Point of Care Molecular Testing: Community-Based Rapid Next-Generation Sequencing to Support Cancer Care
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chart Review
2.2. Sequencing Studies
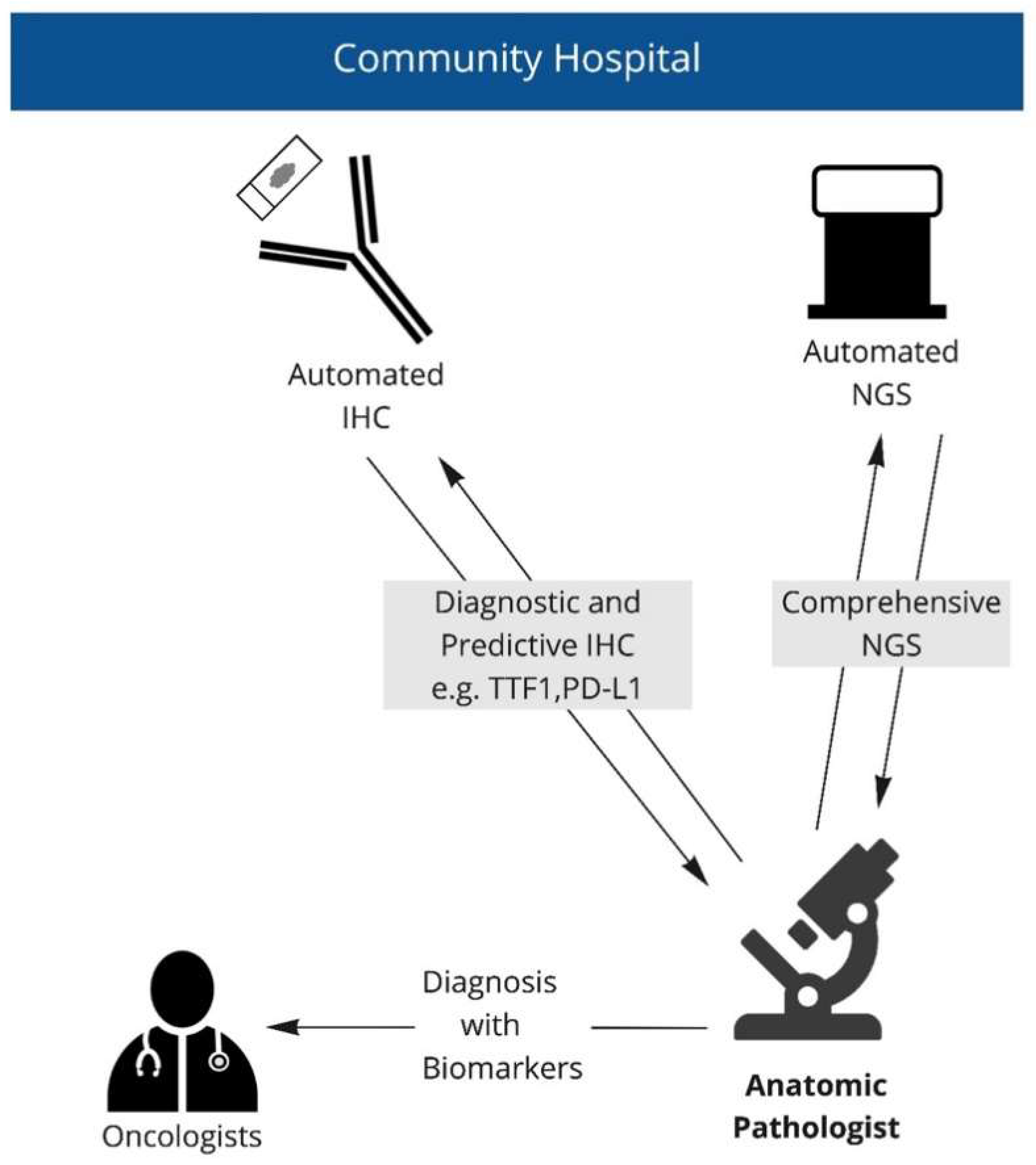
2.3. Specimen Handling and Work Flow
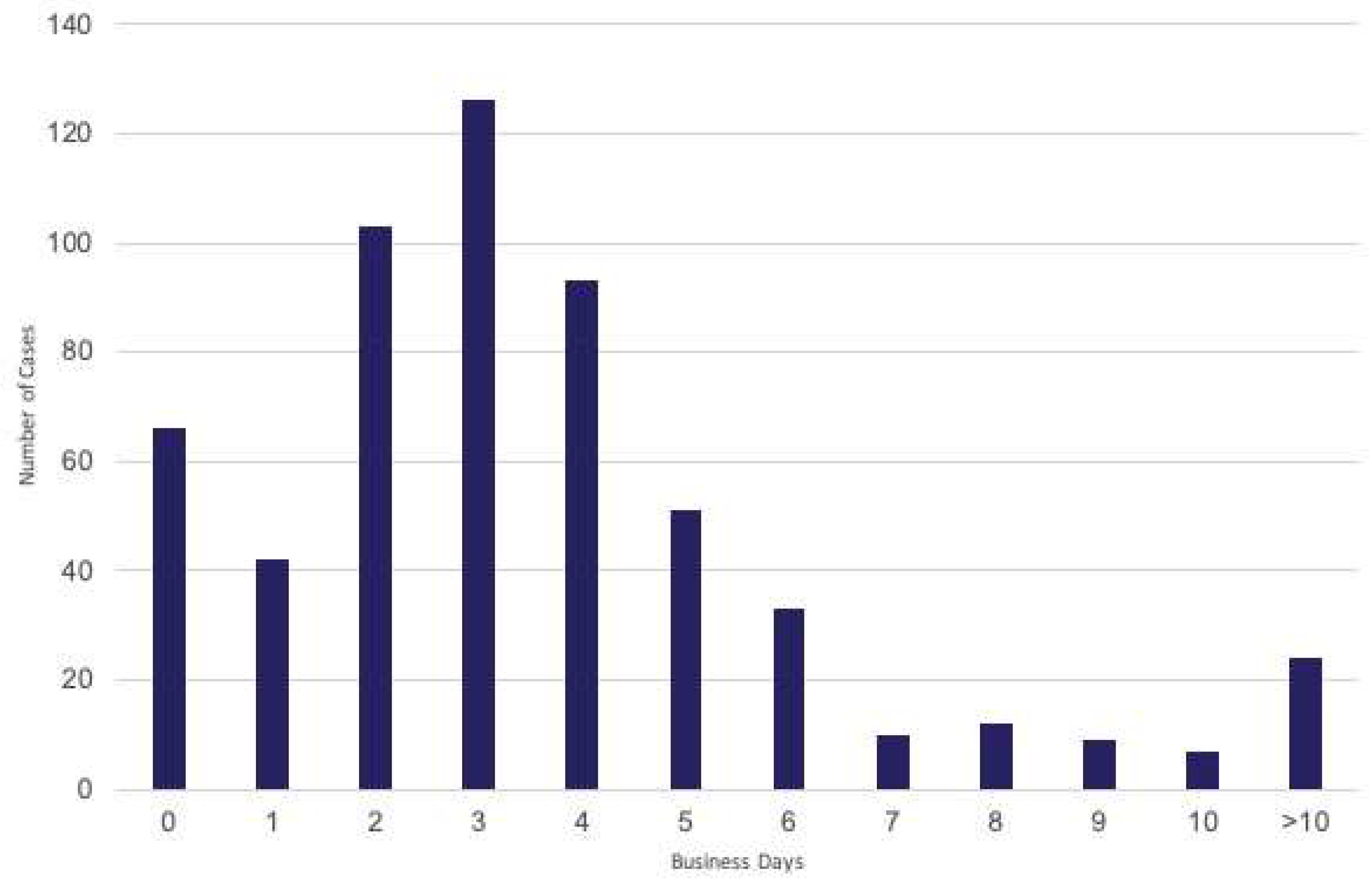
2.4. Turnaround Time Measurements
2.5. Single Gene Testing
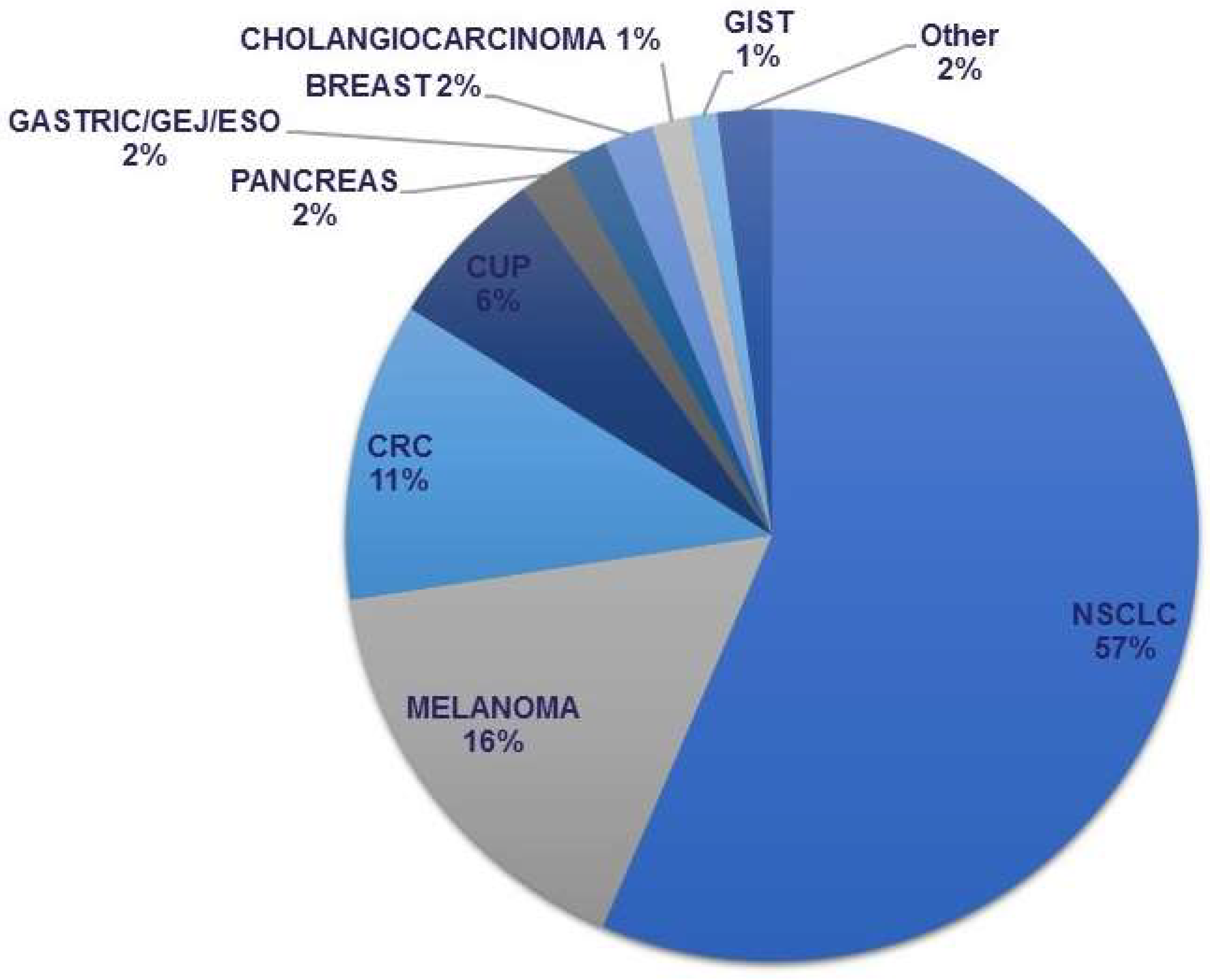
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small Molecules in Targeted Cancer Therapy: Advances, Challenges, and Future Perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Pennell, N.A.; Mutebi, A.; Zhou, Z.-Y.; Ricculli, M.L.; Tang, W.; Wang, H.; Guerin, A.; Arnhart, T.; Dalal, A.; Sasane, M.; et al. Economic Impact of Next-Generation Sequencing Versus Single-Gene Testing to Detect Genomic Alterations in Metastatic Non–Small-Cell Lung Cancer Using a Decision Analytic Model. JCO Precis. Oncol. 2019, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the Use of Next-Generation Sequencing (NGS) for Patients with Metastatic Cancers: A Report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. NCCN Guidelines Insights: Non–Small Cell Lung Cancer, Version 2.2021: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2021, 19, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, A.; Szamreta, E.A.; Shah, R.; Ning, N.; Aggarwal, J.; Hussain, A.; Adeboyeje, G. Barriers to Next-Generation Sequencing despite Increased Utilization: U.S. Physician Survey Results. JCO 2021, 39 (Suppl. S15), e18754. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2019–2021; American Cancer Society: Atlanta, GA, USA, 2019. [Google Scholar]
- Johnston, K.M.; Sheffield, B.S.; Yip, S.; Lakzadeh, P.; Qian, C.; Nam, J. Costs of In-House Genomic Profiling and Implications for Economic Evaluation: A Case Example of Non-Small Cell Lung Cancer (NSCLC). J. Med. Econ. 2020, 23, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Tsao, M.S.; Le, L.W.; Shepherd, F.A.; Feld, R.; Burkes, R.L.; Liu, G.; Kamel-Reid, S.; Hwang, D.; Tanguay, J.; et al. Biomarker Testing and Time to Treatment Decision in Patients with Advanced Nonsmall-Cell Lung Cancer. Ann. Oncol. 2015, 26, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, M.; Mehnert, J.; Silk, A.W.; Jabbour, S.K.; Ganesan, S.; Popli, P.; Riedlinger, G.; Stephenson, R.; de Meritens, A.B.; Leiser, A.; et al. Real-World Application of Tumor Mutation Burden-High (TMB-high) and Microsatellite Instability (MSI) Confirms their Utility as Immunotherapy Biomarkers. ESMO Open 2021, 7, 100336. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.J.; Maziak, D.; Gomes, M.; Fung-Kee-Fung, M.; Dennie, C.; Sekhon, H.; Lo, B.; Bradford, J.-P.; Moore, S.; Reaume, N. The Cost of Delaying Therapy for Advanced Non-Small Cell Lung Cancer (NSCLC): A Population Kinetics Assessment. Cancer Res. 2020, 80, 5489. [Google Scholar] [CrossRef]
- Anand, K.; Phung, T.L.; Bernicker, E.H.; Cagle, P.T.; Olsen, R.J.; Thomas, J.S. Clinical Utility of Reflex Ordered Testing for Molecular Biomarkers in Lung Adenocarcinoma. Clin. Lung Cancer 2020, 21, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Matthews, P.; Beharry, A.; Nematollahi, M.; Bendzsak, K.; Irshad, P.; Cheema, P. Clinical impact of rapid biomarker testing in non–small cell lung cancer in a community setting. Lab. Investig. 2020, 100 (Suppl. S1), 1802. [Google Scholar]
- Rachiglio, A.M.; De Sabato, L.; Roma, C.; Cennamo, M.; Fiorenza, M.; Terracciano, D.; Pasquale, R.; Bergantino, F.; Cavalcanti, E.; Botti, G.; et al. SARS-CoV-2 Complete Genome Sequencing from the Italian Campania Region Using a Highly Automated next Generation Sequencing System. J. Transl. Med. 2021, 19, 246. [Google Scholar] [CrossRef] [PubMed]
- Mileham, K.F.; Basu Roy, U.K.; Bruinooge, S.S.; Freeman-Daily, J.; Garon, E.B.; Garrett-Mayer, L.; Jalal, S.I.; Johnson, B.E.; Moore, A.; Osarogiagbon, R.U.; et al. Physician Concern about Delaying Lung Cancer Treatment While Awaiting Biomarker Testing: Results of a Survey of U.S. Oncologists. JCO 2021, 39 (Suppl. S15), 9067. [Google Scholar] [CrossRef]
- Lee, Y.; Clark, E.W.; Milan, M.S.D.; Champagne, C.; Michael, K.S.; Awad, M.M.; Barbie, D.A.; Cheng, M.L.; Kehl, K.L.; Marcoux, J.P.; et al. Turnaround Time of Plasma Next-Generation Sequencing in Thoracic Oncology Patients: A Quality Improvement Analysis. JCO Precis. Oncol. 2020, 4, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Akahane, T.; Kitazono, I.; Kobayashi, Y.; Nishida-Kirita, Y.; Yamaguchi, T.; Yanazume, S.; Tabata, K.; Kobayashi, H.; Tanimoto, A. Direct Next-generation Sequencing Analysis Using Endometrial Liquid-based Cytology Specimens for Rapid Cancer Genomic Profiling. Diagn. Cytopathol. 2021, 49, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Bormann Chung, C.; Lee, J.; Barritault, M.; Bringuier, P.-P.; Xu, Z.; Huang, W.-Y.; Beharry, A.; Castillo, J.; Christiansen, J.; Lin, J.C.; et al. Evaluating Targeted Next-Generation Sequencing Assays and Reference Materials for NTRK Fusion Detection. J. Mol. Diagn. 2022, 24, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, M.; Borgeaud, M.; Addeo, A.; Friedlaender, A. Oncogenic Driver Mutations in Non-Small Cell Lung Cancer: Past, Present and Future. WJCO 2021, 12, 217–237. [Google Scholar] [CrossRef] [PubMed]

| Gene | Alteration | No (%) | No (%) |
|---|---|---|---|
| KRAS | 116 (37%) | ||
| G12C | 54 (17%) | ||
| Other | 62 (20%) | ||
| EGFR | 51 (16%) | ||
| x19del | 26(8%) | ||
| L858R | 20(6%) | ||
| Other | 5 (2%) | ||
| BRAF | 14 (5%) | ||
| V600E | 4 (1%) | ||
| Other | 10 (3%) | ||
| ERBB2 | 10 (3%) | ||
| Mutation | 7(2%) | ||
| Amplification | 3(1%) | ||
| MET | 13 (4%) | ||
| x14skip | 10(3%) | ||
| Amplification | 3(1%) | ||
| ALK | 9 (3%) | ||
| ROS-1 | 5 (2%) | ||
| RET | 2 (1%) | ||
| Other | 23 (7%) | ||
| None identified | 66 (21%) | ||
| 310 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheffield, B.S.; Beharry, A.; Diep, J.; Perdrizet, K.; Iafolla, M.A.J.; Raskin, W.; Dudani, S.; Brett, M.A.; Starova, B.; Olsen, B.; et al. Point of Care Molecular Testing: Community-Based Rapid Next-Generation Sequencing to Support Cancer Care. Curr. Oncol. 2022, 29, 1326-1334. https://doi.org/10.3390/curroncol29030113
Sheffield BS, Beharry A, Diep J, Perdrizet K, Iafolla MAJ, Raskin W, Dudani S, Brett MA, Starova B, Olsen B, et al. Point of Care Molecular Testing: Community-Based Rapid Next-Generation Sequencing to Support Cancer Care. Current Oncology. 2022; 29(3):1326-1334. https://doi.org/10.3390/curroncol29030113
Chicago/Turabian StyleSheffield, Brandon S., Andrea Beharry, Joanne Diep, Kirstin Perdrizet, Marco A. J. Iafolla, William Raskin, Shaan Dudani, Mary Anne Brett, Blerta Starova, Brian Olsen, and et al. 2022. "Point of Care Molecular Testing: Community-Based Rapid Next-Generation Sequencing to Support Cancer Care" Current Oncology 29, no. 3: 1326-1334. https://doi.org/10.3390/curroncol29030113
APA StyleSheffield, B. S., Beharry, A., Diep, J., Perdrizet, K., Iafolla, M. A. J., Raskin, W., Dudani, S., Brett, M. A., Starova, B., Olsen, B., & Cheema, P. K. (2022). Point of Care Molecular Testing: Community-Based Rapid Next-Generation Sequencing to Support Cancer Care. Current Oncology, 29(3), 1326-1334. https://doi.org/10.3390/curroncol29030113





