Predictors of Functional Recovery among Musculoskeletal Oncology Patients Undergoing Lower Extremity Endoprosthetic Reconstruction
Abstract
1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Data Sources and Variables
2.4. Functional Outcomes
2.5. Statistical Analysis
3. Results
3.1. Cohort Characteristics
3.2. Functional Outcomes
3.3. Binomial Logistic Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weinschenk, R.C.; Wang, W.-L.; Lewis, V.O. Chondrosarcoma. JAAOS-J. Am. Acad. Orthop. Surg. 2021, 29, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Messerschmitt, P.J.; Garcia, R.M.; Abdul-Karim, F.W.; Greenfield, E.M.; Getty, P.J. Osteosarcoma. JAAOS-J. Am. Acad. Orthop. Surg. 2009, 17, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Grimer, R.J.; Aydin, B.K.; Wafa, H.; Carter, S.R.; Jeys, L.; Abudu, A.; Parry, M. Very Long-Term Outcomes after Endoprosthetic Replacement for Malignant Tumours of Bone. Bone Jt. J. 2016, 98, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.R.; Lazarides, A.L.; Visgauss, J.D.; Somarelli, J.A.; Blazer, D.G.; Brigman, B.E.; Eward, W.C. Limb Salvage versus Amputation in Patients with Osteosarcoma of the Extremities: An Update in the Modern Era Using the National Cancer Database. BMC Cancer 2020, 20, 995. [Google Scholar] [CrossRef] [PubMed]
- Calderón, S.A.L.; Kuechle, J.; Raskin, K.A.; Hornicek, F.J. Lower Extremity Megaprostheses in Orthopaedic Oncology. JAAOS-J. Am. Acad. Orthop. Surg. 2018, 26, e249–e257. [Google Scholar] [CrossRef]
- Mahendra, A.; Griffin, A.M.; Yu, C.; Gortzak, Y.; Bell; Ferguson, P.C.; Wunder, J.S.; Davis, A. Correlation of Msts-87 & Tess Functional Evaluation Scores Following Endoprosthetic Replacement for Bone Sarcoma. Orthop. Proc. 2010, 92-B, 64. [Google Scholar] [CrossRef]
- Sharil, A.R.; Nawaz, A.H.; Azman, M.N.; Zulmi, W.; Faisham, W.I. Early Functional Outcome of Resection and Endoprosthesis Replacement for Primary Tumor around the Knee. Malays. Orthop. J. 2013, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Topkar, O.M.; Sofulu, Ö.; Şirin, E.; Erol, B. Limb Salvage Surgery of Primary and Metastatic Bone Tumors of the Lower Extremity: Functional Outcomes and Survivorship of Modular Endoprosthetic Reconstruction. Acta Orthop. Traumatol. Turc. 2021, 55, 147–153. [Google Scholar] [CrossRef]
- Morri, M.; Forni, C.; Ruisi, R.; Giamboi, T.; Giacomella, F.; Donati, D.M.; Benedetti, M.G. Postoperative Function Recovery in Patients with Endoprosthetic Knee Replacement for Bone Tumour: An Observational Study. BMC Musculoskelet. Disord. 2018, 19, 353. [Google Scholar] [CrossRef]
- Wilson, P.J.; Steadman, P.; Beckman, E.M.; Connick, M.J.; Carty, C.P.; Tweedy, S.M. Fitness, Function, and Exercise Training Responses after Limb Salvage With a Lower Limb Megaprosthesis: A Systematic Review. PM&R 2019, 11, 533–547. [Google Scholar]
- Xu, S.; Yu, X.; Xu, M.; Fu, Z.; Chen, Y.; Sun, Y.; Su, Q. Limb Function and Quality of Life after Various Reconstruction Methods According to Tumor Location Following Resection of Osteosarcoma in Distal Femur. BMC Musculoskelet. Disord. 2014, 15, 453. [Google Scholar] [CrossRef] [PubMed]
- Pala, E.; Trovarelli, G.; Calabrò, T.; Angelini, A.; Abati, C.N.; Ruggieri, P. Survival of Modern Knee Tumor Megaprostheses: Failures, Functional Results, and a Comparative Statistical Analysis. Clin. Orthop. Relat. Res. 2015, 473, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Matsumine, A.; Uchida, A.; Kawai, A.; Nishida, Y.; Kunisada, T.; Araki, N.; Sugiura, H.; Tomita, M.; Yokouchi, M. Clinical Outcomes of Kyocera Modular Limb Salvage System after Resection of Bone Sarcoma of the Distal Part of the Femur: The Japanese Musculoskeletal Oncology Group Study. Int. Orthop. 2014, 38, 825–830. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carty, C.P.; Dickinson, I.C.; Watts, M.C.; Crawford, R.W.; Steadman, P. Impairment and Disability Following Limb Salvage Procedures for Bone Sarcoma. Knee 2009, 16, 405–408. [Google Scholar] [CrossRef]
- Ghert, M.; Deheshi, B.; Holt, G.; Randall, R.L.; Ferguson, P.; Wunder, J.; Turcotte, R.; Werier, J.; Clarkson, P.; Damron, T. Prophylactic Antibiotic Regimens in Tumour Surgery (PARITY): Protocol for a Multicentre Randomised Controlled Study. BMJ Open 2012, 2, e002197. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef]
- Davis, A.M.; Wright, J.G.; Williams, J.I.; Bombardier, C.; Griffin, A.; Bell, R.S. Development of a Measure of Physical Function for Patients with Bone and Soft Tissue Sarcoma. Qual. Life Res. 1996, 5, 508–516. [Google Scholar] [CrossRef]
- Clayer, M.; Doyle, S.; Sangha, N.; Grimer, R. The Toronto Extremity Salvage Score in Unoperated Controls: An Age, Gender, and Country Comparison. Sarcoma 2012, 2012, e717213. [Google Scholar] [CrossRef]
- Gazendam, A.; Schneider, P.; Bhandari, M.; Busse, J.; Ghert, M. Defining Minimally Important Differences in Functional Outcomes for Musculoskeletal Oncology Patients Undergoing Lower Extremity Endoprosthetic Reconstruction. JBJS 2022, 104, 1659–1666. [Google Scholar] [CrossRef]
- Heaver, C.; Isaacson, A.; Gregory, J.J.; Cribb, G.; Cool, P. Patient Factors Affecting the Toronto Extremity Salvage Score Following Limb Salvage Surgery for Bone and Soft Tissue Tumors. J. Surg. Oncol. 2016, 113, 804–810. [Google Scholar] [CrossRef]
- Ferguson, P.C.; Griffin, A.M.; O’Sullivan, B.; Catton, C.N.; Davis, A.M.; Murji, A.; Bell, R.S.; Wunder, J.S. Bone Invasion in Extremity Soft-Tissue Sarcoma: Impact on Disease Outcomes. Cancer 2006, 106, 2692–2700. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.M.; Sennik, S.; Griffin, A.M.; Wunder, J.S.; O’Sullivan, B.; Catton, C.N.; Bell, R.S. Predictors of Functional Outcomes Following Limb Salvage Surgery for Lower-Extremity Soft Tissue Sarcoma. J. Surg. Oncol. 2000, 73, 206–211. [Google Scholar] [CrossRef]
- Peduzzi, P.; Concato, J.; Feinstein, A.R.; Holford, T.R. Importance of Events per Independent Variable in Proportional Hazards Regression Analysis II. Accuracy and Precision of Regression Estimates. J. Clin. Epidemiol. 1995, 48, 1503–1510. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Leopold, S.S. Importance of Validating the Scores We Use to Assess Patients with Musculoskeletal Tumors. Clin. Orthop. Relat. Res. 2019, 477, 669. [Google Scholar] [CrossRef] [PubMed]
- Jentzsch, T.; Erschbamer, M.; Seeli, F.; Fuchs, B. Extensor Function after Medial Gastrocnemius Flap Reconstruction of the Proximal Tibia. Clin. Orthop. Relat. Res. 2013, 471, 2333–2339. [Google Scholar] [CrossRef] [PubMed]
- Cipriano, C.A.; Dalton, J.; McDonald, D.J. Does Patellar Tendon Repair with Gastrocnemius Flap Augmentation Effectively Restore Active Extension after Proximal Tibial Sarcoma Resection? Clin. Orthop. Relat. Res. 2019, 477, 584. [Google Scholar] [CrossRef]
- Röder, C.; Staub, L.P.; Eggli, S.; Dietrich, D.; Busato, A.; Müller, U. Influence of Preoperative Functional Status on Outcome After Total Hip Arthroplasty. JBJS 2007, 89, 11–17. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Davis, A.M.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Kandel, R.; Goddard, K.; Sadura, A. Preoperative versus Postoperative Radiotherapy in Soft-Tissue Sarcoma of the Limbs: A Randomised Trial. Lancet 2002, 359, 2235–2241. [Google Scholar] [CrossRef]
- Davis, A.M.; O’Sullivan, B.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Hammond, A.; Benk, V.; Kandel, R.; et al. Late Radiation Morbidity Following Randomization to Preoperative versus Postoperative Radiotherapy in Extremity Soft Tissue Sarcoma. Radiother. Oncol. 2005, 75, 48–53. [Google Scholar] [CrossRef]
- Tunn, P.U.; Pomraenke, D.; Goerling, U.; Hohenberger, P. Functional Outcome after Endoprosthetic Limb-Salvage Therapy of Primary Bone Tumours—A Comparative Analysis Using the MSTS Score, the TESS and the RNL Index. Int. Orthop. 2008, 32, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.L.; Abdel, M.P.; Frank, R.D.; Chamberlain, A.M.; Habermann, E.B.; Mantilla, C.B. Impact of Frailty on Outcomes after Primary and Revision Total Hip Arthroplasty. J. Arthroplast. 2019, 34, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Milder, D.A.; Pillinger, N.L.; Kam, P.C.A. The Role of Prehabilitation in Frail Surgical Patients: A Systematic Review. Acta Anaesthesiol. Scand. 2018, 62, 1356–1366. [Google Scholar] [CrossRef] [PubMed]
| Variable | Entire Cohort (n = 555) | Proximal Femur Reconstruction (n = 144) | Distal Femur Reconstruction (n = 312) | Proximal Tibia Reconstruction (n = 99) | Subgroup Differences (p-Value) |
|---|---|---|---|---|---|
| Age (SD) | 40.7 (21.6) | 51.3 (20.4) | 37.1 (20.9) | 36.6 (20.6) | <0.001 ** |
| Gender (M/F) | 332/223 | 91/53 | 177/135 | 0.237 * | |
| Diagnosis (%) | |||||
| Primary bone sarcoma | 407 (73) | 97 (67) | 239 (77) | 71 (71) | <0.001 * |
| Soft tissue sarcoma | 54 (10) | 11 (8) | 33 (11) | 10 (10) | |
| Metastatic bone disease | 51 (9) | 32 (22) | 16 (5) | 3 (3) | |
| Giant cell tumor | 43 (8) | 4 (3) | 24 (7) | 15 (15) | |
| Pre-operative TESS (%) | |||||
| Poor (0–39) | 113 (20) | 44 (31) | 58 (19) | 11 (11) | 0.003 * |
| Fair (40–59) | 96 (17) | 22 (15) | 53 (17) | 21 (21) | |
| Good (60–79) | 140 (25) | 23 (16) | 96 (31) | 21 (21) | |
| Excellent (80–100) Missing | 180 (32) 26 (5) | 45 (31) 10 (7) | 92 (29) 13 (4) | 43 (43) 3 (3) | |
| Systemic Metastases (%) | |||||
| Yes | 97 (17) | 42 (29) | 45 (14) | 10 (10) | <0.001 * |
| No | 458 (83) | 102 (71) | 267 (86) | 89 (90) | |
| Death from disease progression (%) | 51 (9) | 23 (16) | 24 (8) | 4 (4) | 0.003 * |
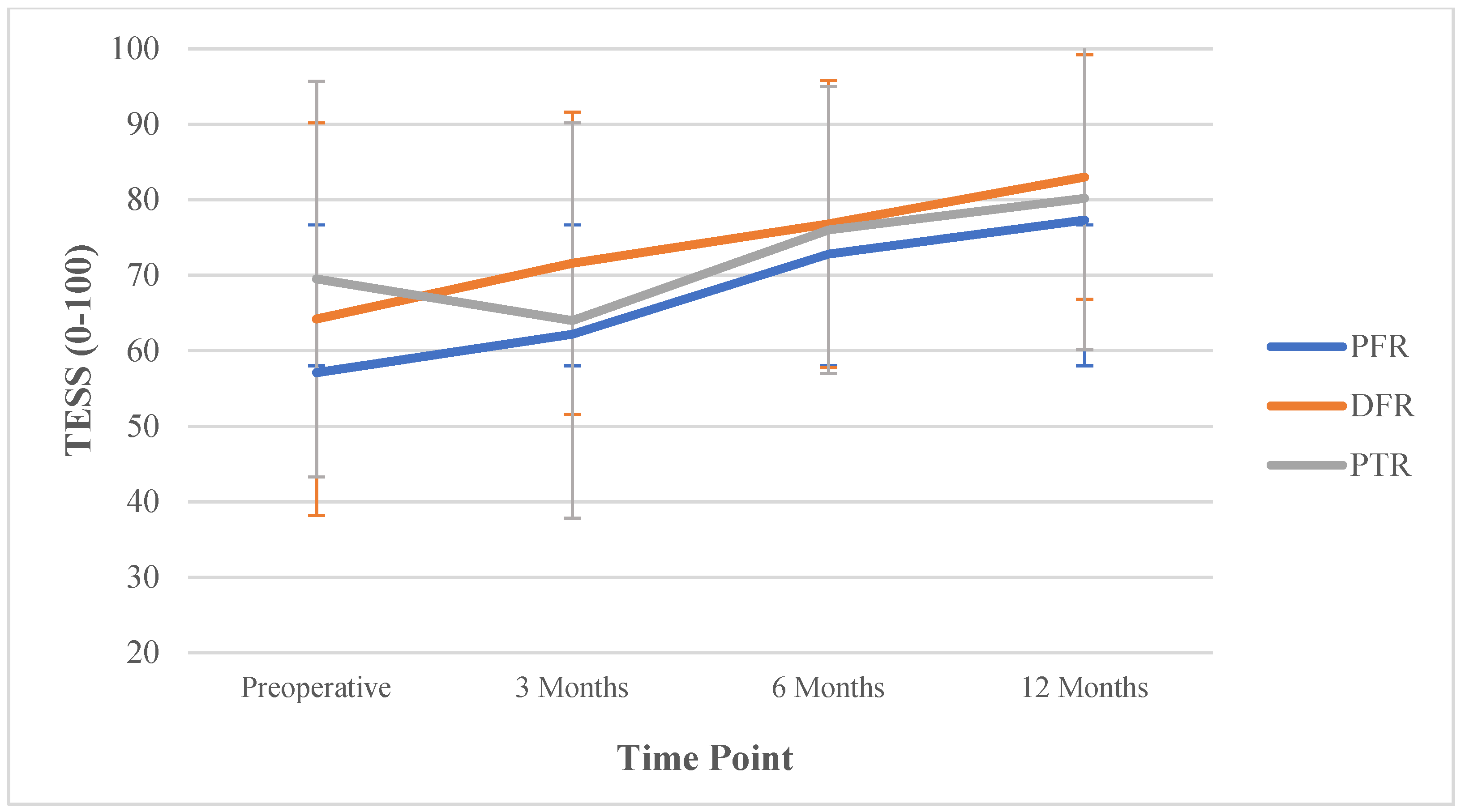
| Functional Score | Overall Mean Score (SD) | PFR Mean Score (SD) | DFR Mean Score (SD) | PTR Mean Score (SD) |
|---|---|---|---|---|
| TESS | ||||
| Preoperative | 63.5 (27.7) | 57.1 (31.2) | 64.2 (26.0) | 69.5 (26.2) |
| 3 months | 67.9 (21.3) | 62.2 (21.7) | 71.6 (20.0) | 64.0 (26.2) |
| 6 months | 75.7 (19.2) | 72.8 (19.3) | 76.8 (19.0) | 76.0 (19.0) |
| 12 months | 81.1 (17.8) | 77.3 (18.5) | 83.0 (16.2) | 80.2 (20.1) |
| Functional Score | Mean Differences (95%CIs) | |||||
|---|---|---|---|---|---|---|
| 0–3 Months | p-Value | 0–6 Months | p-Value | 0–12 Months | p-Value | |
| TESS | ||||||
| Overall | 3.4 (0.7, 6.2) | 0.015 | 10.0 (7.4, 12.6) | <0.001 | 14.9 * (12.2, 17.6) | <0.001 |
| PFR | 2.7 (−3.7, 9.0) | 0.410 | 12.3 * (6.1, 18.6) | <0.001 | 16.6 * (10.6, 22.6) | <0.001 |
| DFR | 7.1 (3.8, 10.5) | <0.001 | 10.8 (7.5, 14.0) | <0.001 | 16.5 * (13.0, 20.0) | <0.001 |
| PTR | −7.0 (−13, −0.5) | 0.034 | 4.8 (−1.1, 10.7) | 0.11 | 8.2 (1.8, 14.6) | 0.013 |
| Factor | OR (95%CI) | p-Value | Absolute Risk, % (95%CI) * |
|---|---|---|---|
| Age (per 10-year increase from age 12) | 1.32 (1.17, 1.49) | <0.001 | 4.5 (2.4, 6.6) |
| Sex Female Male | reference category 1.00 (0.63, 1.60) | 0.999 | |
| Tumor Type Bone sarcoma Soft-tissue sarcoma Metastatic bone disease Giant cell tumor | reference category 2.27 (1.03, 5.01) 0.78 (0.28, 2.20) 0.40 (0.17, 0.95) | 0.042 0.628 0.038 | 15.3 (0.4, 34.4) −9.9 (−14.4, −0.7) |
| Type of Reconstruction Distal femur Proximal femur Proximal tibia | reference category 0.98 (0.55, 1.75) 1.3 (0.72, 2.4)) | 0.947 0.368 | |
| Pre-operative TESS Score Excellent (80–100) Good (60–79) Fair (40–59) Poor (0–39) | reference category 1.04 (0.57, 1.91) 1.83 (0.96, 3.50) 3.30 (1.6, 6.60) | 0.889 0.068 0.001 | 24.0 (8.0, 41.2) |
| Metastases at Presentation | 1.30 (0.61, 2.62) | 0.537 | |
| Antibiotic Duration 24 h regime 5-day regime | Reference 0.91 (0.58, 1.42) | 0.668 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazendam, A.M.; Schneider, P.; Heels-Ansdell, D.; Bhandari, M.; Busse, J.W.; Ghert, M. Predictors of Functional Recovery among Musculoskeletal Oncology Patients Undergoing Lower Extremity Endoprosthetic Reconstruction. Curr. Oncol. 2022, 29, 7598-7606. https://doi.org/10.3390/curroncol29100600
Gazendam AM, Schneider P, Heels-Ansdell D, Bhandari M, Busse JW, Ghert M. Predictors of Functional Recovery among Musculoskeletal Oncology Patients Undergoing Lower Extremity Endoprosthetic Reconstruction. Current Oncology. 2022; 29(10):7598-7606. https://doi.org/10.3390/curroncol29100600
Chicago/Turabian StyleGazendam, Aaron M., Patricia Schneider, Diane Heels-Ansdell, Mohit Bhandari, Jason W. Busse, and Michelle Ghert. 2022. "Predictors of Functional Recovery among Musculoskeletal Oncology Patients Undergoing Lower Extremity Endoprosthetic Reconstruction" Current Oncology 29, no. 10: 7598-7606. https://doi.org/10.3390/curroncol29100600
APA StyleGazendam, A. M., Schneider, P., Heels-Ansdell, D., Bhandari, M., Busse, J. W., & Ghert, M. (2022). Predictors of Functional Recovery among Musculoskeletal Oncology Patients Undergoing Lower Extremity Endoprosthetic Reconstruction. Current Oncology, 29(10), 7598-7606. https://doi.org/10.3390/curroncol29100600





