CDK4/6 Inhibitors as Upfront Treatment in a Patient with Breast Cancer Presenting with a Clinical Critic Situation: A Case Report and Review of the Literature
Abstract
1. Introduction
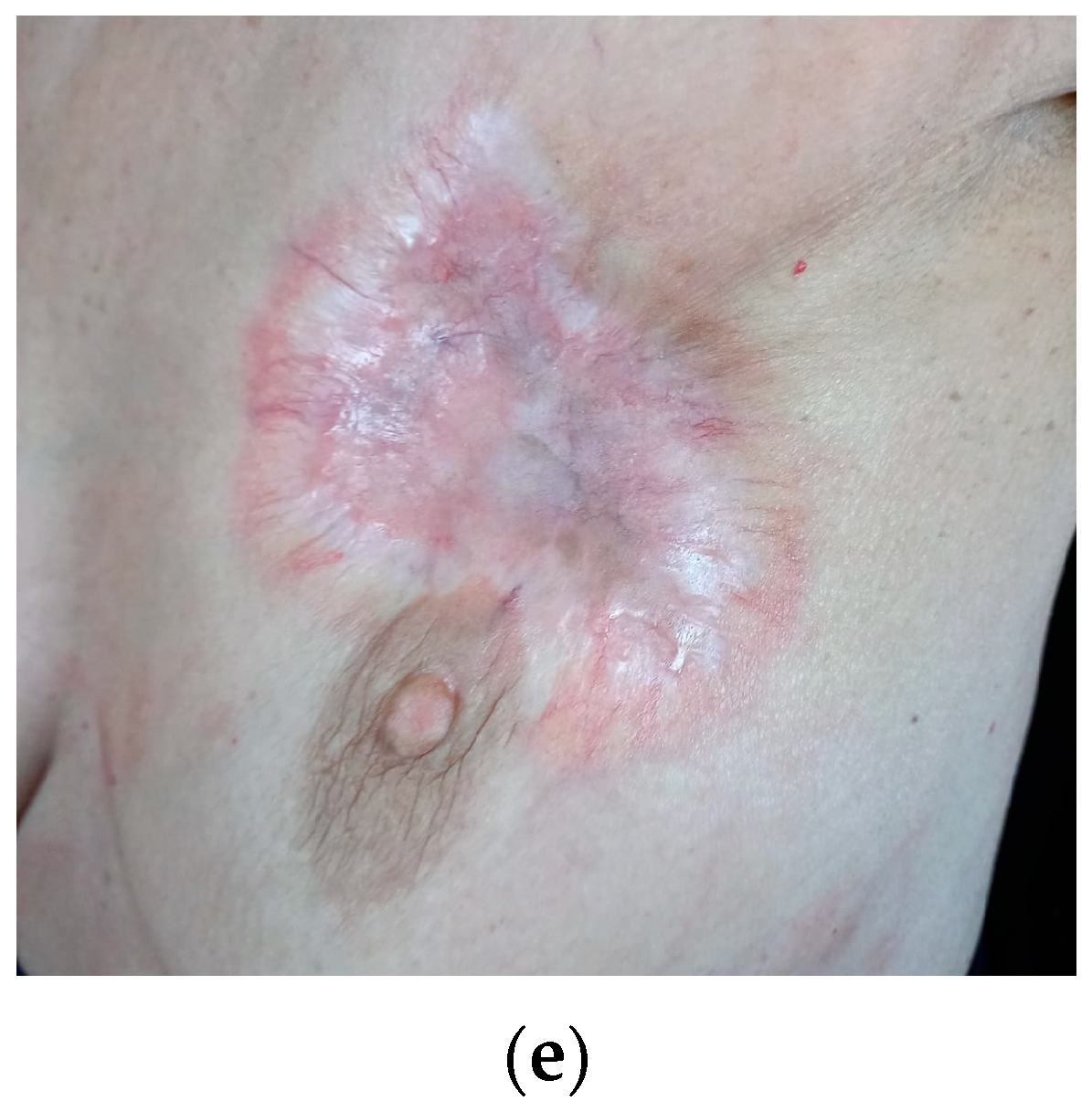
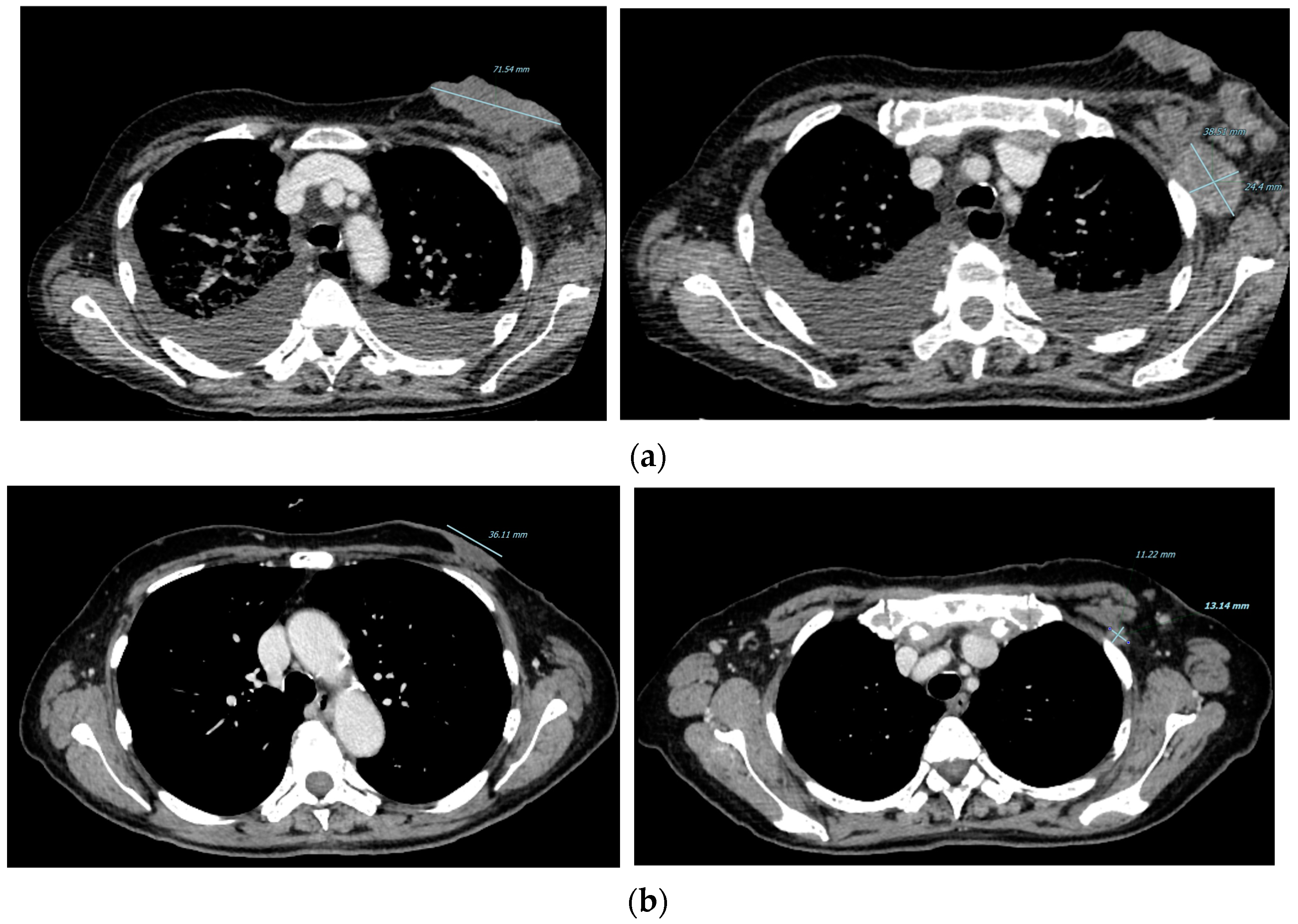
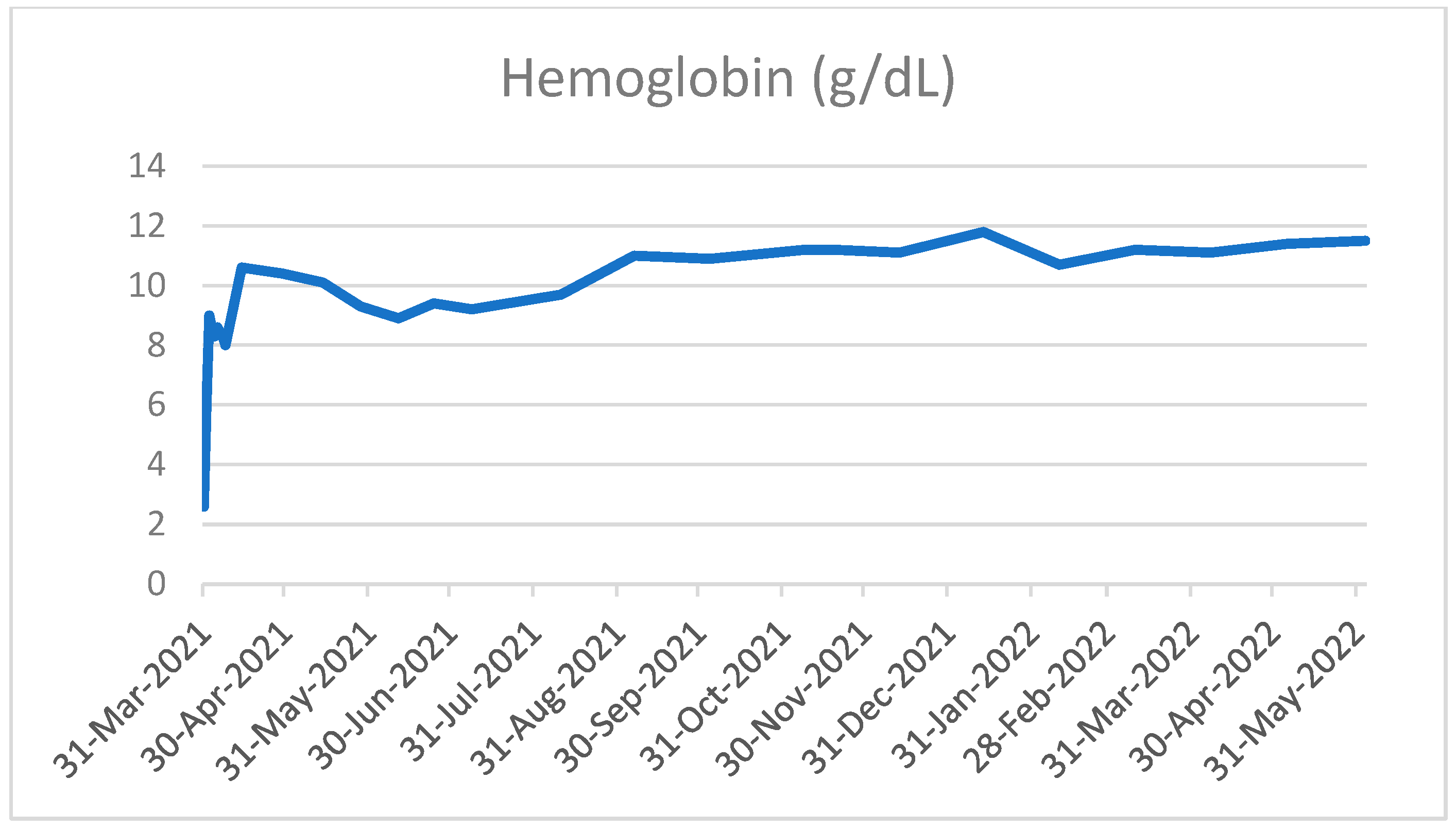
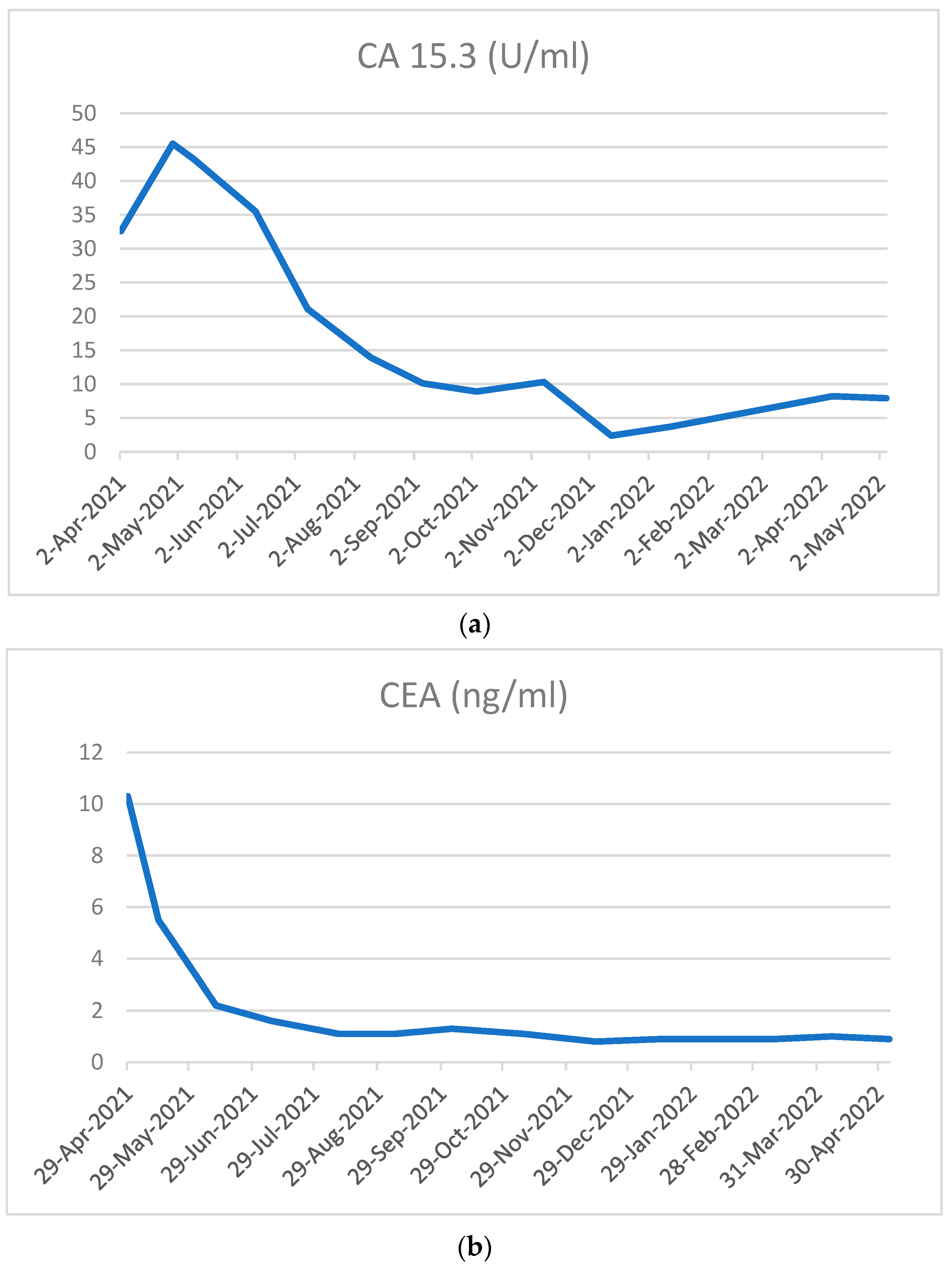
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, J.; Steeg, P.S.; Price, J.E.; Krishnamurthy, S.; Mani, S.A.; Reuben, J.; Cristofanilli, M.; Dontu, G.; Bidaut, L.; Valero, V.; et al. Breast Cancer Metastasis: Challenges and Opportunities. Cancer Res. 2009, 69, 4951–4953. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Finn, R.S.; Diéras, V.; Ettl, J.; Lipatov, O.; Joy, A.A.; Harbeck, N.; Castrellon, A.; Iyer, S.; Lu, D.R.; et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res. Treat. 2019, 174, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Petrakova, K.; Blackwell, K.L.; Winer, E.P.; et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 2018, 29, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Trédan, O.; Chen, S.-C.; Manso, L.; et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.-A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Blackwell, K.L.; André, F.; Winer, E.P.; et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1738–1748. [Google Scholar] [CrossRef]
- Tripathy, D.; Im, S.-A.; Colleoni, M.; Franke, F.; Bardia, A.; Harbeck, N.; Hurvitz, S.A.; Chow, L.; Sohn, J.; Lee, K.S.; et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial. Lancet Oncol. 2018, 19, 904–915. [Google Scholar] [CrossRef]
- Johnston, S.; Martin, M.; Di Leo, A.; Im, S.-A.; Awada, A.; Forrester, T.; Frenzel, M.; Hardebeck, M.C.; Cox, J.; Barriga, S.; et al. MONARCH 3 final PFS: A randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 2019, 5, 1–8. [Google Scholar] [CrossRef]
- Sledge, G.W., Jr.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2− Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J. Clin. Oncol. 2017, 35, 2875–2884. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016, 17, 425–439. [Google Scholar] [CrossRef]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.-A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martín, M.; et al. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer: MONALEESA-3. J. Clin. Oncol. 2018, 36, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, Q.; Hu, X.; Li, W.; Tong, Z.; Sun, T.; Teng, Y.; Wu, X.; Ouyang, Q.; Yan, X.; et al. 229P Dalpiciclib plus fulvestrant in HR+/HER2− advanced breast cancer (ABC): Updated analysis from the phase III DAWNA-1 trial. Ann. Oncol. 2022, 33, S642–S643. [Google Scholar] [CrossRef]
- Partridge, A.H.; Rumble, R.B.; Carey, L.A.; Come, S.E.; Davidson, N.E.; Di Leo, A.; Gralow, J.; Hortobagyi, G.N.; Moy, B.; Yee, D.; et al. Chemotherapy and Targeted Therapy for Women With Human Epidermal Growth Factor Receptor 2–Negative (or unknown) Advanced Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2014, 32, 3307–3329. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; André, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef]
- Jacquet, E.; Lardy-Cléaud, A.; Pistilli, B.; Franck, S.; Cottu, P.; Delaloge, S.; Debled, M.; Vanlemmens, L.; Leheurteur, M.; Guizard, A.; et al. Endocrine therapy or chemotherapy as first-line therapy in hormone receptor–positive HER2-negative metastatic breast cancer patients. Eur. J. Cancer 2018, 95, 93–101. [Google Scholar] [CrossRef]
- André, F.; Neven, P.; Marinsek, N.; Zhang, J.; Baladi, J.-F.; Degun, R.; Benelli, G.; Saletan, S.; Jerusalem, G. Disease management patterns for postmenopausal women in Europe with hormone-receptor-positive, human epidermal growth factor receptor-2 negative advanced breast cancer. Curr. Med Res. Opin. 2014, 30, 1007–1016. [Google Scholar] [CrossRef]
- Balleyguier, C.; Ayadi, S.; Van Nguyen, K.; Vanel, D.; Dromain, C.; Sigal, R. BIRADS™ classification in mammography. Eur. J. Radiol. 2007, 61, 192–194. [Google Scholar] [CrossRef]
- Park, Y.H.; Kim, T.-Y.; Kim, G.M.; Kang, S.Y.; Park, I.H.; Kim, J.H.; Lee, K.E.; Ahn, H.K.; Lee, M.H.; Kim, H.-J.; et al. Palbociclib plus exemestane with gonadotropin-releasing hormone agonist versus capecitabine in premenopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer (KCSG-BR15-10): A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2019, 20, 1750–1759. [Google Scholar] [CrossRef]
- Martin, M.; Zielinski, C.; Ruiz-Borrego, M.; Carrasco, E.; Turner, N.; Ciruelos, E.M.; Muñoz, M.; Bermejo, B.; Margeli, M.; Anton, A.; et al. Palbociclib in combination with endocrine therapy versus capecitabine in hormonal receptor-positive, human epidermal growth factor 2-negative, aromatase inhibitor-resistant metastatic breast cancer: A phase III randomised controlled trial—PEARL. Ann. Oncol. 2021, 32, 488–499. [Google Scholar] [CrossRef]
- Giuliano, M.; Schettini, F.; Rognoni, C.; Milani, M.; Jerusalem, G.; Bachelot, T.; De Laurentiis, M.; Thomas, G.; De Placido, P.; Arpino, G.; et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: A systematic review and network meta-analysis. Lancet Oncol. 2019, 20, 1360–1369. [Google Scholar] [CrossRef]
- Piezzo, M.; Chiodini, P.; Riemma, M.; Cocco, S.; Caputo, R.; Cianniello, D.; Di Gioia, G.; Di Lauro, V.; Di Rella, F.; Fusco, G.; et al. Progression-Free Survival and Overall Survival of CDK 4/6 Inhibitors Plus Endocrine Therapy in Metastatic Breast Cancer: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 6400. [Google Scholar] [CrossRef] [PubMed]
- Janni, W.; Alba, E.; Bachelot, T.; Diab, S.; Gil-Gil, M.; Beck, T.J.; Ryvo, L.; Lopez, R.; Tsai, M.; Esteva, F.J.; et al. First-line ribociclib plus letrozole in postmenopausal women with HR+, HER2− advanced breast cancer: Tumor response and pain reduction in the phase 3 MONALEESA-2 trial. Breast Cancer Res. Treat. 2018, 169, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.; Toi, M.; Huober, J.; Sohn, J.; Tredan, O.; Park, I.; Campone, M.; Chen, S.; Sanchez, L.M.; Paluch-Shimon, S.; et al. LBA15 MONARCH 3: Interim overall survival (OS) results of abemaciclib plus a nonsteroidal aromatase inhibitor (NSAI) in patients (pts) with HR+, HER2- advanced breast cancer (ABC). Ann. Oncol. 2022, 33, S1384. [Google Scholar] [CrossRef]
- Gao, J.J.; Cheng, J.; Bloomquist, E.; Sanchez, J.; Wedam, S.B.; Singh, H.; Amiri-Kordestani, L.; Ibrahim, A.; Sridhara, R.; Goldberg, K.B.; et al. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: A US Food and Drug Administration pooled analysis. Lancet Oncol. 2020, 21, 250–260. [Google Scholar] [CrossRef]
- Hortobagyi, J.O.G.N.; Stemmer, S.M.; Burris, H.A., III; Yap, Y.S.; Sonke, G.S.; Hart, L.; Campone, M.; Petrakova, K.; Winer, E.P.; Janni, W.; et al. Overall survival (OS) results from the phase III MONALEESA-2 (ML-2) trial of postmenopausal patients (pts) with hormone receptor positive/human epidermal growth factor receptor 2 negative (HR+/HER2−) advanced breast cancer (ABC) treated with endocrine the endocrine therapy (ET) ± ribociclib (RIB). Ann. Oncol. 2021, 32, S1283–S1346. [Google Scholar]
- Slamon, D.J.; Neven, P.; Chia, S.K.L.; Jerusalem, G.H.M.; De Laurentiis, M.; Im, S.-A.; Petrakova, K.; Bianchi, G.V.; Martin, M.; Nusch, A.; et al. Updated overall survival (OS) results from the phase III MONALEESA-3 trial of postmenopausal patients (pts) with HR+/HER2- advanced breast cancer (ABC) treated with fulvestrant (FUL) ± ribociclib (RIB). J. Clin. Oncol. 2021, 39, 1001. [Google Scholar] [CrossRef]
- Lu, Y.-S.; Im, S.-A.; Colleoni, M.; Franke, F.; Bardia, A.; Cardoso, F.; Harbeck, N.; Hurvitz, S.; Chow, L.; Sohn, J.; et al. Updated Overall Survival of Ribociclib plus Endocrine Therapy versus Endocrine Therapy Alone in Pre- and Perimenopausal Patients with HR+/HER2− Advanced Breast Cancer in MONALEESA-7: A Phase III Randomized Clinical Trial. Clin. Cancer Res. 2022, 28, 851–859. [Google Scholar] [CrossRef]
- Sledge, G.W.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor–Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy—MONARCH 2: A Randomized Clinical Trial. JAMA Oncol. 2020, 6, 116–124. [Google Scholar] [CrossRef]
- Hamilton, E.; Infante, J.R. Targeting CDK4/6 in patients with cancer. Cancer Treat. Rev. 2016, 45, 129–138. [Google Scholar] [CrossRef]
- Spring, L.M.; Wander, S.A.; Andre, F.; Moy, B.; Turner, N.C.; Bardia, A. Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: Past, present, and future. Lancet 2020, 395, 817–827. [Google Scholar] [CrossRef]
- Onesti, C.E.; Jerusalem, G. CDK4/6 inhibitors in breast cancer: Differences in toxicity profiles and impact on agent choice. A systematic review and meta-analysis. Expert Rev. Anticancer Ther. 2021, 21, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.; O’Shaughnessy, J.; Martin, M.; Huober, J.; Toi, M.; Sohn, J.; André, V.A.M.; Martin, H.R.; Hardebeck, M.C.; Goetz, M.P. Abemaciclib as initial therapy for advanced breast cancer: MONARCH 3 updated results in prognostic subgroups. NPJ Breast Cancer 2021, 7, 80. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Targato, G.; Bortot, L.; Dri, A.; Bonotto, M.; Minisini, A.M.; Fasola, G.; Mansutti, M. CDK4/6 Inhibitors as Upfront Treatment in a Patient with Breast Cancer Presenting with a Clinical Critic Situation: A Case Report and Review of the Literature. Curr. Oncol. 2022, 29, 9630-9639. https://doi.org/10.3390/curroncol29120756
Targato G, Bortot L, Dri A, Bonotto M, Minisini AM, Fasola G, Mansutti M. CDK4/6 Inhibitors as Upfront Treatment in a Patient with Breast Cancer Presenting with a Clinical Critic Situation: A Case Report and Review of the Literature. Current Oncology. 2022; 29(12):9630-9639. https://doi.org/10.3390/curroncol29120756
Chicago/Turabian StyleTargato, Giada, Lucia Bortot, Arianna Dri, Marta Bonotto, Alessandro Marco Minisini, Gianpiero Fasola, and Mauro Mansutti. 2022. "CDK4/6 Inhibitors as Upfront Treatment in a Patient with Breast Cancer Presenting with a Clinical Critic Situation: A Case Report and Review of the Literature" Current Oncology 29, no. 12: 9630-9639. https://doi.org/10.3390/curroncol29120756
APA StyleTargato, G., Bortot, L., Dri, A., Bonotto, M., Minisini, A. M., Fasola, G., & Mansutti, M. (2022). CDK4/6 Inhibitors as Upfront Treatment in a Patient with Breast Cancer Presenting with a Clinical Critic Situation: A Case Report and Review of the Literature. Current Oncology, 29(12), 9630-9639. https://doi.org/10.3390/curroncol29120756




