_cll_fn_rec.pdf (accessed on 10 December 2021).
- pCODR Expert Review Committee Recommendation for Nivolumab for cHL, 2018. Available online: https://cadth.ca/sites/default/files/pcodr/pcodr_nivolumab_opdivo_cHL_fn_rec.pdf (accessed on 10 December 2021).
- pCODR Expert Review Committee Recommendation for Pembrolizumab for cHL, 2018. Available online: https://cadth.ca/sites/default/files/pcodr/pcodr_pembrolizumab_keytruda_chl_fn_rec.pdf (accessed on 10 December 2021).
- pCODR Expert Review Committee Recommendation for Olaratumab for Sarcoma, 2018. Available online: https://cadth.ca/sites/default/files/pcodr/pcodr_olaratumab_lartruvo_sts_fn_rec.pdf (accessed on 10 December 2021).
- O’Rourke, B.; Oortwijn, W.; Schuller, T.; The International Joint Task Group. The new definition of health technology assessment: A milestone in international collaboration. Int. J. Technol. Assess. Health Care 2020, 36, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Office of the Commissioner. Project Orbis. 2021. Available online: https://www.fda.gov/about-fda/oncology-center-excellence/project-orbis (accessed on 10 December 2021).
- Health Canada. Access Consortium. 2020. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/international-activities/australia-canada-singapore-switzerland-consortium.html (accessed on 10 December 2021).




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sehdev, S.; Chambers, A. Is It Time to Commit to a Process to Re-Evaluate Oncology Drugs? A Descriptive Analysis of Systemic Therapies for Solid Tumour Indications Reviewed in Canada from 2017 to 2021. Curr. Oncol. 2022, 29, 1919-1931. https://doi.org/10.3390/curroncol29030156
Sehdev S, Chambers A. Is It Time to Commit to a Process to Re-Evaluate Oncology Drugs? A Descriptive Analysis of Systemic Therapies for Solid Tumour Indications Reviewed in Canada from 2017 to 2021. Current Oncology. 2022; 29(3):1919-1931. https://doi.org/10.3390/curroncol29030156
Chicago/Turabian StyleSehdev, Sandeep, and Alexandra Chambers. 2022. "Is It Time to Commit to a Process to Re-Evaluate Oncology Drugs? A Descriptive Analysis of Systemic Therapies for Solid Tumour Indications Reviewed in Canada from 2017 to 2021" Current Oncology 29, no. 3: 1919-1931. https://doi.org/10.3390/curroncol29030156
APA StyleSehdev, S., & Chambers, A. (2022). Is It Time to Commit to a Process to Re-Evaluate Oncology Drugs? A Descriptive Analysis of Systemic Therapies for Solid Tumour Indications Reviewed in Canada from 2017 to 2021. Current Oncology, 29(3), 1919-1931. https://doi.org/10.3390/curroncol29030156




