Adherence to Mediterranean Diet and Nutritional Status in Women with Breast Cancer: What Is Their Impact on Disease Progression and Recurrence-Free Patients’ Survival?
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Statistical Analysis
3. Results
3.1. Evaluation of the Study Population’s Anthropometry, Disease and Lifestyle Characteristics and Recurrence-Free Survival
3.2. Associations between Examined Characteristics and Mediterranean Diet (MD) Adherence
3.3. Associations between Examined Characteristics and Nutritional Status
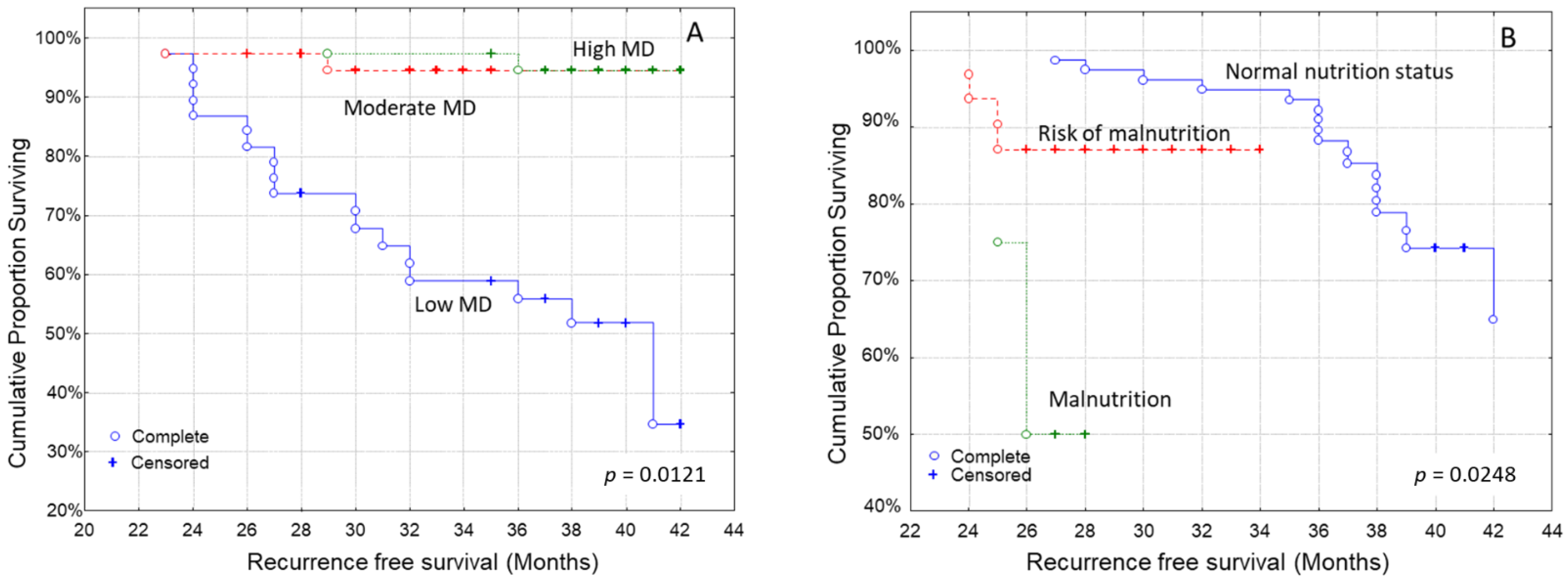
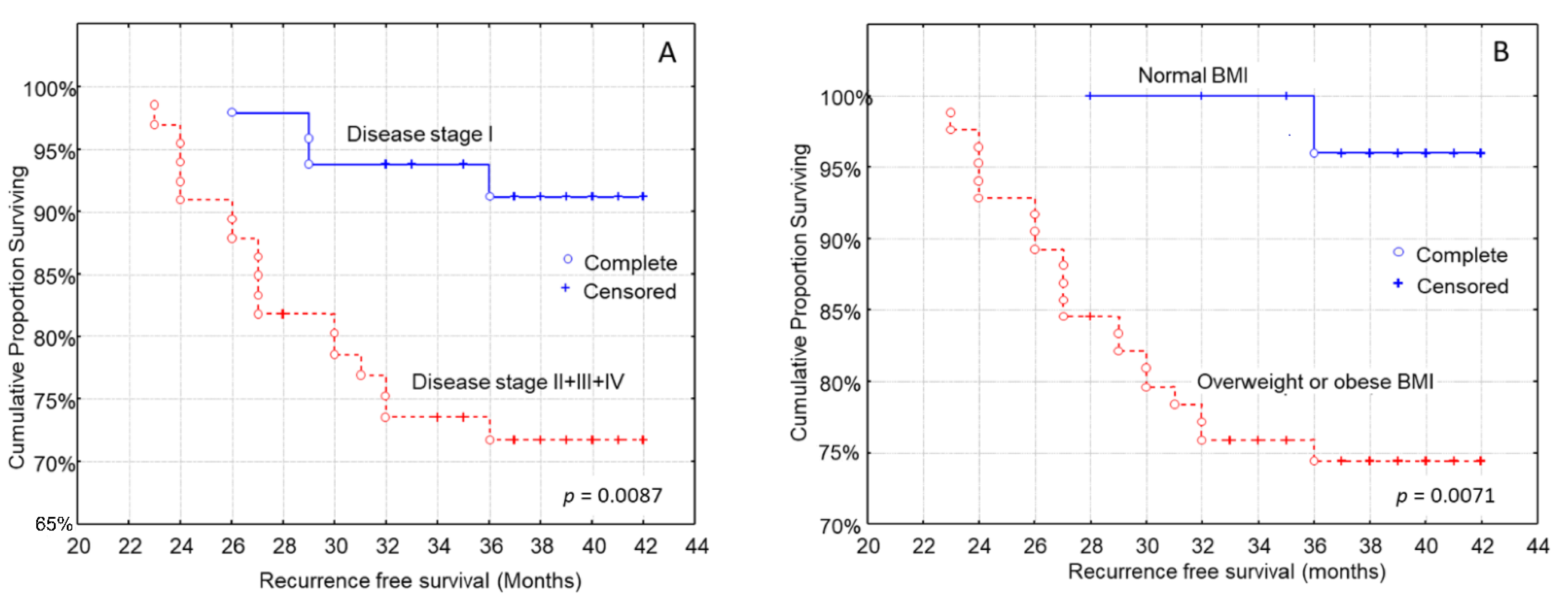
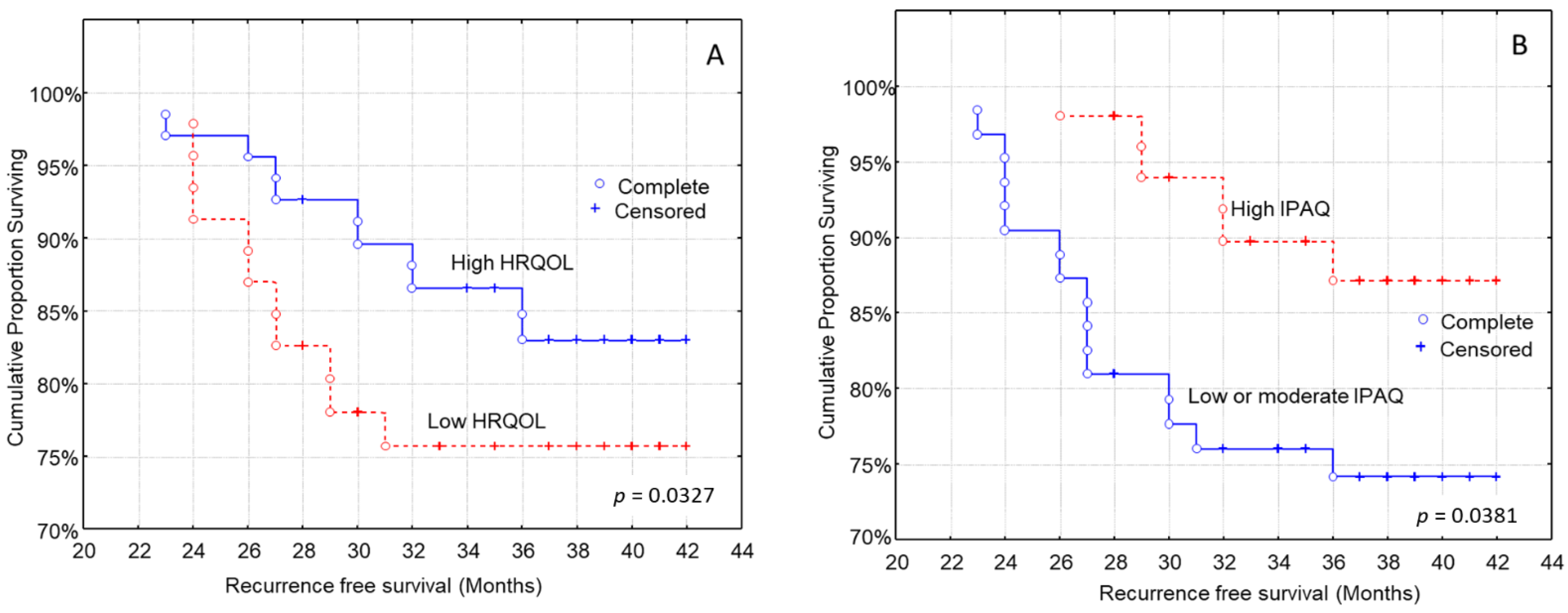
3.4. Kaplan–Meier Analysis for the Time to Recurrence after Diagnosis
3.5. Multivariate Analysis for the Time to Recurrence after Diagnosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Tomorrow; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, R.M.; Webb-Vargas, Y.; Wheeler, W.; Gail, M.H. Proportion of U.S. Trends in Breast Cancer Incidence Attributable to Long-term Changes in Risk Factor Distributions. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Heer, E.; Harper, A.; Escandor, N.; Sung, H.; McCormack, V.; Fidler-Benaoudia, M.M. Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. Lancet Glob. Health 2020, 8, e1027–e1037. [Google Scholar] [CrossRef]
- Arnold, M.; Rutherford, M.J.; Bardot, A.; Ferlay, J.; Andersson, T.M.; Myklebust, T.Å.; Tervonen, H.; Thursfield, V.; Ransom, D.; Shack, L. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): A population-based study. Lancet Oncol. 2019, 20, 1493–1505. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- CANCER RESEARCH, UK. Breast cancer survival statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer/survival/%20Office%20for%20National%20Statistics%2C%202019 (accessed on 15 June 2022).
- Cokkinides, V.; Albano, J.; Samuels, A.; Ward, M.; Thum, J. American Cancer Society: Cancer Facts and Figures; American Cancer Society: Atlanta, GA, USA, 2005. [Google Scholar]
- AMERICAN CANCER SOCIETY. Inflammatory Breast Cancer. Available online: https://www.cancer.org/cancer/breast-cancer/about/types-of-breast-cancer/inflammatory-breast-cancer.html#references (accessed on 14 December 2021).
- Gotsis, E.; Anagnostis, P.; Mariolis, A.; Vlachou, A.; Katsiki, N.; Karagiannis, A. Health benefits of the Mediterranean Diet: An update of research over the last 5 years. Angiology 2015, 66, 304–318. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Naska, A.; Orfanos, P.; Trichopoulos, D. Mediterranean diet in relation to body mass index and waist-to-hip ratio: The Greek European Prospective Investigation into Cancer and Nutrition Study. Am. J. Clin. Nutr. 2005, 82, 935–940. [Google Scholar] [CrossRef]
- Potentas, E.; Witkowska, A.M.; Zujko, M.E. Mediterranean diet for breast cancer prevention and treatment in postmenopausal women. Prz. Menopauzalny 2015, 14, 247–253. [Google Scholar] [CrossRef]
- Shapira, N. The potential contribution of dietary factors to breast cancer prevention. Eur. J. Cancer Prev. 2017, 26, 385–395. [Google Scholar] [CrossRef]
- Mourouti, N.; Kontogianni, M.D.; Papavagelis, C.; Panagiotakos, D.B. Diet and breast cancer: A systematic review. Int. J. Food Sci. Nutr. 2015, 66, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Cathcart-Rake, E.J.; Ruddy, K.J.; Johnson, R.H. Modifiable risk factors for the development of breast cancer in young women. Cancer J. 2018, 24, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Bella, F.; Godos, J.; Sciacca, S.; Del Rio, D.; Ray, S.; Galvano, F.; Giovannucci, E.L. Possible role of diet in cancer: Systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr. Rev. 2017, 75, 405–419. [Google Scholar] [CrossRef]
- Fund, W.C.R. Cancer Prevention Recommendations. Available online: https://www.wcrf.org/dietandcancer/cancer-prevention-recommendations (accessed on 12 April 2022).
- Schwedhelm, C.; Boeing, H.; Hoffmann, G.; Aleksandrova, K.; Schwingshackl, L. Effect of diet on mortality and cancer recurrence among cancer survivors: A systematic review and meta-analysis of cohort studies. Nutr. Rev. 2016, 74, 737–748. [Google Scholar] [CrossRef]
- Fung, T.T.; Hu, F.B.; McCullough, M.L.; Newby, P.K.; Willett, W.C.; Holmes, M.D. Diet quality is associated with the risk of estrogen receptor-negative breast cancer in postmenopausal women. J. Nutr. 2006, 136, 466–472. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Bamia, C.; Lagiou, P.; Trichopoulos, D. Conformity to traditional Mediterranean diet and breast cancer risk in the Greek EPIC (European Prospective Investigation into Cancer and Nutrition) cohort. Am. J. Clin. Nutr. 2010, 92, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Castelló, A.; Pollán, M.; Buijsse, B.; Ruiz, A.; Casas, A.M.; Baena-Cañada, J.M.; Lope, V.; Antolín, S.; Ramos, M.; Muñoz, M.; et al. Spanish Mediterranean diet and other dietary patterns and breast cancer risk: Case-control EpiGEICAM study. Br. J. Cancer 2014, 111, 1454–1462. [Google Scholar] [CrossRef]
- Hastert, T.A.; Beresford, S.A.; Patterson, R.E.; Kristal, A.R.; White, E. Adherence to WCRF/AICR cancer prevention recommendations and risk of postmenopausal breast cancer. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1498–1508. [Google Scholar] [CrossRef]
- Du, M.; Liu, S.H.; Mitchell, C.; Fung, T.T. Associations between Diet Quality Scores and Risk of Postmenopausal Estrogen Receptor-Negative Breast Cancer: A Systematic Review. J. Nutr. 2018, 148, 100–108. [Google Scholar] [CrossRef]
- Kroenke, C.H.; Fung, T.T.; Hu, F.B.; Holmes, M.D. Dietary patterns and survival after breast cancer diagnosis. J. Clin. Oncol. 2005, 23, 9295–9303. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Willett, W.C.; Fung, T.; Rosner, B.; Holmes, M.D. Diet quality indices and postmenopausal breast cancer survival. Nutr. Cancer 2011, 63, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Kwan, M.L.; Greenlee, H.; Lee, V.S.; Castillo, A.; Gunderson, E.P.; Habel, L.A.; Kushi, L.H.; Sweeney, C.; Tam, E.K.; Caan, B.J. Multivitamin use and breast cancer outcomes in women with early-stage breast cancer: The Life after Cancer Epidemiology study. Breast Cancer Res. Treat. 2011, 130, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Izano, M.A.; Fung, T.T.; Chiuve, S.S.; Hu, F.B.; Holmes, M.D. Are diet quality scores after breast cancer diagnosis associated with improved breast cancer survival? Nutr. Cancer 2013, 65, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Vrieling, A.; Buck, K.; Seibold, P.; Heinz, J.; Obi, N.; Flesch-Janys, D.; Chang-Claude, J. Dietary patterns and survival in German postmenopausal breast cancer survivors. Br. J. Cancer 2013, 108, 188–192. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Adherence to Mediterranean diet and risk of cancer: An updated systematic review and meta-analysis of observational studies. Cancer Med. 2015, 4, 1933–1947. [Google Scholar] [CrossRef]
- Dewys, W.D.; Begg, C.; Lavin, P.T.; Band, P.R.; Bennett, J.M.; Bertino, J.R.; Cohen, M.H.; Douglass, H.O., Jr.; Engstrom, P.F.; Ezdinli, E.Z. Prognostic effect of weight loss prior tochemotherapy in cancer patients. Am. J. Med. 1980, 69, 491–497. [Google Scholar] [CrossRef]
- Argiles, J. Cancer-associated malnutrition. Eur. J. Oncol. Nurs. 2005, 9, S39–S50. [Google Scholar] [CrossRef]
- Laviano, A.; Di Lazzaro, L.; Koverech, A. Nutrition support and clinical outcome in advanced cancer patients. Proc. Nutr. Soc. 2018, 77, 388–393. [Google Scholar] [CrossRef]
- Santarpia, L.; Contaldo, F.; Pasanisi, F. Nutritional screening and early treatment of malnutrition in cancer patients. J. Cachexia Sarcopenia Muscle 2011, 2, 27–35. [Google Scholar] [CrossRef]
- Gandy, J. Manual of Dietetic Practice; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Arends, J.; Bodoky, G.; Bozzetti, F.; Fearon, K.; Muscaritoli, M.; Selga, G.; Von Meyenfeldt, M.; Zürcher, G.; Fietkau, R.; Aulbert, E. ESPEN guidelines on enteral nutrition: Non-surgical oncology. Clin. Nutr. 2006, 25, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Andreyev, H.; Norman, A.; Oates, J.; Cunningham, D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur. J. Cancer 1998, 34, 503–509. [Google Scholar] [CrossRef]
- Mantzorou, M.; Koutelidakis, A.; Theocharis, S.; Giaginis, C. Clinical value of nutritional status in cancer: What is its impact and how it affects disease progression and prognosis? Nutr. Cancer 2017, 69, 1151–1176. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, B.; Hou, L.; Xie, Y.; Cao, X. Pre-operative prognostic nutritional index predicts the outcomes for triple-negative breast cancer. Tumor Biol. 2014, 35, 12165–12171. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K. Cancer cachexia: Developing multimodal therapy for a multidimensional problem. Eur. J. Cancer 2008, 44, 1124–1132. [Google Scholar] [CrossRef]
- Consul, N.; Guo, X.; Coker, C.; Lopez-Pintado, S.; Hibshoosh, H.; Zhao, B.; Kalinsky, K.; Acharyya, S. Monitoring metastasis and cachexia in a patient with breast cancer: A case study. Clin. Med. Insights Oncol. 2016, 10, 83–94. [Google Scholar] [CrossRef]
- Jain, M.; Miller, A.B. Tumor characteristics and survival of breast cancer patients in relation to premorbid diet and body size. Breast Cancer Res. Treat. 1997, 42, 43–55. [Google Scholar] [CrossRef]
- Bamia, C. Dietary patterns in association to cancer incidence and survival: Concept, current evidence, and suggestions for future research. Eur. J. Clin. Nutr. 2018, 72, 818–825. [Google Scholar] [CrossRef]
- Pierce, J.P.; Natarajan, L.; Caan, B.J.; Parker, B.A.; Greenberg, E.R.; Flatt, S.W.; Rock, C.L.; Kealey, S.; Al-Delaimy, W.K.; Bardwell, W.A. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: The Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA 2007, 298, 289–298. [Google Scholar] [CrossRef]
- Torbahn, G.; Strauß, T.; Sieber, C.; Volkert, D.; Kiesswetter, E. Use of mini nutritional assessment (MNA)® in oncological patients–an evidence map. Clin. Nutr. 2018, 37, S122. [Google Scholar] [CrossRef]
- Fatmah, F. Training program to support posbindu cadre knowledge and community health centre staff in the Geriatric Nutrition Service. ASEAN J. Community Engagem. 2020, 4, 500–518. [Google Scholar] [CrossRef]
- Boulahssass, R.; Gonfrier, S.; Ferrero, J.-M.; Sanchez, M.; Mari, V.; Moranne, O.; Rambaud, C.; Auben, F.; Bereder, J.-M.; Bereder, I. Predicting early death in older adults with cancer. Eur. J. Cancer 2018, 100, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Guigoz, Y.; Vellas, B. The Mini Nutritional Assessment (MNA) for grading the nutritional state of elderly patients: Presentation of the MNA, history and validation. In Proceedings of the Nestle Nutrition Workshop Series. Clinical Performance Programme, Laussane, Switzerland, October 1997; pp. 3–11. [Google Scholar] [CrossRef]
- Turner-Bowker, D.M.; Hao, Y.; Foley, C.; Galipeau, N.; Mazar, I.; Krohe, M.; Globe, D.; Shields, A.L. The use of patient-reported outcomes in advanced breast cancer clinical trials: A review of the published literature. Curr. Med. Res. Opin. 2016, 32, 1709–1717. [Google Scholar] [CrossRef]
- Kontodimopoulos, N.; Ntinoulis, K.; Niakas, D. Validity of the Greek EORTC QLQ-C30 and QLQ-BR23 for measuring health-related quality of life in breast cancer patients. Eur. J. Cancer Care 2011, 20, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Buckland, G.; González, C.A.; Agudo, A.; Vilardell, M.; Berenguer, A.; Amiano, P.; Ardanaz, E.; Arriola, L.; Barricarte, A.; Basterretxea, M. Adherence to the Mediterranean diet and risk of coronary heart disease in the Spanish EPIC Cohort Study. Am. J. Epidemiol. 2009, 170, 1518–1529. [Google Scholar] [CrossRef]
- Murray, J.M.; Coleman, H.G.; Hunter, R.F. Physical activity and cancer risk: Findings from the UK Biobank, a large prospective cohort study. Cancer Epidemiol. 2020, 68, 101780. [Google Scholar] [CrossRef] [PubMed]
- Gavala-González, J.; Torres-Pérez, A.; Fernández-García, J.C. Impact of Rowing Training on Quality of Life and Physical Activity Levels in Female Breast Cancer Survivors. Int. J. Environ. Res. Public Health 2021, 18, 7188. [Google Scholar] [CrossRef]
- Sellen, D. Physical Status: The Use and Interpretation of Anthropometry. Report of a WHO Expert Committee. WHO Technical Report Series No. 854. Pp. 452. (WHO, Geneva, 1995.) Swiss Fr 71.00. J. Biosoc. Sci. 1998, 30, 135–144. [Google Scholar] [CrossRef]
- Centers for Disease Control Prevention. National Health and Nutrition Examination Survey (NHANES): Anthropometry Procedures Manual; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2007.
- Pan, H.; Gray, R.; Braybrooke, J.; Davies, C.; Taylor, C.; McGale, P.; Peto, R.; Pritchard, K.I.; Bergh, J.; Dowsett, M. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. New Engl. J. Med. 2017, 377, 1836–1846. [Google Scholar] [CrossRef]
- Ho-Huynh, A.; Tran, A.; Bray, G.; Abbot, S.; Elston, T.; Gunnarsson, R.; de Costa, A. Factors influencing breast cancer outcomes in Australia: A systematic review. Eur. J. Cancer Care 2019, 28, e13038. [Google Scholar] [CrossRef]
- Dar, H.; Johansson, A.; Nordenskjöld, A.; Iftimi, A.; Yau, C.; Perez-Tenorio, G.; Benz, C.; Nordenskjöld, B.; Stål, O.; Esserman, L.J. Assessment of 25-year survival of women with estrogen receptor–positive/ERBB2-negative breast cancer treated with and without tamoxifen therapy: A secondary analysis of data from the Stockholm tamoxifen randomized clinical trial. JAMA Netw. Open 2021, 4, e2114904. [Google Scholar] [CrossRef] [PubMed]
- Hastings, J.; Iganej, S.; Huang, C.; Huang, R.; Slezak, J. Risk factors for locoregional recurrence after mastectomy in stage T1 N0 breast cancer. Am. J. Clin. Oncol. 2014, 37, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Jiralerspong, S.; Goodwin, P.J. Obesity and breast cancer prognosis: Evidence, challenges, and opportunities. J. Clin. Oncol. 2016, 34, 4203–4216. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Vieira, A.; Aune, D.; Bandera, E.; Greenwood, D.; McTiernan, A.; Rosenblatt, D.N.; Thune, I.; Vieira, R.; Norat, T. Body mass index and survival in women with breast cancer—Systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 2014, 25, 1901–1914. [Google Scholar] [CrossRef] [PubMed]
- Warren, L.E.; Ligibel, J.A.; Chen, Y.-H.; Truong, L.; Catalano, P.J.; Bellon, J.R. Body mass index and locoregional recurrence in women with early-stage breast cancer. Ann. Surg. Oncol. 2016, 23, 3870–3879. [Google Scholar] [CrossRef]
- Picon-Ruiz, M.; Morata-Tarifa, C.; Valle-Goffin, J.J.; Friedman, E.R.; Slingerland, J.M. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J. Clin. 2017, 67, 378–397. [Google Scholar] [CrossRef]
- Fallone, F.; Deudon, R.; Muller, C.; Vaysse, C. Cancer du sein, obésité et tissu adipeux-Un trio à haut risque. Médecine/Sci. 2018, 34, 1079–1086. [Google Scholar] [CrossRef]
- Filippidis, F.; Tzavara, C.; Dimitrakaki, C.; Tountas, Y. Compliance with a healthy lifestyle in a representative sample of the Greek population: Preliminary results of the Hellas Health I study. Public Health 2011, 125, 436–441. [Google Scholar] [CrossRef]
- Kyriacou, A.; Evans, J.M.; Economides, N.; Kyriacou, A. Adherence to the Mediterranean diet by the Greek and Cypriot population: A systematic review. Eur. J. Public Health 2015, 25, 1012–1018. [Google Scholar] [CrossRef]
- Castro-Espin, C.; Agudo, A. The Role of Diet in Prognosis among Cancer Survivors: A Systematic Review and Meta-Analysis of Dietary Patterns and Diet Interventions. Nutrients 2022, 14, 348. [Google Scholar] [CrossRef]
- Kwan, M.; Weltzien, E.; Kushi, L.; Castillo, A.; Slattery, M.L.; Caan, B.J. Dietary patterns and breast cancer recurrence and survival among women with early-stage breast cancer. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Weigl, J.; Hauner, H.; Hauner, D. Can nutrition lower the risk of recurrence in breast cancer. Breast Care 2018, 13, 86–91. [Google Scholar] [CrossRef]
- Augimeri, G.; Montalto, F.I.; Giordano, C.; Barone, I.; Lanzino, M.; Catalano, S.; Andò, S.; De Amicis, F.; Bonofiglio, D. Nutraceuticals in the Mediterranean Diet: Potential Avenues for Breast Cancer Treatment. Nutrients 2021, 13, 2557. [Google Scholar] [CrossRef] [PubMed]
- Escrich, E.; Solanas, M.; Moral, R.; Escrich, R. Modulatory effects and molecular mechanisms of olive oil and other dietary lipids in breast cancer. Curr. Pharm. Des. 2011, 17, 813–830. [Google Scholar] [CrossRef]
- Donovan, M.G.; Selmin, O.I.; Stillwater, B.J.; Neumayer, L.A.; Romagnolo, D.F. Do olive and fish oils of the mediterranean diet have a role in triple negative breast cancer prevention and therapy? An exploration of evidence in cells and animal models. Front. Nutr. 2020, 7, 571455. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, M.; McKoy, J.M.; Bhulani, N.N.A.; Valero, V.; Barcenas, C.H.; Popat, U.R.; Sri, M.K.; Shah, J.B.; Dinney, C.P. Malnutrition in older patients with cancer: Appraisal of the Mini Nutritional Assessment, weight loss, and body mass index. J. Geriatr. Oncol. 2018, 9, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Lorenzon, L.; Brandl, A.; Guiral, D.C.; Hoogwater, F.; Lundon, D.; Marano, L.; Montagna, G.; Polom, K.; Primavesi, F.; Schrage, Y. Nutritional assessment in surgical oncology: An ESSO-EYSAC global survey. Eur. J. Surg. Oncol. 2020, 46, 2074–2082. [Google Scholar] [CrossRef]
- Wang, Y.; Battseren, B.; Yin, W.; Lin, Y.; Zhou, L.; Yang, F.; Wang, Y.; Sun, L.; Lu, J. Predictive and prognostic value of prognostic nutritional index for locally advanced breast cancer. Gland Surg. 2019, 8, 618. [Google Scholar] [CrossRef]
- Liu, X.; Guo, X.; Zhang, Z. Preoperative serum hypersensitive-c-reactive-protein (Hs-CRP) to albumin ratio predicts survival in patients with Luminal B subtype breast cancer. OncoTargets Ther. 2021, 14, 4137. [Google Scholar] [CrossRef]
- Huang, Z.-Z.; Song, C.-G.; Huang, J.-J.; Xia, W.; Bi, X.-W.; Hua, X.; He, Z.-Y.; Yuan, Z.-Y. Prognostic significance of the Controlling Nutritional Status (CONUT) score in surgically treated breast cancer patients. Gland. Surg. 2020, 9, 1370. [Google Scholar] [CrossRef]
- Aaldriks, A.; Giltay, E.; Le Cessie, S.; van der Geest, L.; Portielje, J.; Tanis, B.; Nortier, J.; Maartense, E. Prognostic value of geriatric assessment in older patients with advanced breast cancer receiving chemotherapy. Breast 2013, 22, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Bering, T.; Fernandes Maurício, S.; Silva, J.B.d.; Correia, M.I.T.D. El estado nutricional y metabólico de las mujeres con cáncer de mama. Nutr. Hosp. 2015, 31, 751–758. [Google Scholar]
- Blanco-Montenegro, I.; De Ritis, R.; Chiappini, M. Imaging and modelling the subsurface structure of volcanic calderas with high-resolution aeromagnetic data at Vulcano (Aeolian Islands, Italy). Bull. Volcanol. 2007, 69, 643–659. [Google Scholar] [CrossRef]
- Limon-Miro, A.T.; Lopez-Teros, V.; Astiazaran-Garcia, H. Dietary guidelines for breast cancer patients: A critical review. Adv. Nutr. 2017, 8, 613–623. [Google Scholar] [PubMed]
- Trestini, I.; Sperduti, I.; Caldart, A.; Bonaiuto, C.; Fiorio, E.; Parolin, V.; Zambonin, V.; Zanelli, S.; Tregnago, D.; Avancini, A. Evidence-based tailored nutrition educational intervention improves adherence to dietary guidelines, anthropometric measures and serum metabolic biomarkers in early-stage breast cancer patients: A prospective interventional study. Breast 2021, 60, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Ravasco, P. Nutrition in cancer patients. J. Clin. Med. 2019, 8, 1211. [Google Scholar] [CrossRef] [PubMed]
- DuMontier, C.; Clough-Gorr, K.M.; Silliman, R.A.; Stuck, A.E.; Moser, A. Health-related quality of life in a predictive model for mortality in older breast cancer survivors. J. Am. Geriatr. Soc. 2018, 66, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Rodriguez, J.L.; O’Brien, K.M.; Nichols, H.B.; Hodgson, M.E.; Weinberg, C.R.; Sandler, D.P. Health-related quality of life outcomes among breast cancer survivors. Cancer 2021, 127, 1114–1125. [Google Scholar] [CrossRef]
- Mokhatri-Hesari, P.; Montazeri, A. Health-related quality of life in breast cancer patients: Review of reviews from 2008 to 2018. Health Qual. Life Outcomes 2020, 18, 338. [Google Scholar] [CrossRef]
- Villar, R.R.; Fernández, S.P.; Garea, C.C.; Pillado, M.; Barreiro, V.B.; Martín, C.G. Quality of life and anxiety in women with breast cancer before and after treatment. Rev. Lat. Am. Enferm. 2017, 25, e2958. [Google Scholar] [CrossRef]
- Zheng, C.; Yu, L.-X.; Jia, H.-Y.; Cui, S.-D.; Tian, F.-G.; Fan, Z.-M.; Geng, C.-Z.; Cao, X.-C.; Yang, Z.-L.; Wang, X. Relationship Between Lifestyle Habits and Health-Related Quality of Life of Recently Diagnosed Breast Cancer Patients: A Comparison Between Younger and Older Women in China. Front. Public Health 2021, 9, 767151. [Google Scholar] [CrossRef] [PubMed]
- Mierzynska, J.; Taye, M.; Pe, M.; Coens, C.; Martinelli, F.; Pogoda, K.; Velikova, G.; Bjelic-Radisic, V.; Cardoso, F.; Brain, E. Reference values for the EORTC QLQ-C30 in early and metastatic breast cancer. Eur. J. Cancer 2020, 125, 69–82. [Google Scholar] [CrossRef] [PubMed]
- De Groef, A.; Geraerts, I.; Demeyer, H.; Van der Gucht, E.; Dams, L.; de Kinkelder, C.; Dukers-van Althuis, S.; Van Kampen, M.; Devoogdt, N. Physical activity levels after treatment for breast cancer: Two-year follow-up. Breast 2018, 40, 23–28. [Google Scholar] [CrossRef]
- Palesh, O.; Kamen, C.; Sharp, S.; Golden, A.; Neri, E.; Spiegel, D.; Koopman, C. Physical activity and survival in women with advanced breast cancer. Cancer Nurs. 2018, 41, E31. [Google Scholar] [CrossRef] [PubMed]
- Lahart, I.M.; Metsios, G.S.; Nevill, A.M.; Carmichael, A.R. Physical activity, risk of death and recurrence in breast cancer survivors: A systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015, 54, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Pinto, B.M.; Trunzo, J.J.; Reiss, P.; Shiu, S.Y. Exercise participation after diagnosis of breast cancer: Trends and effects on mood and quality of life. Psycho-Oncol. J. Psychol. Soc. Behav. Dimens. Cancer 2002, 11, 389–400. [Google Scholar] [CrossRef]
- Harrison, S.; Hayes, S.C.; Newman, B. Level of physical activity and characteristics associated with change following breast cancer diagnosis and treatment. Psycho-Oncol. J. Psychol. Soc. Behav. Dimens. Cancer 2009, 18, 387–394. [Google Scholar] [CrossRef]
- Andrykowski, M.A.; Beacham, A.O.; Jacobsen, P.B. Prospective, longitudinal study of leisure-time exercise in women with early-stage breast cancer. Cancer Epidemiol. Prev. Biomark. 2007, 16, 430–438. [Google Scholar] [CrossRef]
- Irwin, M.L.; Crumley, D.; McTiernan, A.; Bernstein, L.; Baumgartner, R.; Gilliland, F.D.; Kriska, A.; Ballard-Barbash, R. Physical activity levels before and after a diagnosis of breast carcinoma: The Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2003, 97, 1746–1757. [Google Scholar] [CrossRef]
- Littman, A.J.; Tang, M.-T.; Rossing, M.A. Longitudinal study of recreational physical activity in breast cancer survivors. J. Cancer Surviv. 2010, 4, 119–127. [Google Scholar] [CrossRef]
- Spei, M.-E.; Samoli, E.; Bravi, F.; La Vecchia, C.; Bamia, C.; Benetou, V. Physical activity in breast cancer survivors: A systematic review and meta-analysis on overall and breast cancer survival. Breast 2019, 44, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Van Mackelenbergh, M.; Wesch, D.; Mundhenke, C. Physical activity influences the immune system of breast cancer patients. J. Cancer Res. Ther. 2017, 13, 392. [Google Scholar] [PubMed]
- Rangel, J.; Tomás, M.T.; Fernandes, B. Physical activity and physiotherapy: Perception of women breast cancer survivors. Breast Cancer 2019, 26, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.E.; Wiskemann, J.; Armbrust, P.; Schneeweiss, A.; Ulrich, C.M.; Steindorf, K. Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: A randomized controlled trial. Int. J. Cancer 2015, 137, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Montagnese, C.; Porciello, G.; Vitale, S.; Palumbo, E.; Crispo, A.; Grimaldi, M.; Calabrese, I.; Pica, R.; Prete, M.; Falzone, L. Quality of life in women diagnosed with breast cancer after a 12-month treatment of lifestyle modifications. Nutrients 2020, 13, 136. [Google Scholar] [CrossRef]
- Awick, E.A.; Phillips, S.M.; Lloyd, G.R.; McAuley, E. Physical activity, self-efficacy and self-esteem in breast cancer survivors: A panel model. Psycho-Oncology 2017, 26, 1625–1631. [Google Scholar] [CrossRef]
- Althubaiti, A. Information bias in health research: Definition, pitfalls, and adjustment methods. J. Multidiscip. Healthc. 2016, 9, 211. [Google Scholar] [CrossRef]
- Koutoukidis, D.A.; Knobf, M.T.; Lanceley, A. Obesity, diet, physical activity, and health-related quality of life in endometrial cancer survivors. Nutr. Rev. 2015, 73, 399–408. [Google Scholar] [CrossRef]
- Pisegna, J.; Xu, M.; Spees, C.; Krok-Schoen, J.L. Mental health-related quality of life is associated with diet quality among survivors of breast cancer. Support. Care Cancer 2021, 29, 2021–2028. [Google Scholar] [CrossRef]
- Brown, J.C.; Sarwer, D.B.; Troxel, A.B.; Sturgeon, K.; DeMichele, A.M.; Denlinger, C.S.; Schmitz, K.H. A randomized trial of exercise and diet on health-related quality of life in survivors of breast cancer with overweight or obesity. Cancer 2021, 127, 3856–3864. [Google Scholar] [CrossRef]
| Characteristics, n = 114 | Mediterranean Diet (MD) Adherence | |||
|---|---|---|---|---|
| Low (33.3%) | Medium (33.3%) | High (33.3%) | p-Value | |
| Age (years, IQR *) | 66.7 (53–72) | 64.4 (55–76) | 64.2 (57–78) | p = 0.119 |
| BMI (Kg/m2, IQR) | 32.5 (27.2–36.9) | 28.4 (24.3–32.1) | 27.1 (23.5–30.8) | p ˂ 0.001 |
| Histopatological type (n, %) | p = 0.682 | |||
| Ductal breast carcinoma | 30 (78,9) | 29 (76.3) | 32 (84.2) | |
| Lobular breast carcinoma | 8 (21.1) | 9 (23.7) | 6 (15.8) | |
| Tumor grade of differentiation (n, %) | p = 0.062 | |||
| High | 0 (0.0) | 6 (15.8) | 2 (5.3) | |
| Medium | 25 (65.8) | 25 (65.8) | 24 (63.2) | |
| Low | 13 (34.2) | 7 (18.4) | 12 (31.6) | |
| Tumor stage (n, %) | p = 0.008 | |||
| Stage I | 1 (2.6) | 19 (50.0) | 27 (71.0) | |
| Stage II | 21 (55.3) | 14 (36.8) | 11 (29.0) | |
| Stage III + IV | 16 (42.1) | 5 (13.2) | 0 (0.0) | |
| Tumor size (n, %) | p = 0.017 | |||
| T1, ≤2 cm | 7 (18.4) | 24 (63.2) | 31 (81.6) | |
| T2, >2 cm and ≤5 cm | 21 (55.3) | 12 (31.6) | 6 (15.8) | |
| T3 + 4, >5 cm | 10 (26.3) | 2 (5.3) | 1 (2.6) | |
| Presence of lymph node metastasis (n, %) | p = 0.026 | |||
| No | 12 (31.2) | 23 (60.5) | 25 (65.8) | |
| Yes | 26 (68.4) | 15 (39.5) | 13 (34.2) | |
| Presence of distant metastasis (n, %) | p = 0.059 | |||
| No | 30 (78.9) | 37 (97.4) | 35 (92.1) | |
| Yes | 8 (21.1) | 1 (2.6) | 3 (7.9) | |
| Physical activity levels (IPAQ, n, %) | p = 0.015 | |||
| Low | 29 (76.3) | 15 (39.5) | 19 (50.0) | |
| Moderate | 6 (15.8) | 9 (23.7) | 10 (26.3) | |
| High | 3 (7.9) | 14 (36.8) | 9 (23.7) | |
| Health-related quality of life (EORTC QLQ-C30, n, %) | p = 0.084 | |||
| Low | 18 (47.4) | 25 (65.8) | 15 (39.5) | |
| High | 20 (52.6) | 13 (34.2) | 23 (60.5) | |
| Nutritional status score (median, IQR) | 23.5 (20.0–26.0) | 24.0 (20.5–28.0) | 25.5 (21.5–28.5) | p = 0.036 |
| Recurrence-free survival (months, IQR) | 33.0 (30.0–36.5) | 35.5 (33.0–38.5) | 38.5 (35.0–41.5) | p = 0.001 |
| Characteristics, n = 114 | Nutritional Status | |||
|---|---|---|---|---|
| Well-Nourished Score > 23 (n = 78, 68.4%) | Risk of Malnutrition 17 ≤ Score ≤ 23 (n = 32, 28.1%) | Malnourished Score < 17 (n = 4, 3.5%) | p-Value | |
| Age (years, IQR **) | 63.5 (57.0–71.5) | 64.5 (58.0–71.0) | 65.5 (59.5–72.5) | p = 0.125 |
| BMI (Kg/m2, IQR) | 32.3 (26.3–33.8) | 31.8 (25.2–33,2) | 29.7 (24.1–34,4) | p = 0.022 |
| Histopatological type (n, %) | p = 0.195 | |||
| Ductal breast carcinoma | 65 (83.3) | 24 (75.0) | 2 (50.5) | |
| Lobular breast carcinoma | 13 (16.7) | 8 (25.0) | 2 (50.5) | |
| Tumor grade of differentiation (n, %) | p = 0.408 | |||
| High | 4 (5.1) | 3 (9.4) | 1 (25.0) | |
| Medium | 52 (66.7) | 19 (59.4) | 3 (75.0) | |
| Low | 22 (28.2) | 10 (31.2) | 0 (0.0) | |
| Tumor stage (n, %) | p = 0.672 | |||
| Stage I | 35 (44.9) | 11 (34.4) | 1 (25.0) | |
| Stage II | 28 (35.9) | 16 (50.0) | 2 (50.0) | |
| Stage III + IV | 15 (19.2) | 5 (15.6) | 1 (25.0) | |
| Tumor size (n, %) | p = 0.450 | |||
| T1, ≤2 cm | 46 (59.0) | 15 (46.9) | 1 (25.0) | |
| T2, >2 cm and ≤5 cm | 23 (29.5) | 14 (43.7) | 2 (50.0) | |
| T3 + 4, >5 cm | 9 (11.5) | 3 (9.4) | 1 (25.0) | |
| Presence of lymph node metastasis (n, %) | p = 0.888 | |||
| No | 40 (51.3) | 18 (56.2) | 2 (50.0) | |
| Yes | 38 (48.7) | 14 (43.8) | 2 (50.0) | |
| Presence of distant metastasis (n, %) | p = 0.737 | |||
| No | 70 (89.7) | 28 (87.5) | 4 (100.0) | |
| Yes | 8 (10.3) | 4 (12.5) | 0 (0.0) | |
| Physical activity levels (IPAQ, n, %) | p = 0.570 | |||
| Low | 41 (52,6) | 19 (59.4) | 3 (75.0) | |
| Moderate | 16 (20.5) | 8 (25.0) | 1 (25.0) | |
| High | 21 (26.9) | 5 (15.6) | 0 (0.0) | |
| Health-related quality of life (EORTC QLQ-C30, n, %) | p = 0.050 | |||
| Low | 44 (56.4) | 13 (40.6) | 1 (25.0) | |
| High | 34 (43.6) | 19 (59.4) | 3 (75.0) | |
| Mediterranean diet score (median, IQR) | 27.0 (23.0–31.0) | 26.0 (22.0–30.0) | 26.0 (21.0–29.0) | p = 0.046 |
| Recurrence-free survival (months, IQR) | 36.5 (31.5–42.0) | 36.0 (31.0–41.0) | 34.5 (29.0–39.5) | p = 0.022 |
| Characteristics | Recurrence-Free Survival | |
|---|---|---|
| HR * (95% CI **) | p-Value | |
| Age (below/over median value) | 1.10 (0.38–3.03) | p = 0.558 |
| BMI (normal weight/overweight and obese) | 3.07 (2.12–4.91) | p = 0.002 |
| Histological type (ductal/lobular) | 2.98 (1.48–5.23) | p = 0.043 |
| Histological grade (high and medium/Low) | 1.35 (0.24–2.43) | p = 0.312 |
| Tumor size (≤2 cm/>2 cm) | 0.94 (0.13–5.77) | p = 0.748 |
| Presence of lymph node metastases (No/Yes) | 1.06 (0.20–5.89) | p = 0.620 |
| Presence of distant metastases (No/Yes) | 0.97 (0.21–5.12) | p = 0.407 |
| Physical activity levels (low/moderate and high) | 1.12 (0.32–2.59) | p = 0.573 |
| Health-related quality of life (low/high) | 0.66 (0.21–1.54) | p = 0.112 |
| MD adherence | p = 0.017 | |
| Low (reference) | 1.00 | |
| Moderate | 0.47 (0.18–0.79) | |
| High | 0.39 (0.15–0.72) | |
| Nutritional status | p = 0.046 | |
| Malnutrition (reference) | 1.00 | |
| Risk of malnutrition | 0.62 (0.21–1.02) | |
| Well-nourished | 0.57 (0.18–0.97) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantzorou, M.; Tolia, M.; Poultsidi, A.; Vasios, G.K.; Papandreou, D.; Theocharis, S.; Kavantzas, N.; Troumbis, A.Y.; Giaginis, C. Adherence to Mediterranean Diet and Nutritional Status in Women with Breast Cancer: What Is Their Impact on Disease Progression and Recurrence-Free Patients’ Survival? Curr. Oncol. 2022, 29, 7482-7497. https://doi.org/10.3390/curroncol29100589
Mantzorou M, Tolia M, Poultsidi A, Vasios GK, Papandreou D, Theocharis S, Kavantzas N, Troumbis AY, Giaginis C. Adherence to Mediterranean Diet and Nutritional Status in Women with Breast Cancer: What Is Their Impact on Disease Progression and Recurrence-Free Patients’ Survival? Current Oncology. 2022; 29(10):7482-7497. https://doi.org/10.3390/curroncol29100589
Chicago/Turabian StyleMantzorou, Maria, Maria Tolia, Antigoni Poultsidi, Georgios K. Vasios, Dimitrios Papandreou, Stamatios Theocharis, Nikolaos Kavantzas, Andreas Y. Troumbis, and Constantinos Giaginis. 2022. "Adherence to Mediterranean Diet and Nutritional Status in Women with Breast Cancer: What Is Their Impact on Disease Progression and Recurrence-Free Patients’ Survival?" Current Oncology 29, no. 10: 7482-7497. https://doi.org/10.3390/curroncol29100589
APA StyleMantzorou, M., Tolia, M., Poultsidi, A., Vasios, G. K., Papandreou, D., Theocharis, S., Kavantzas, N., Troumbis, A. Y., & Giaginis, C. (2022). Adherence to Mediterranean Diet and Nutritional Status in Women with Breast Cancer: What Is Their Impact on Disease Progression and Recurrence-Free Patients’ Survival? Current Oncology, 29(10), 7482-7497. https://doi.org/10.3390/curroncol29100589










