Understanding Treatment Patterns and Outcomes among Patients with De Novo Unresectable Locally Advanced or Metastatic Urothelial Cancer: A Population-Level Retrospective Analysis from Alberta, Canada
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Sources
2.2. Patients
Outcomes
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Treatment Patterns
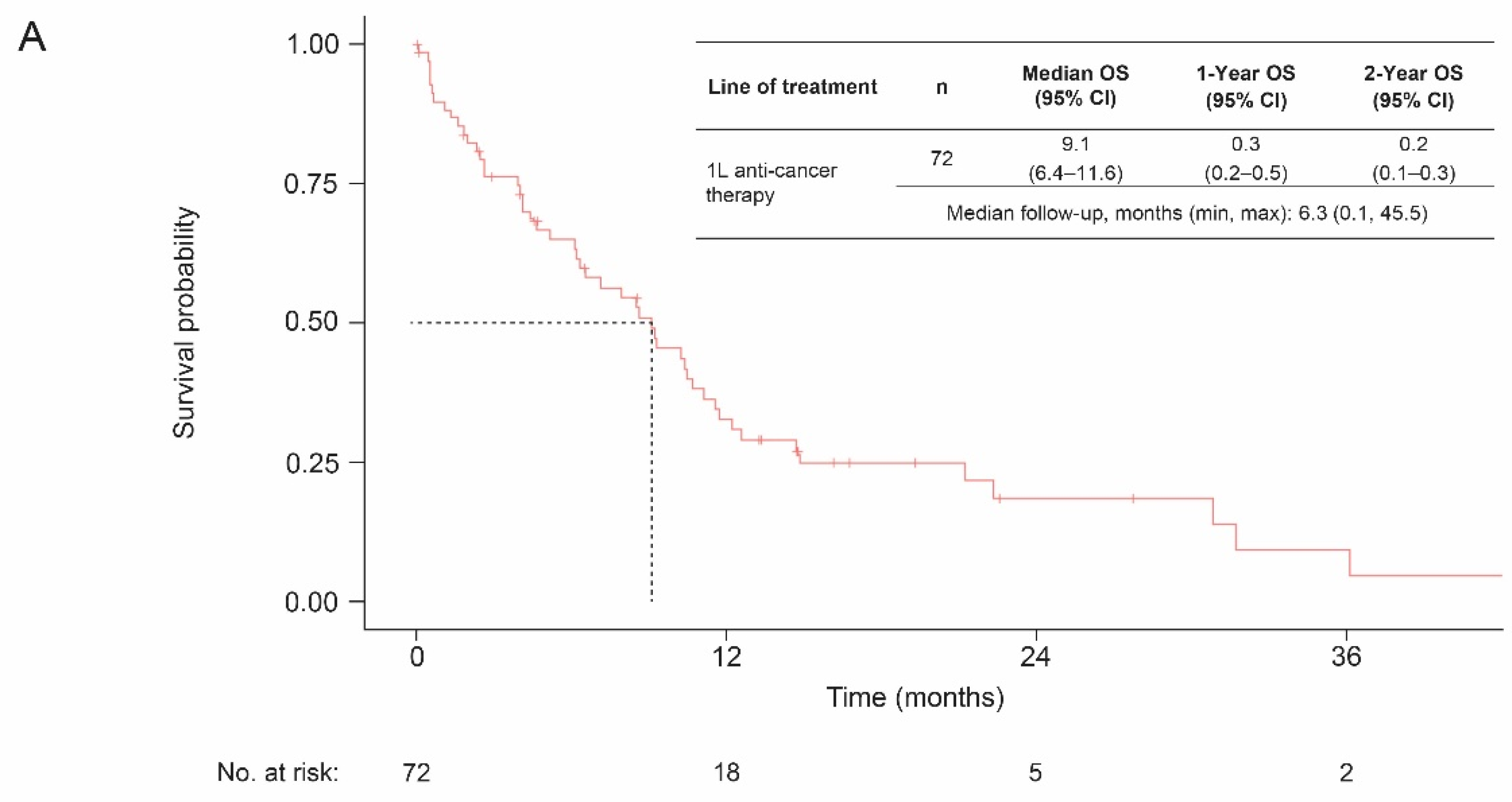
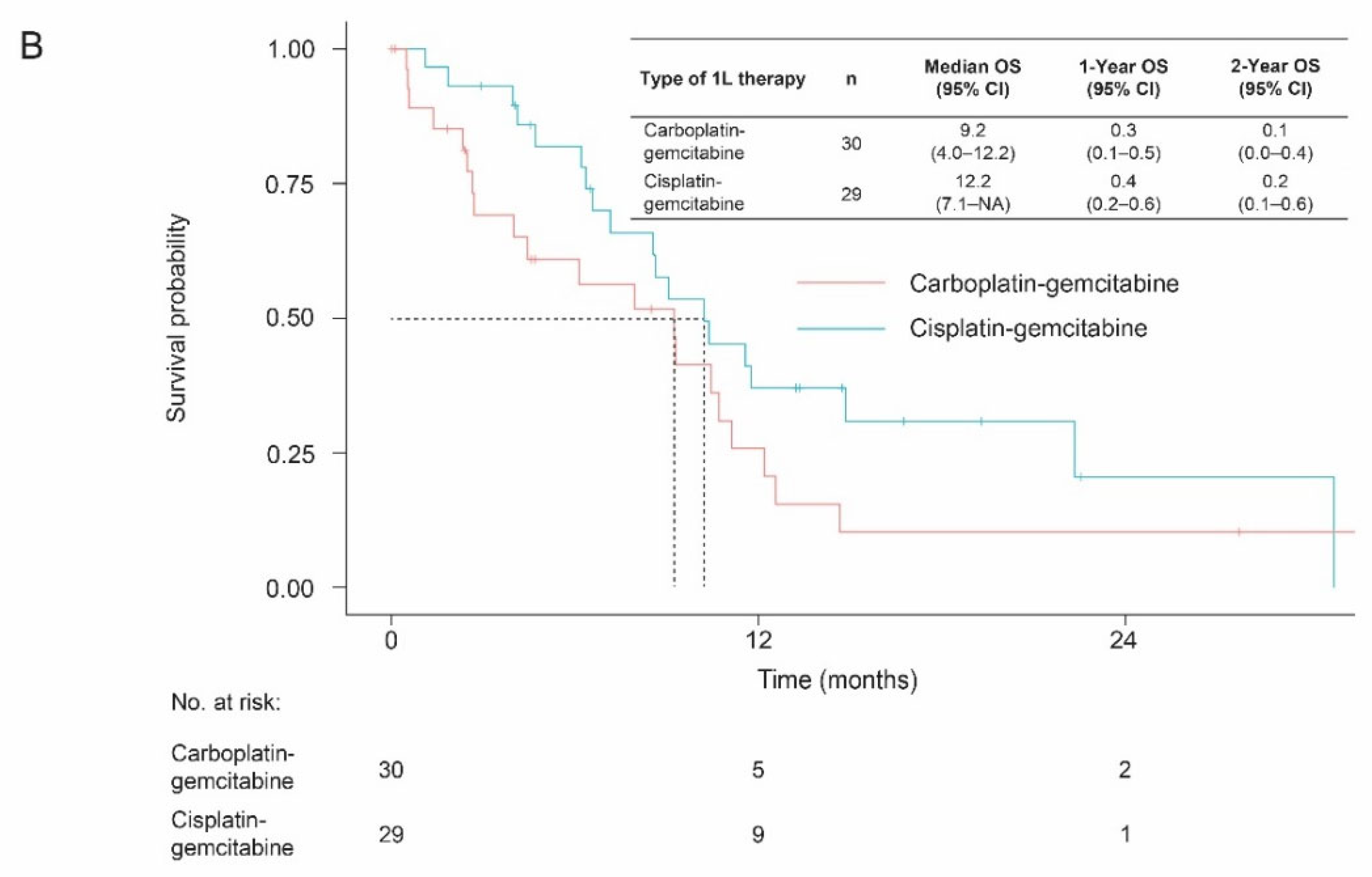
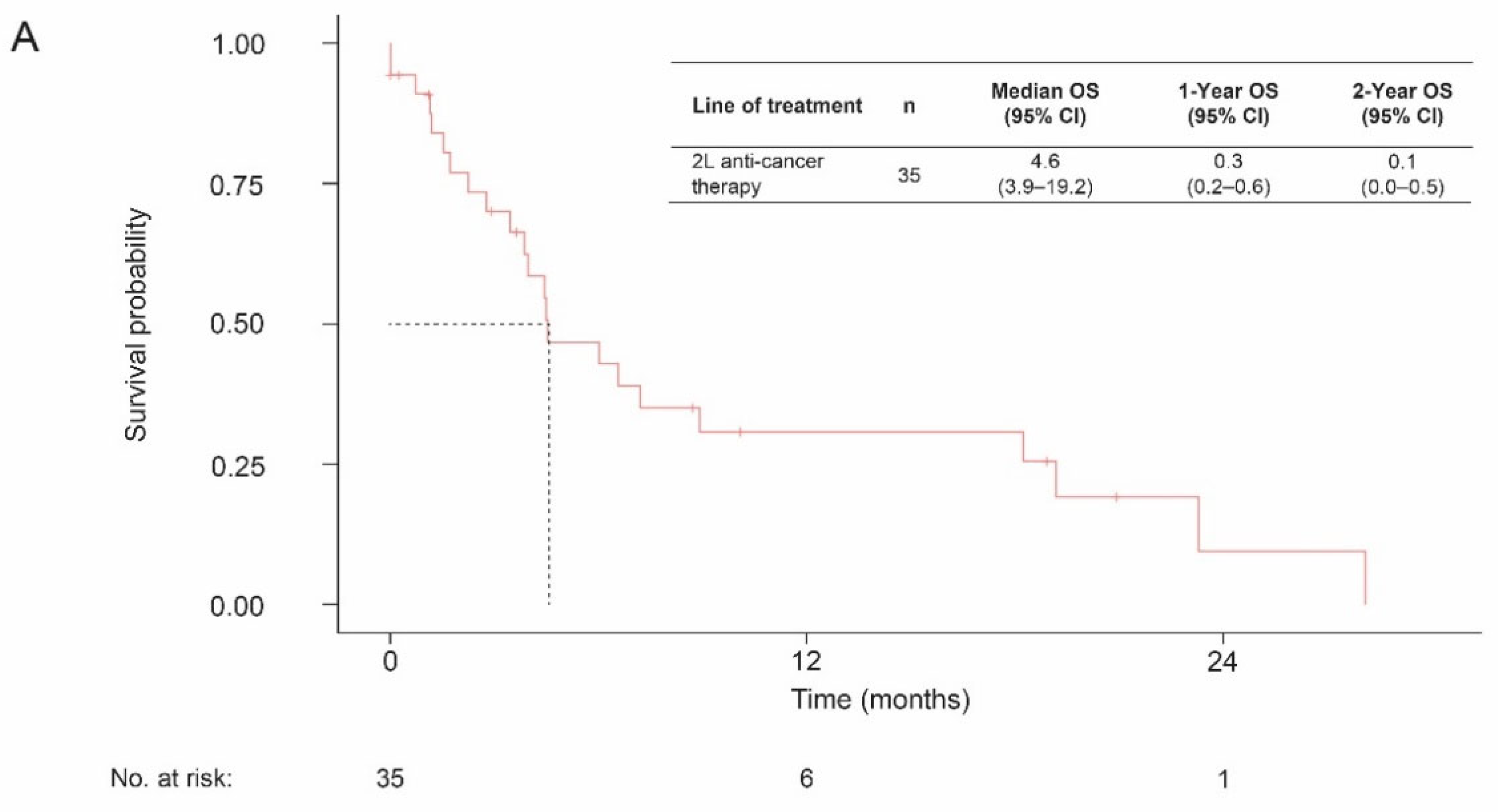
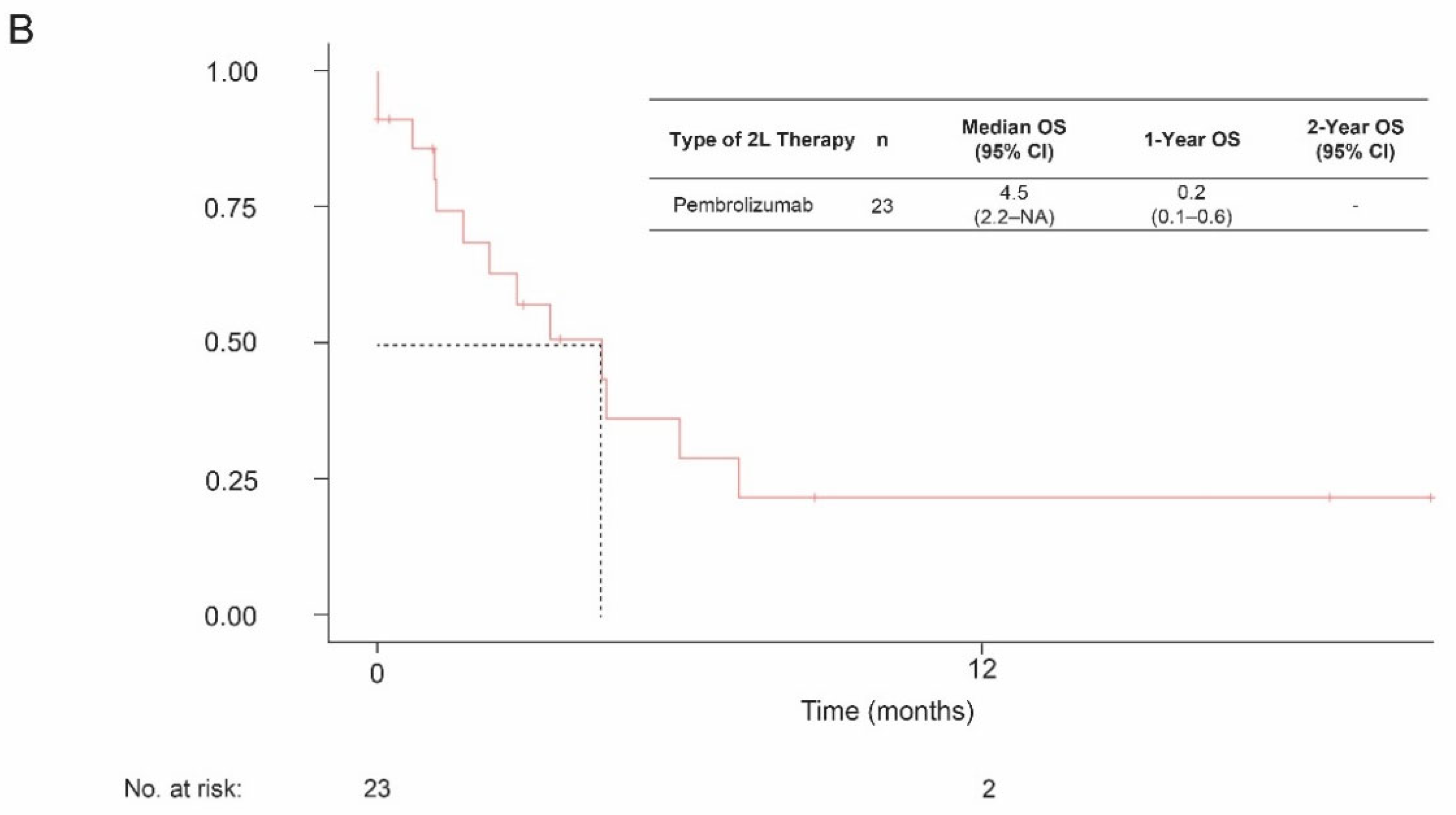
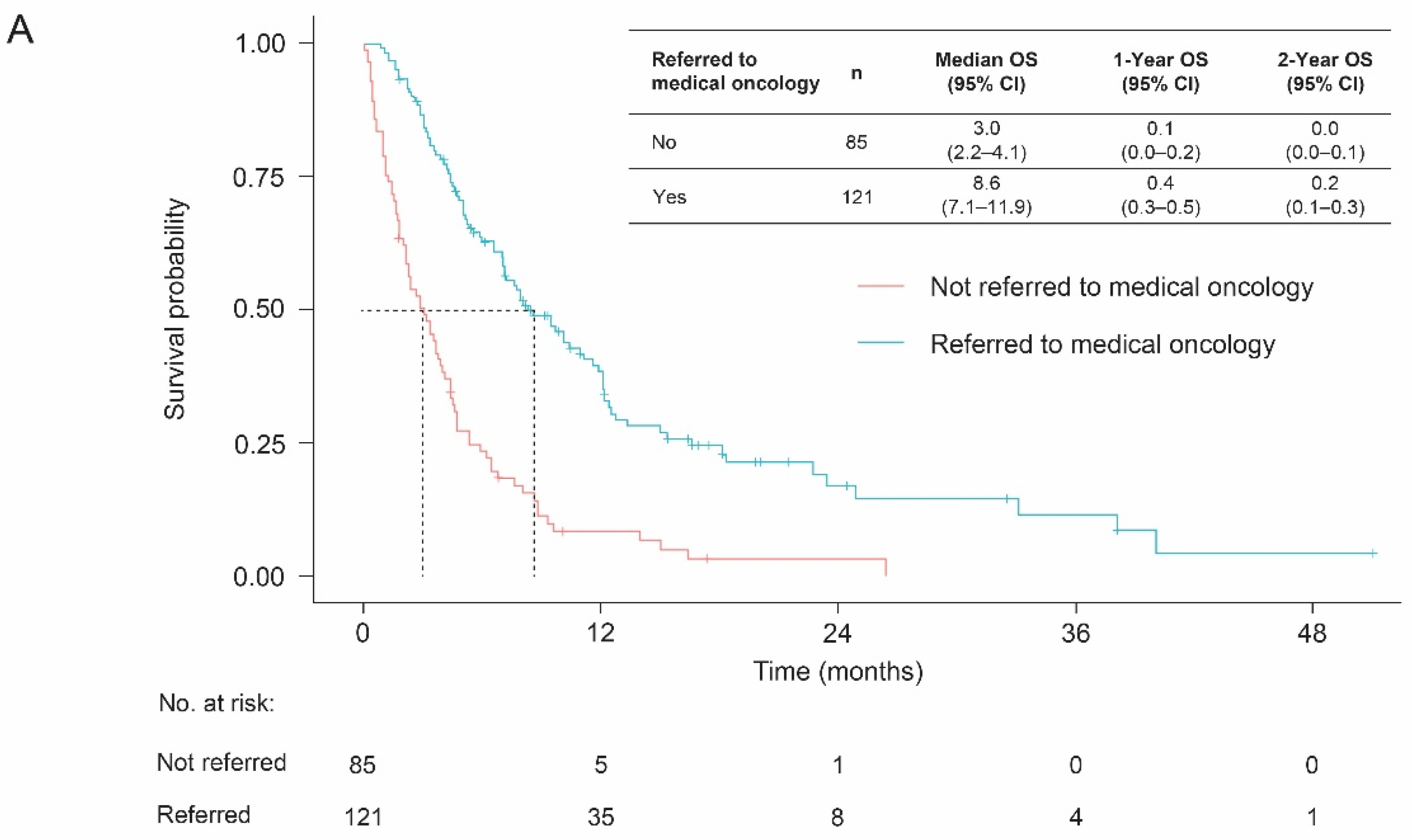
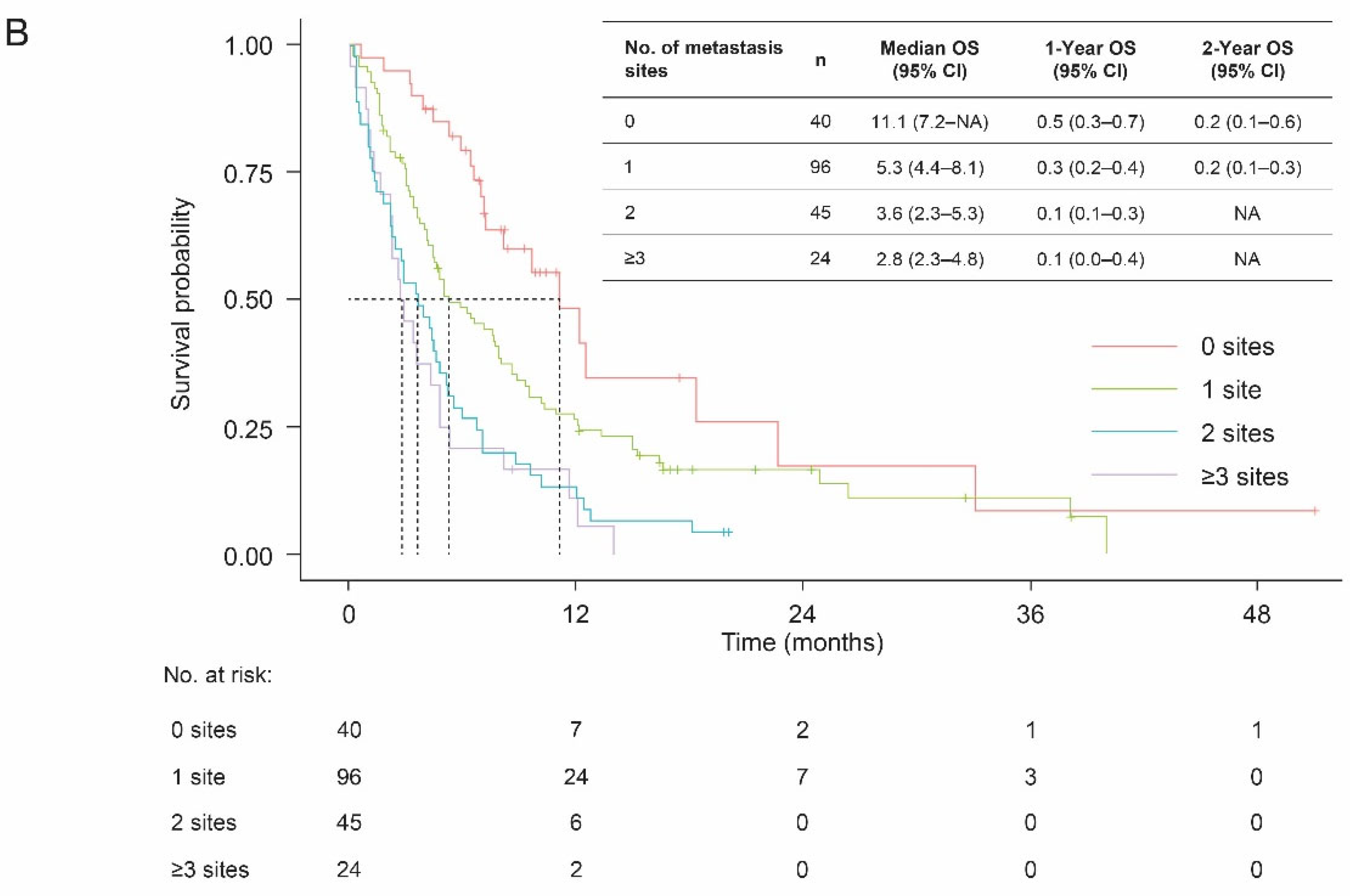
3.3. Overall Survival
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Government of Canada. Canadian Cancer Statistics 2021. Available online: https://cdn.cancer.ca/-/media/files/research/cancer-statistics/2021-statistics/2021_cancer-specific-stats.pdf?rev=bbadfd869b66415e97be9655a842858c&hash=3E552CE702E1BD7657E4E91FB7BA624B&_ga=2.166916030.1881271137.1651145809-343036952.1650888439 (accessed on 27 April 2022).
- Richters, A.; Aben, K.K.H.; Kiemeney, L.A.L.M. The global burden of urinary bladder cancer: An update. World J. Urol. 2020, 38, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.S.; Fung, F.D.H.; Leung, C.; Cheung, W.W.L.; Goggins, W.B.; Ng, C.F. The global epidemiology of bladder cancer: A joinpoint regression analysis of its incidence and mortality trends and projection. Sci. Rep. 2018, 8, 1129. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Bladder Cancer V.5.2021. Available online: https://www.nccn.org/guidelines/category_1 (accessed on 2 November 2021).
- American Cancer Society. Survival Rates for Bladder Cancer. Available online: https://www.cancer.org/cancer/bladder-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 3 February 2021).
- American Cancer Society. Bladder Cancer Stages. Available online: https://www.cancer.org/cancer/bladder-cancer/detection-diagnosis-staging/staging.html (accessed on 28 April 2022).
- Cancer Care Alberta. Locally Advanced/Metastatic Bladder Cancer (T4bNxM0, TxN2-3M0, TxNxM1). Clinical Practice Guideline GU–014 Version 2. Effective Date: October. 2021. Available online: https://www.albertahealthservices.ca/assets/info/hp/cancer/if-hp-cancer-guide-gu014-ambc.pdf (accessed on 24 June 2022).
- National Cancer Institute SEER Program. Cancer Stat Facts: Bladder Cancer. Available online: https://seer.cancer.gov/statfacts/html/urinb.html (accessed on 26 January 2022).
- Koufopoulou, M.; Miranda, P.A.; Kazmierska, P.; Deshpande, S.; Gaitonde, P. Clinical evidence for the first-line treatment of advanced urothelial carcinoma: Current paradigms and emerging treatment options. Cancer Treat. Rev. 2020, 89, 102072. [Google Scholar] [CrossRef]
- Morgans, A.; Galsky, M.; Hepp, Z.; Chang, N.; Campbell, M.; Wirtz, H.; Fuldeore, R.; Sesterhenn, S.; Surinach, A.; Liu, Y. Treatment patterns among patients with advanced urothelial carcinoma (aUC) in the USA. Ann. Oncol. 2021, 32, S714–S715. [Google Scholar] [CrossRef]
- Swami, U.; Grivas, P.; Pal, S.K.; Agarwal, N. Utilization of systemic therapy for treatment of advanced urothelial carcinoma: Lessons from real world experience. Cancer Treat. Res. Commun. 2021, 27, 100325. [Google Scholar] [CrossRef] [PubMed]
- Canada’s Drug and Health Technology Agency. Avelumab (Bavencio) for Urothelial Carcinoma—Details. Available online: https://www.cadth.ca/avelumab-bavencio-urothelial-carcinoma-details (accessed on 17 May 2022).
- Warren, M.; Kolinsky, M.; Canil, C.M.; Czaykowski, P.; Sridhar, S.S.; Black, P.C.; Booth, C.M.; Kassouf, W.; Eapen, L.; Mukherjee, S.D.; et al. Canadian Urological Association/Genitourinary Medical Oncologists of Canada consensus statement: Management of unresectable locally advanced and metastatic urothelial carcinoma. Can. Urol. Assoc. J. 2019, 13, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Canada’s Drug and Health Technology Agency. Keytruda for Metastatic Urothelial Carcinoma—Details. Available online: https://www.cadth.ca/keytruda-metastatic-urothelial-carcinoma-details (accessed on 17 May 2022).
- Canada’s Drug and Health Technology Agency. Enfortumab Vedotin. Available online: https://www.cadth.ca/enfortumab-vedotin (accessed on 24 June 2022).
- Janssen. Press Release: Janssen Receives Health Canada Approval of BALVERSA™ (Erdafitinib). Available online: https://www.janssen.com/canada/sites/www_janssen_com_canada/files/balversa_news_release_-_final-_feb._21_2020.pdf (accessed on 24 June 2022).
- Sonpavde, G.P.; Galsky, M.D.; Wright, P.; Hepp, Z.; Chang, N.N.; Willmon, C.; Sesterhenn, S.; Liu, Y.; Morgans, A.K. Real-world treatment patterns and clinical outcomes with first-line therapy in cisplatin-eligible and ineligible patients with advanced urothelial carcinoma. J. Clin. Oncol. 2022, 40, 4565. [Google Scholar] [CrossRef]
- Yip, S.M.; Kaiser, J.; Li, H.; North, S.; Heng, D.Y.C.; Alimohamed, N.S. Real-world Outcomes in Advanced Urothelial Cancer and the Role of Neutrophil to Lymphocyte Ratio. Clin. Genitourin. Cancer 2018, 16, e637–e644. [Google Scholar] [CrossRef]
- von der Maase, H.; Hansen, S.W.; Roberts, J.T.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Bodrogi, I.; Albers, P.; Knuth, A.; Lippert, C.M.; et al. Gemcitabine and Cisplatin Versus Methotrexate, Vinblastine, Doxorubicin, and Cisplatin in Advanced or Metastatic Bladder Cancer: Results of a Large, Randomized, Multinational, Multicenter, Phase III Study. J. Clin. Oncol. 2000, 18, 3068–3077. [Google Scholar] [CrossRef] [PubMed]
- De Santis, M.; Bellmunt, J.; Mead, G.; Kerst, J.M.; Leahy, M.; Maroto, P.; Gil, T.; Marreaud, S.; Daugaard, G.; Skoneczna, I.; et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J. Clin. Oncol. 2012, 30, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Niegisch, G.; Gerullis, H.; Lin, S.-W.; Pavlova, J.; Gondos, A.; Rudolph, A.; Haas, G.; Hennies, N.; Kramer, M.W. A real-world data study to evaluate treatment patterns, clinical characteristics and survival outcomes for first-and second-line treatment in locally advanced and metastatic urothelial cancer patients in Germany. J. Cancer 2018, 9, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, S.; Thompson, M.; Sopwith, W.; Godden, P.; Seshagiri, D.; Adedokun, L.; Zucker, K.; Jain, S.; Kotwal, S.; Prescott, S.; et al. Current treatment and outcomes benchmark for locally advanced or metastatic urothelial cancer from a large UK-based single centre. Front. Oncol. 2020, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Barone, B.; Calogero, A.; Scafuri, L.; Ferro, M.; Lucarelli, G.; Di Zazzo, E.; Sicignano, E.; Falcone, A.; Romano, L.; De Luca, L.; et al. Immune Checkpoint Inhibitors as a Neoadjuvant/Adjuvant Treatment of Muscle-Invasive Bladder Cancer: A Systematic Review. Cancers 2022, 14, 2545. [Google Scholar] [CrossRef] [PubMed]
- Iacovino, M.L.; Miceli, C.C.; De Felice, M.; Barone, B.; Pompella, L.; Chiancone, F.; Di Zazzo, E.; Tirino, G.; Della Corte, C.M.; Imbimbo, C.; et al. Novel Therapeutic Opportunities in Neoadjuvant Setting in Urothelial Cancers: A New Horizon Opened by Molecular Classification and Immune Checkpoint Inhibitors. Int. J. Mol. Sci. 2022, 23, 1133. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Durán, I.; Lee, J.L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. A Study of Erdafitinib Compared With Vinflunine or Docetaxel or Pembrolizumab in Participants With Advanced Urothelial Cancer and Selected Fibroblast Growth Factor Receptor (FGFR) Gene Aberrations. Available online: https://clinicaltrials.gov/ct2/show/NCT03390504 (accessed on 15 December 2021).
| Variable | De Novo Cohort (N = 206) | |
|---|---|---|
| Age | Mean (SD), years | 73.0 (10.1) |
| <60 years, n (%) | 45 (21.8) | |
| ≥60 years, n (%) | 161 (78.2) | |
| Sex, n (%) | Female | 43 (20.9) |
| Male | 163 (79.1) | |
| Year of diagnosis, n (%) [median follow-up, months] | 2015 | 32 (15.5) [4.0] |
| 2016 | 31 (15.0) [6.0] | |
| 2017 | 43 (20.9) [4.4] | |
| 2018 | 33 (16.0) [4.6] | |
| 2019 | 67 (32.5) [5.9] | |
| AJCC TNM stage at diagnosis, n (%) | Unresected locally advanced | 40 (19.4) |
| Metastatic UC | 166 (80.6) | |
| Disease histology, n (%) | TCC | 206 (100) |
| No. of metastasis sites at diagnosis, n (%) | 0 | 40 (19.4) |
| 1 | 96 (46.6) | |
| 2 | 45 (21.8) | |
| ≥3 | 24 (11.7) | |
| Missing | 1 (0.5) | |
| Sites of metastases at diagnosis a, n (%) | Lymph nodes | 83 (40.5) |
| Bone | 55 (26.8) | |
| Lung | 44 (21.5) | |
| Hepatic | 36 (17.6) | |
| Peritoneum | 20 (9.8) | |
| Adrenals | <10 | |
| Brain | <10 | |
| Other | 18 (8.8) | |
| Overall, n (%) | Year of Diagnosis, n (%) a | ||||
|---|---|---|---|---|---|
| 2015–2016 | 2017 | 2018 | 2019 | ||
| No systemic therapy | 134 (65.0) | 41 (65.1) | 30 (69.8) | 22 (66.7) | 41 (61.2) |
| 1L systemic therapy | 72 (35.0) | 22 (34.9) | 13 (30.2) | 11 (33.3) | 26 (38.8) |
| Treatment | N (%) | Duration of Treatment, Months | ||
|---|---|---|---|---|
| Mean (SD) | Median [25th–75th Percentile] | Median (Kaplan-Meier) | ||
| 1L anti-cancer therapies | 72 (100.0) | 3.2 (2.3) | 2.8 [1.1–4.4] | 3.6 |
| Carboplatin-gemcitabine | 30 (41.7) | 3.5 (2.6) | 3.0 [1.1–4.6] | 4.2 |
| Cisplatin-gemcitabine | 29 (40.3) | 3.4 (1.9) | 3.1 [1.9–4.4] | 3.5 |
| Other a | 13 (18.1) | - | - | - |
| 2L anti-cancer therapies | 35 (100.0) | 4.2 (4.7) | 3.0 [1.6–5.0] | 4.4 |
| Pembrolizumab | 23 (65.7) | 4.4 (5.4) | 3.0 [1.4–5.0] | 5.4 |
| Other a | 12 (34.3) | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alimohamed, N.; Grewal, S.; Wirtz, H.S.; Hepp, Z.; Sauvageau, S.; Boyne, D.J.; Brenner, D.R.; Cheung, W.Y.; Jarada, T.N. Understanding Treatment Patterns and Outcomes among Patients with De Novo Unresectable Locally Advanced or Metastatic Urothelial Cancer: A Population-Level Retrospective Analysis from Alberta, Canada. Curr. Oncol. 2022, 29, 7587-7597. https://doi.org/10.3390/curroncol29100599
Alimohamed N, Grewal S, Wirtz HS, Hepp Z, Sauvageau S, Boyne DJ, Brenner DR, Cheung WY, Jarada TN. Understanding Treatment Patterns and Outcomes among Patients with De Novo Unresectable Locally Advanced or Metastatic Urothelial Cancer: A Population-Level Retrospective Analysis from Alberta, Canada. Current Oncology. 2022; 29(10):7587-7597. https://doi.org/10.3390/curroncol29100599
Chicago/Turabian StyleAlimohamed, Nimira, Simrun Grewal, Heidi S. Wirtz, Zsolt Hepp, Stephanie Sauvageau, Devon J. Boyne, Darren R. Brenner, Winson Y. Cheung, and Tamer N. Jarada. 2022. "Understanding Treatment Patterns and Outcomes among Patients with De Novo Unresectable Locally Advanced or Metastatic Urothelial Cancer: A Population-Level Retrospective Analysis from Alberta, Canada" Current Oncology 29, no. 10: 7587-7597. https://doi.org/10.3390/curroncol29100599
APA StyleAlimohamed, N., Grewal, S., Wirtz, H. S., Hepp, Z., Sauvageau, S., Boyne, D. J., Brenner, D. R., Cheung, W. Y., & Jarada, T. N. (2022). Understanding Treatment Patterns and Outcomes among Patients with De Novo Unresectable Locally Advanced or Metastatic Urothelial Cancer: A Population-Level Retrospective Analysis from Alberta, Canada. Current Oncology, 29(10), 7587-7597. https://doi.org/10.3390/curroncol29100599





