Prediction of Postoperative Pathologic Risk Factors in Cervical Cancer Patients Treated with Radical Hysterectomy by Machine Learning
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Considered Features
2.2. Data Splitting
2.3. Supervised Machine Learning Classifiers
2.4. Model Assessment
2.5. Confidence of Prediction and Shannon’s Information Gain
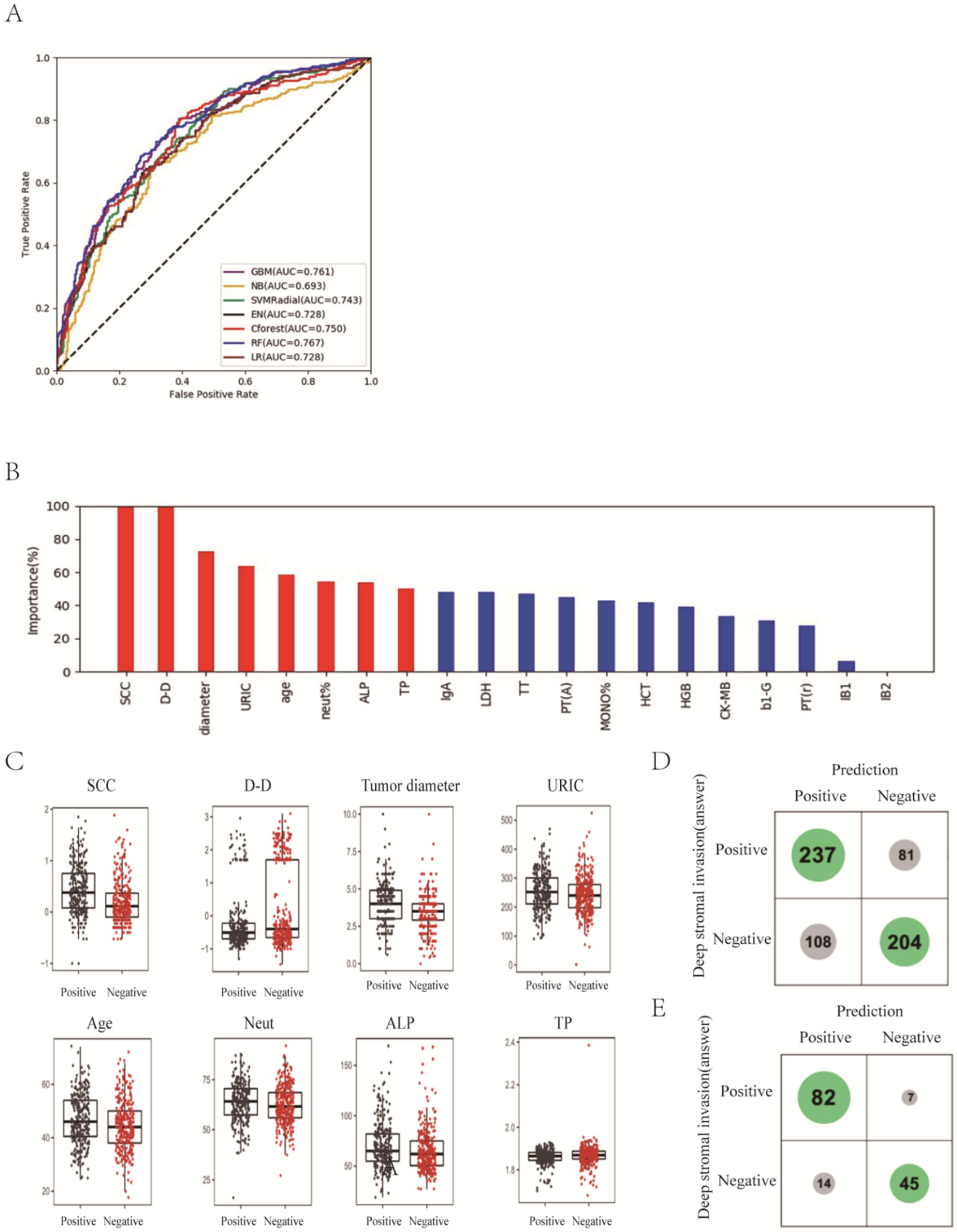
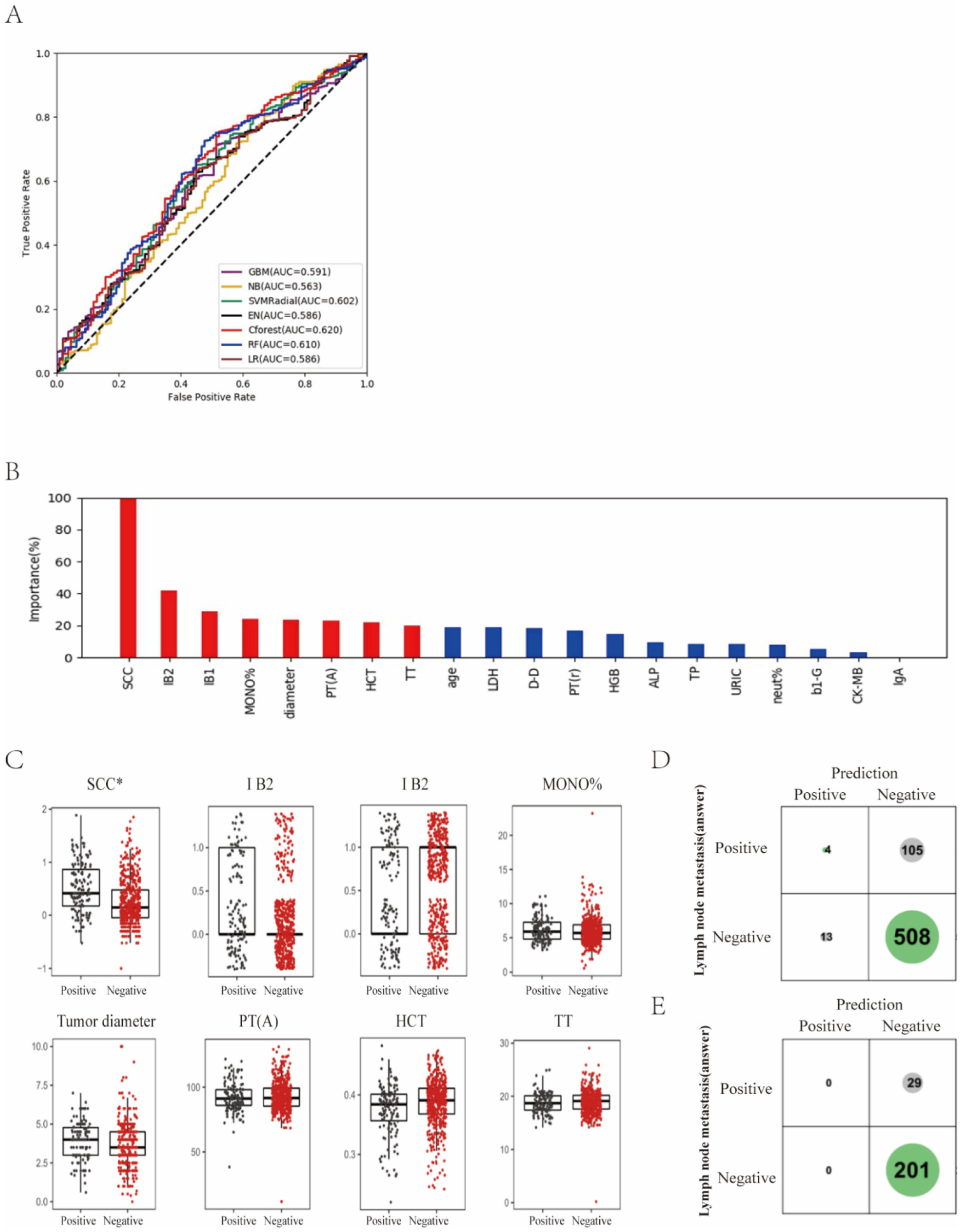
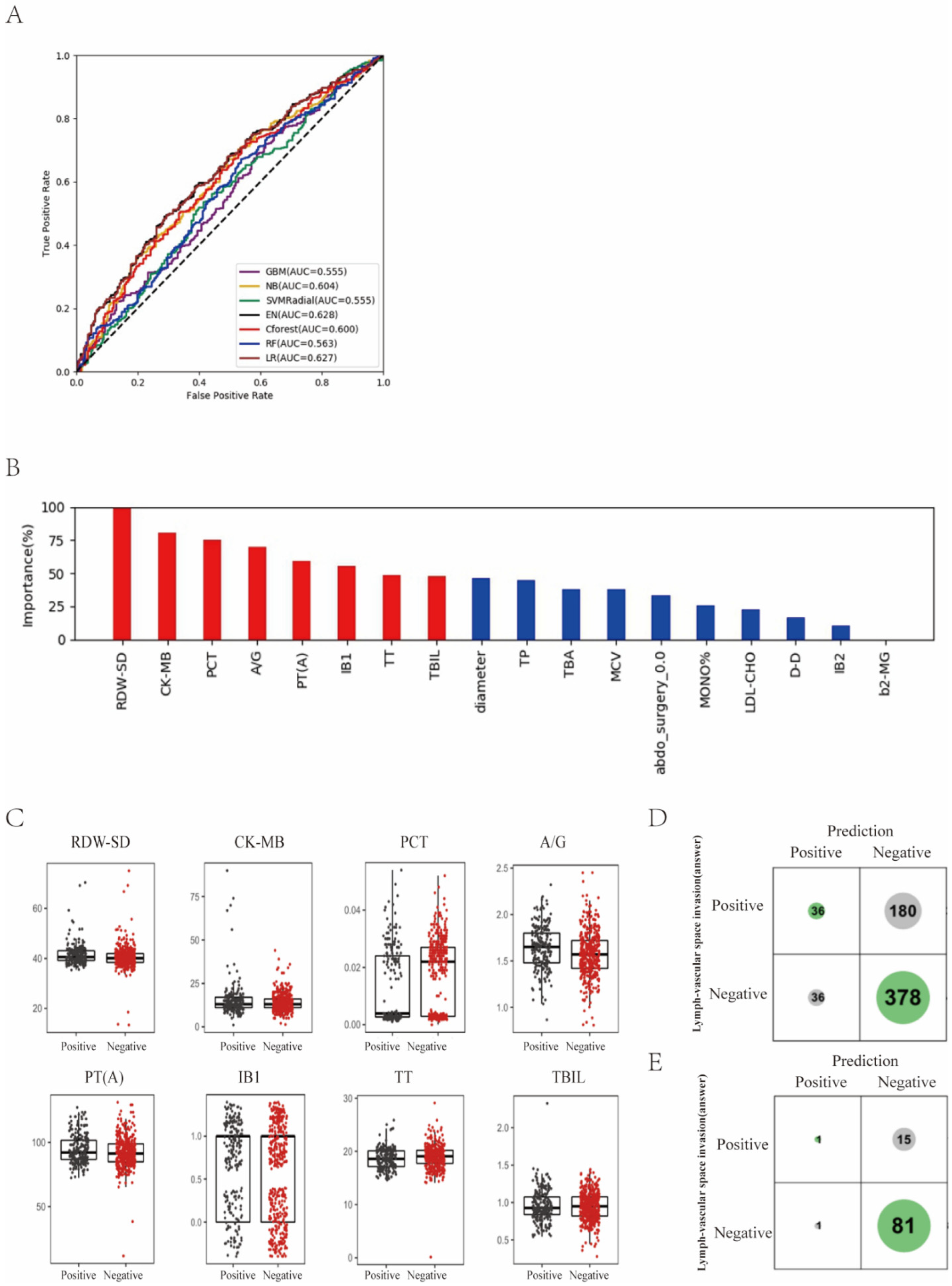
3. Results
3.1. Prediction of Deep Stromal Infiltration of Cervical Cancer Based on Multiple Preoperative Blood Markers Using Machine Learning Methods
3.2. Differentiation of Lymph Node Metastasis of Cervical Cancer with Machine Learning Methods
3.3. Prediction of Lymph-Vascular Space Invasion of Cervical Cancer Based on Preoperative Blood Markers Using Machine Learning Methods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri. Int. J. Gynaecol. Obstet. 2018, 143 (Suppl. 2), 22–36. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.H.; Wang, X.X.; Zhu, J.S.; Gao, L. Neo-adjuvant chemotherapy plus surgery versus surgery alone for cervical cancer: Meta-analysis of randomized controlled trials. J. Obstet. Gynaecol. Res. 2016, 42, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Landoni, F.; Colombo, A.; Milani, R.; Placa, F.; Zanagnolo, V.; Mangioni, C. Randomized study between radical surgery and radiotherapy for the treatment of stage IB-IIA cervical cancer: 20-year update. J. Gynecol. Oncol. 2017, 28, e34. [Google Scholar] [CrossRef] [PubMed]
- Barter, J.F.; Soong, S.J.; Shingleton, H.M.; Hatch, K.D.; Orr, J.W., Jr. Complications of combined radical hysterectomy-postoperative radiation therapy in women with early stage cervical cancer. Gynecol. Oncol. 1989, 32, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, A.; Al, R.A.; Baykal, C.; Demirtas, E.; Ayhan, A.; Yüce, K. Prognostic factors in FIGO stage IB cervical cancer without lymph node metastasis and the role of adjuvant radiotherapy after radical hysterectomy. Int. J. Gynecol. Cancer 2004, 14, 286–292. [Google Scholar] [CrossRef]
- Kim, D.Y.; Shim, S.H.; Kim, S.O.; Lee, S.W.; Park, J.Y.; Suh, D.S.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Nam, J.H. Preoperative nomogram for the identification of lymph node metastasis in early cervical cancer. Br. J. Cancer 2014, 110, 34–41. [Google Scholar] [CrossRef]
- Hutchcraft, M.L.; Smith, B.; McLaughlin, E.M.; Hade, E.M.; Backes, F.J.; O’Malley, D.M.; Cohn, D.E.; Fowler, J.M.; Copeland, L.J.; Salani, R. Conization pathologic features as a predictor of intermediate and high risk features on radical hysterectomy specimens in early stage cervical cancer. Gynecol. Oncol. 2019, 153, 255–258. [Google Scholar] [CrossRef]
- Li, X.; Zhou, J.; Huang, K.; Tang, F.; Zhou, H.; Wang, S.; Jia, Y.; Sun, H.; Ma, D.; Li, S. The predictive value of serum squamous cell carcinoma antigen in patients with cervical cancer who receive neoadjuvant chemotherapy followed by radical surgery: A single-institute study. PLoS ONE 2015, 10, e0122361. [Google Scholar] [CrossRef]
- Obrzut, B.; Kusy, M.; Semczuk, A.; Obrzut, M.; Kluska, J. Prediction of 5-year overall survival in cervical cancer patients treated with radical hysterectomy using computational intelligence methods. BMC Cancer 2017, 17, 840. [Google Scholar] [CrossRef]
- Matsuo, K.; Purushotham, S.; Jiang, B.; Mandelbaum, R.S.; Takiuchi, T.; Liu, Y.; Roman, L.D. Survival outcome prediction in cervical cancer: Cox models vs deep-learning model. Am. J. Obstet. Gynecol. 2019, 220, 381.e1–381.e14. [Google Scholar] [CrossRef]
- Papadia, A.; Bellati, F.; Bogani, G.; Ditto, A.; Martinelli, F.; Lorusso, D.; Donfrancesco, C.; Gasparri, M.L.; Raspagliesi, F. When Does Neoadjuvant Chemotherapy Really Avoid Radiotherapy? Clinical Predictors of Adjuvant Radiotherapy in Cervical Cancer. Ann. Surg. Oncol. 2015, 22 (Suppl. 3), S944–S951. [Google Scholar] [CrossRef]
- Friedman, J.H. Greedy function approximation: A gradient boosting machine. Ann. Stat. 2001, 29, 1189–1232. [Google Scholar] [CrossRef]
- Natekin, A.; Knoll, A. Gradient boosting machines, a tutorial. Front. Neurorobot. 2013, 7, 21. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V. Support-Vector Networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Liu, L.; Chen, L.; Zhang, K.; Liusan, N.; Yang, Z. Conditional Random Forest Based Smiling Face Detector, Has Random Forest Smile Classification Module for Detecting Dynamic Smiling Face Classifying Random Forest Non-Classification Face Area of Smiling Face. China Patent CN106650637-A, 10 May 2017. [Google Scholar]
- Dv, L. Fiducial distributions and Bayes’ theorem. J. R. Stat. Soc. 1958, 1, 102–107. [Google Scholar]
- Zou, H.; Hastie, T. Regularization and variable selection via the elastic net. J. R. Stat. Soc. 2005, 67, 301–320. [Google Scholar] [CrossRef]
- Feutrill, A.; Roughan, M. A Review of Shannon and Differential Entropy Rate Estimation. Entropy 2021, 23, 1046. [Google Scholar] [CrossRef]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri: 2021 update. Int. J. Gynecol. Obstet. 2021, 155, 28–44. [Google Scholar] [CrossRef]
- Sedlis, A.; Bundy, B.N.; Rotman, M.Z.; Lentz, S.S.; Muderspach, L.I.; Zaino, R.J. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A Gynecologic Oncology Group Study. Gynecol. Oncol. 1999, 73, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, Q.D.; Trimbos, J.B.M.Z.; Dijkman, A.; Creutzberg, C.L.; Gaarenstroom, K.N.; Peters, A.A.W.; Kenter, G.G. Postoperative radiation therapy improves prognosis in patients with adverse risk factors in localized, early-stage cervical cancer: A retrospective comparative study. Int. J. Gynecol. Cancer 2006, 16, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.-Y.; Park, S.-I.; Nam, B.-H.; Cho, C.-K.; Kim, K.; Kim, B.-J.; Kim, M.-H.; Choi, S.-C.; Lee, E.-D.; Lee, K.-H. Is adjuvant chemoradiotherapy overtreatment in cervical cancer patients with intermediate risk factors? Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Peters, W.A.; Liu, P.Y.; Barrett, R.J.; Stock, R.J.; Monk, B.J.; Berek, J.S.; Souhami, L.; Grigsby, P.; Gordon, W.; Alberts, D.S. Concurrent Chemotherapy and Pelvic Radiation Therapy Compared With Pelvic Radiation Therapy Alone as Adjuvant Therapy After Radical Surgery in High-Risk Early-Stage Cancer of the Cervix. J. Clin. Oncol. 2000, 18, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Landoni, F.; Maneo, A.; Colombo, A.; Placa, F.; Milani, R.; Perego, P.; Favini, G.; Ferri, L.; Mangioni, C. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet 1997, 350, 535–540. [Google Scholar] [CrossRef]
- Kong, T.-W.; Lee, J.-D.; Son, J.-H.; Paek, J.; Chun, M.; Chang, S.-J.; Ryu, H.-S. Treatment outcomes in patients with FIGO stage IB–IIA cervical cancer and a focally disrupted cervical stromal ring on magnetic resonance imaging: A propensity score matching study. Gynecol. Oncol. 2016, 143, 77–82. [Google Scholar] [CrossRef]
- Wishart, D.S.; Mandal, R.; Stanislaus, A.; Ramirez-Gaona, M. Cancer Metabolomics and the Human Metabolome Database. Metabolites 2016, 6, 10. [Google Scholar] [CrossRef]
- Yang, K.; Xia, B.; Wang, W.; Cheng, J.; Yin, M.; Xie, H.; Li, J.; Ma, L.; Yang, C.; Li, A.; et al. A Comprehensive Analysis of Metabolomics and Transcriptomics in Cervical Cancer. Sci. Rep. 2017, 7, 43353. [Google Scholar] [CrossRef]
- Yuan, Y.; Cai, X.; Shen, F.; Ma, F. HPV post-infection microenvironment and cervical cancer. Cancer Lett. 2021, 497, 243–254. [Google Scholar] [CrossRef]
- Charakorn, C.; Thadanipon, K.; Chaijindaratana, S.; Rattanasiri, S.; Numthavaj, P.; Thakkinstian, A. The association between serum squamous cell carcinoma antigen and recurrence and survival of patients with cervical squamous cell carcinoma: A systematic review and meta-analysis. Gynecol. Oncol. 2018, 150, 190–200. [Google Scholar] [CrossRef]
- Choi, K.H.; Lee, S.W.; Yu, M.; Jeong, S.; Lee, J.W.; Lee, J.H. Significance of elevated SCC-Ag level on tumor recurrence and patient survival in patients with squamous-cell carcinoma of uterine cervix following definitive chemoradiotherapy: A multi-institutional analysis. J. Gynecol. Oncol. 2019, 30, e1. [Google Scholar] [CrossRef]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Z.; Ye, W.; Xiao, Y.; Zheng, W.; Chen, Q.; Bai, P.; Lin, Z.; Chen, C. Prognostic value of serum uric acid and tumor response to induction chemotherapy in locally advanced nasopharyngeal carcinoma. BMC Cancer 2021, 21, 519. [Google Scholar] [CrossRef]
- Hayashi, M.; Yamada, S.; Tanabe, H.; Takami, H.; Inokawa, Y.; Sonohara, F.; Shimizu, D.; Hattori, N.; Kanda, M.; Tanaka, C.; et al. High Serum Uric Acid Levels Could Be a Risk Factor of Hepatocellular Carcinoma Recurrences. Nutr. Cancer 2021, 73, 996–1003. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, P.; Xu, W.; Liu, Y.; Wang, B.; Jiang, T.; Hua, C.; Wang, X.; Xu, D.; Sun, B. Serum Uric Acid Increases Risk of Cancer Incidence and Mortality: A Systematic Review and Meta-Analysis. Mediat. Inflamm. 2015, 2015, 764250. [Google Scholar] [CrossRef]
- Kang, D.H.; Ha, S.K. Uric Acid Puzzle: Dual Role as Anti-oxidantand Pro-oxidant. Electrolyte Blood Press. 2014, 12, 1–6. [Google Scholar] [CrossRef]
- Kuo, C.F.; See, L.C.; Yu, K.H.; Chou, I.J.; Chiou, M.J.; Luo, S.F. Significance of serum uric acid levels on the risk of all-cause and cardiovascular mortality. Rheumatology 2013, 52, 127–134. [Google Scholar] [CrossRef]
- Watanabe, A.; Araki, K.; Harimoto, N.; Kubo, N.; Igarashi, T.; Ishii, N.; Yamanaka, T.; Hagiwara, K.; Kuwano, H.; Shirabe, K. D-dimer predicts postoperative recurrence and prognosis in patients with liver metastasis of colorectal cancer. Int. J. Clin. Oncol. 2018, 23, 689–697. [Google Scholar] [CrossRef]
- Kim, E.Y.; Song, K.Y. Prognostic value of D-dimer levels in patients with gastric cancer undergoing gastrectomy. Surg. Oncol. 2021, 37, 101570. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, Z.; Qiu, Y.; Zhang, J.; Wu, H.; Liang, R.; Chen, G.; Qin, G.; Li, Y.; Zou, D. Clinical significance of plasma D-dimer and fibrinogen in digestive cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2018, 44, 1494–1503. [Google Scholar] [CrossRef]
- Ma, J.Y.; Ke, L.C.; Liu, Q. The pretreatment platelet-to-lymphocyte ratio predicts clinical outcomes in patients with cervical cancer: A meta-analysis. Medicine 2018, 97, e12897. [Google Scholar] [CrossRef] [PubMed]
- Montagnana, M.; Danese, E. Red cell distribution width and cancer. Ann. Transl. Med. 2016, 4, 399. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.F.; Song, S.Y.; Guo, H.; Wang, T.J.; Liu, N.; Yan, C.X. Prognostic role of pretreatment red blood cell distribution width in patients with cancer: A meta-analysis of 49 studies. J. Cancer 2019, 10, 4305–4317. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.S.V.d.; Mantoani, P.T.S.; Murta, E.F.C.; Nomelini, R.S. Laboratory parameters as predictors of prognosis in uterine cervical neoplasia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 256, 391–396. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Sanchis-Gomar, F.; Picanza, A.; Lippi, G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit. Rev. Clin. Lab. Sci. 2015, 52, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Borné, Y.; Engström, G. The relationship between red cell distribution width and all-cause and cause-specific mortality in a general population. Sci. Rep. 2019, 9, 16208. [Google Scholar] [CrossRef]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, F.; Sheng, X.G.; Zhang, S.Q.; Chen, Y.T.; Liu, B.W. Peripheral platelet/lymphocyte ratio predicts lymph node metastasis and acts as a superior prognostic factor for cervical cancer when combined with neutrophil: Lymphocyte. Medicine 2016, 95, e4381. [Google Scholar] [CrossRef]
- Huang, L.; Mo, Z.; Zhang, L.; Qin, S.; Qin, S.; Li, S. Diagnostic Value of Albumin to Fibrinogen Ratio in Cervical Cancer. Int. J. Biol. Markers 2020, 35, 66–73. [Google Scholar] [CrossRef]
- Chen, X.; Duan, H.; Liu, P.; Lin, L.; Ni, Y.; Li, D.; Dai, E.; Zhan, X.; Li, P.; Huo, Z.; et al. Development and validation of a prognostic nomogram for 2018 FIGO stages IB1, IB2, and IIA1 cervical cancer: A large multicenter study. Ann. Transl. Med. 2022, 10, 121. [Google Scholar] [CrossRef]
- Chu, R.; Zhang, Y.; Qiao, X.; Xie, L.; Chen, W.; Zhao, Y.; Xu, Y.; Yuan, Z.; Liu, X.; Yin, A.; et al. Risk Stratification of Early-Stage Cervical Cancer with Intermediate-Risk Factors: Model Development and Validation Based on Machine Learning Algorithm. Oncologist 2021, 26, e2217–e2226. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Li, B.; Liu, S.H.; Ao, M. Nomogram model for predicting postoperative survival of patients with stage IB-IIA cervical cancer. Am. J. Cancer Res. 2021, 11, 5559–5570. [Google Scholar] [PubMed]
- Du, W.; Wang, Y.; Li, D.; Xia, X.; Tan, Q.; Xiong, X.; Li, Z. Preoperative Prediction of Lymphovascular Space Invasion in Cervical Cancer With Radiomics–Based Nomogram. Front. Oncol. 2021, 11, 637794. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Cui, Y.; Wang, P.; Ren, J.; Wang, L.; Ma, Y.; Jia, Y.; Ma, X.; Zhao, L. Multi-Parametric Magnetic Resonance Imaging-Based Radiomics Analysis of Cervical Cancer for Preoperative Prediction of Lymphovascular Space Invasion. Front. Oncol. 2021, 11, 663370. [Google Scholar] [CrossRef] [PubMed]
- Palsdottir, K.; Fischerova, D.; Franchi, D.; Testa, A.; Di Legge, A.; Epstein, E. Preoperative prediction of lymph node metastasis and deep stromal invasion in women with invasive cervical cancer: Prospective multicenter study using 2D and 3D ultrasound. Ultrasound Obstet. Gynecol. 2015, 45, 470–475. [Google Scholar] [CrossRef]
- Okuno, K.; Joja, I.; Miyagi, Y.; Sakaguchi, Y.; Notohara, K.; Kudo, T.; Hiraki, Y. Cervical carcinoma with full-thickness stromal invasion: Relationship between tumor size on T2-weighted images and parametrial involvement. J. Comput. Assist. Tomogr. 2002, 26, 119–125. [Google Scholar] [CrossRef]
- Bidus, M.A.; Caffrey, A.S.; You, W.B.; Amezcua, C.A.; Chernofsky, M.R.; Barner, R.; Seidman, J.; Rose, G.S. Cervical biopsy and excision procedure specimens lack sufficient predictive value for lymph-vascular space invasion seen at hysterectomy for cervical cancer. Am. J. Obstet. Gynecol. 2008, 199, 151.e1–151.e4. [Google Scholar] [CrossRef]
- Salvo, G.; Ramirez, P.T.; Levenback, C.F.; Munsell, M.F.; Euscher, E.D.; Soliman, P.T.; Frumovitz, M. Sensitivity and negative predictive value for sentinel lymph node biopsy in women with early-stage cervical cancer. Gynecol. Oncol. 2017, 145, 96–101. [Google Scholar] [CrossRef]
- Gortzak-Uzan, L.; Jimenez, W.; Nofech-Mozes, S.; Ismiil, N.; Khalifa, M.A.; Dube, V.; Rosen, B.; Murphy, J.; Laframboise, S.; Covens, A. Sentinel lymph node biopsy vs. pelvic lymphadenectomy in early stage cervical cancer: Is it time to change the gold standard? Gynecol. Oncol. 2010, 116, 28–32. [Google Scholar] [CrossRef]
- Chen, X.L.; Chen, G.W.; Xu, G.H.; Ren, J.; Li, Z.L.; Pu, H.; Li, H. Tumor Size at Magnetic Resonance Imaging Association With Lymph Node Metastasis and Lymphovascular Space Invasion in Resectable Cervical Cancer: A Multicenter Evaluation of Surgical Specimens. Int. J. Gynecol. Cancer 2018, 28, 1545–1552. [Google Scholar] [CrossRef]
| Variables | All Patients (n = 1260) | Training Cohort (n = 630) | Test Cohort (n = 630) | p Value |
|---|---|---|---|---|
| Age (years) | 45 (18–74) | 45 (18–74) | 45 (21–73) | 0.777 |
| BMI (kg/m2) | 23.6 (16.0–42.7) | 23.6 (16.0–47.5) | 23.7 (16.5–42.7) | 0.453 |
| Menopausal status | ||||
| Yes | 353 (28.0%) | 446 (70.8%) | 461 (73.2%) | 0.347 |
| No | 907 (72.0%) | 184 (29.2%) | 169 (26.8%) | |
| Clinical tumor diameter (cm) | 3.5 (0.5–8.0) | 3.5 (0.5–10.0) | 3.5 (0.5–8.0) | 0.211 |
| Histology | ||||
| Squamous carcinoma | 1053 (83.6%) | 525 (83.3%) | 528 (83.8%) | 0.82 |
| Adenocarcinoma | 133 (10.6%) | 69 (11.0%) | 64 (10.2%) | 0.647 |
| Others | 74 (5.8%) | 36 (5.7%) | 38 (6.0%) | 0.811 |
| FIGO stage (2003) | ||||
| IB1 | 707 (56.1%) | 361 (57.3%) | 346 (54.9%) | 0.394 |
| IB2 | 289 (22.9%) | 142 (22.5%) | 147 (23.3%) | 0.738 |
| IIA1 | 135 (10.7%) | 60 (9.5%) | 75 (11.9%) | 0.172 |
| IIA2 | 129 (10.3%) | 67 (10.6%) | 62 (9.8%) | 0.642 |
| Gross type | ||||
| Exophytic | 1163 (92.3%) | 587 (93.2%) | 576 (91.4%) | 0.245 |
| Endophytic | 97 (7.7%) | 43 (6.8%) | 54 (8.6%) | |
| Previous abdominal surgery | ||||
| Yes | 255 (20.2%) | 133 (21.1%) | 122 (19.4%) | 0.441 |
| No | 1005 (79.8%) | 497 (78.9%) | 508 (80.6%) | |
| Histologic grade | ||||
| Good | 87 (6.9%) | 43 (6.8%) | 44 (7.0%) | 0.912 |
| Moderate | 506 (40.2%) | 256 (40.6%) | 250 (39.7%) | 0.73 |
| Poor | 667 (52.9%) | 331 (52.5%) | 336 (53.3%) | 0.778 |
| Deep stromal infiltration | ||||
| Negative | 653 (51.8%) | 335 (53.2%) | 318 (50.5%) | 0.338 |
| Positive | 607 (48.2%) | 295 (46.8%) | 312 (49.5%) | |
| Lymph-vascular space invasion | ||||
| Negative | 829 (65.8%) | 415 (65.9%) | 414 (65.7%) | 0.953 |
| Positive | 431 (34.2%) | 215 (34.1%) | 216 (34.3%) | |
| Lymph node metastasis | ||||
| Negative | 1017 (80.7%) | 496 (78.7%) | 521 (82.7%) | 0.074 |
| Positive | 243 (19.3%) | 134 (21.3%) | 109 (17.3%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, Z.; Mao, W.; Tan, L.; Yang, Y.; Liu, S.; Zhang, Y.; Li, B.; Zhao, D. Prediction of Postoperative Pathologic Risk Factors in Cervical Cancer Patients Treated with Radical Hysterectomy by Machine Learning. Curr. Oncol. 2022, 29, 9613-9629. https://doi.org/10.3390/curroncol29120755
Ou Z, Mao W, Tan L, Yang Y, Liu S, Zhang Y, Li B, Zhao D. Prediction of Postoperative Pathologic Risk Factors in Cervical Cancer Patients Treated with Radical Hysterectomy by Machine Learning. Current Oncology. 2022; 29(12):9613-9629. https://doi.org/10.3390/curroncol29120755
Chicago/Turabian StyleOu, Zhengjie, Wei Mao, Lihua Tan, Yanli Yang, Shuanghuan Liu, Yanan Zhang, Bin Li, and Dan Zhao. 2022. "Prediction of Postoperative Pathologic Risk Factors in Cervical Cancer Patients Treated with Radical Hysterectomy by Machine Learning" Current Oncology 29, no. 12: 9613-9629. https://doi.org/10.3390/curroncol29120755
APA StyleOu, Z., Mao, W., Tan, L., Yang, Y., Liu, S., Zhang, Y., Li, B., & Zhao, D. (2022). Prediction of Postoperative Pathologic Risk Factors in Cervical Cancer Patients Treated with Radical Hysterectomy by Machine Learning. Current Oncology, 29(12), 9613-9629. https://doi.org/10.3390/curroncol29120755




