Effect of Different Durations of Adjuvant Capecitabine Monotherapy on the Outcome of High-Risk Stage II and Stage III Colorectal Cancer: A Retrospective Study Based on a CRC Database
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria
2.3. Protocol Treatment
2.4. Primary/Secondary Endpoint and Statistical Analysis
2.4.1. Primary/Secondary Endpoint
2.4.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
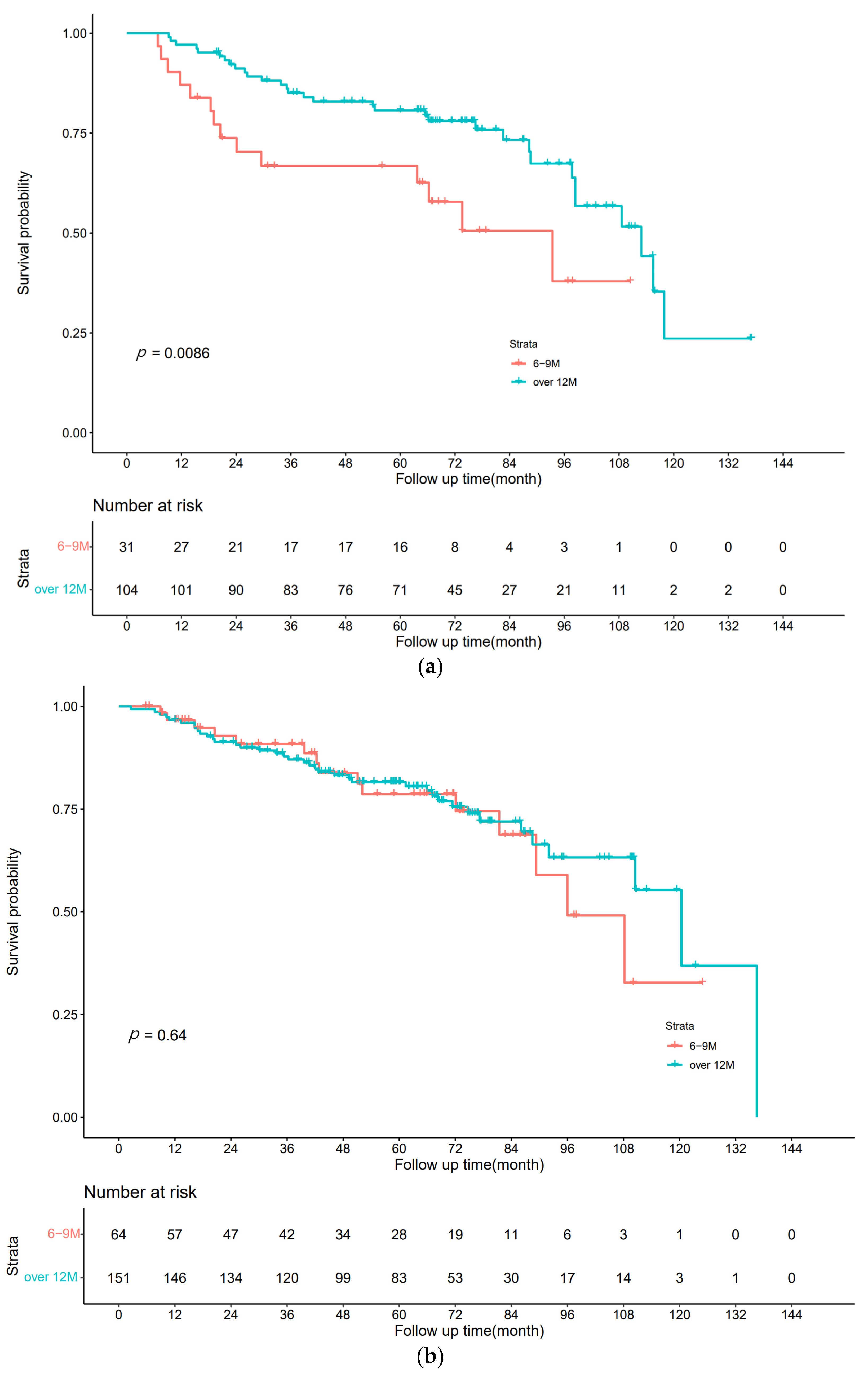
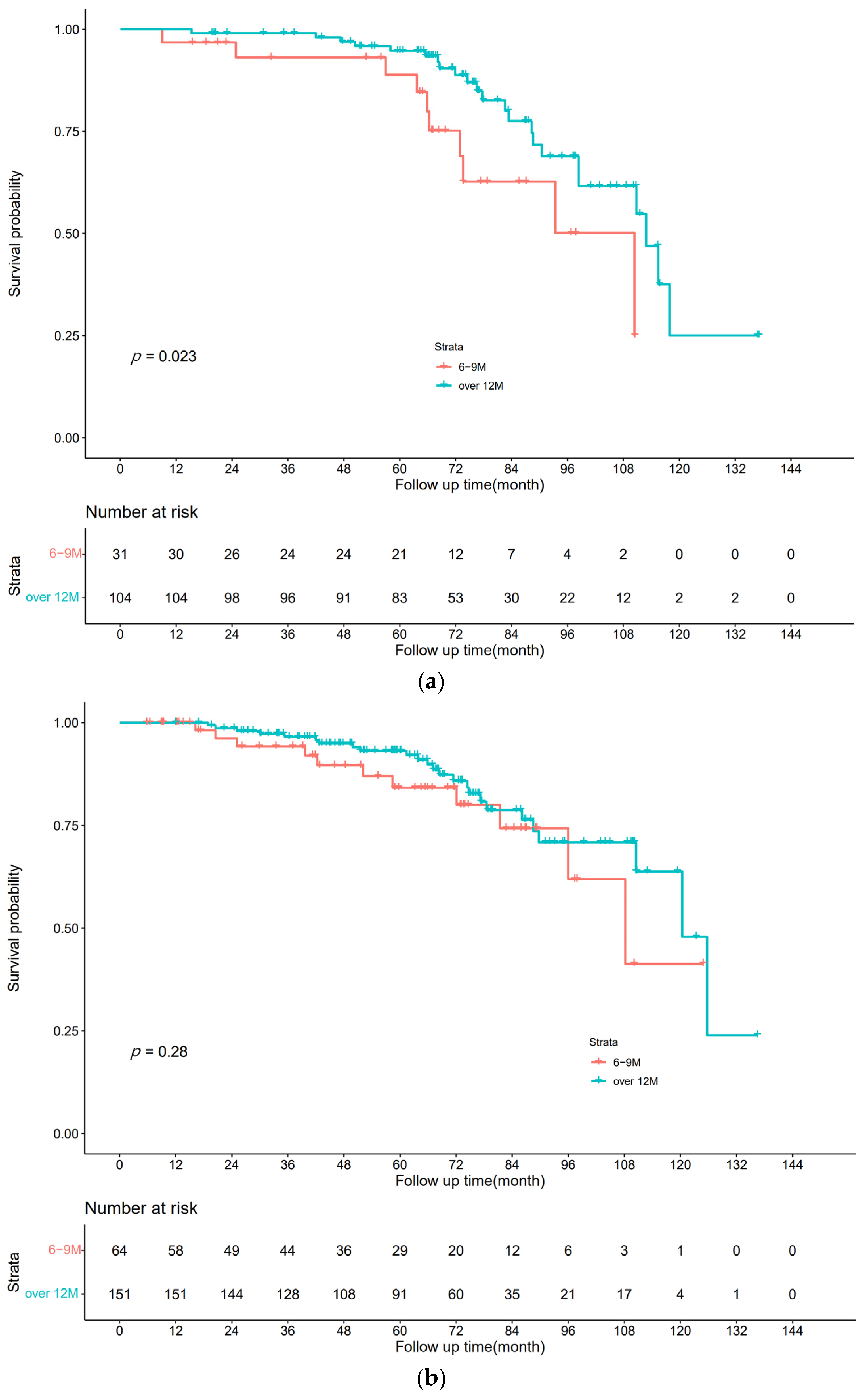
3.2. Disease-Free Survival
3.3. Overall Survival
4. Discussion
4.1. Prognostic Impact of Different Durations of Capecitabine Monotherapy
4.2. Limitations of the Study
4.3. Practical Significance of This Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. CRC. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Collins, G.; Wang, H.; Toh, J.W.T. Pathological Features and Prognostication in CRC. Curr. Oncol. 2021, 28, 5356–5383. [Google Scholar] [CrossRef] [PubMed]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rodel, C.; Cervantes, A.; Arnold, D.; Committee, E.G. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. S4), iv22–iv40. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef] [PubMed]
- Haller, D.G.; Tabernero, J.; Maroun, J.; de Braud, F.; Price, T.; Van Cutsem, E.; Hill, M.; Gilberg, F.; Rittweger, K.; Schmoll, H.J. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J. Clin. Oncol. 2011, 29, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Andre, T.; Boni, C.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Bonetti, A.; Clingan, P.; Bridgewater, J.; Rivera, F.; et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J. Clin. Oncol. 2009, 27, 3109–3116. [Google Scholar] [CrossRef] [PubMed]
- Chionh, F.; Lau, D.; Yeung, Y.; Price, T.; Tebbutt, N. Oral versus intravenous fluoropyrimidines for CRC. Cochrane Database Syst. Rev. 2017, 7, CD008398. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Chen, W.T.; Lee, H.C.; Lin, J.K.; Fang, C.Y.; Chou, Y.H.; Lin, P.C.; Lin, B.W.; Huang, C.C.; Yeh, C.H.; et al. Health-related quality of life and cost comparison of adjuvant capecitabine versus 5-fluorouracil/leucovorin in stage III CRC patients. Qual. Life Res. 2015, 24, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xu, Y.; Zhang, T.; Yang, Y. Impact of the COVID-19 outbreak on adjuvant chemotherapy for patients with stage II or III colon cancer: Experiences from a multicentre clinical trial in China. Curr. Oncol. 2020, 27, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Franssen, E.; Fitch, M.I.; Warner, E. Patient preferences for oral versus intravenous palliative chemotherapy. J. Clin. Oncol. 1997, 15, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Yamanaka, T.; Oki, E.; Kotaka, M.; Manaka, D.; Eto, T.; Hasegawa, J.; Takagane, A.; Nakamura, M.; Kato, T.; et al. Efficacy and Long-term Peripheral Sensory Neuropathy of 3 vs. 6 Months of Oxaliplatin-Based Adjuvant Chemotherapy for Colon Cancer: The ACHIEVE Phase 3 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Tomita, N.; Kunieda, K.; Maeda, A.; Hamada, C.; Yamanaka, T.; Sato, T.; Yoshida, K.; Boku, N.; Nezu, R.; Yamaguchi, S.; et al. Phase III randomised trial comparing 6 vs. 12-month of capecitabine as adjuvant chemotherapy for patients with stage III colon cancer: Final results of the JFMC37-0801 study. Br. J. Cancer 2019, 120, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, L. Part Ⅰ of database building: Tag and structure of personal data for Database from Colorectal Cancer in West China Hospital. Chin. J. Bases Clin. Gen. 2019, 26, 335–342. [Google Scholar] [CrossRef]
- Twelves, C.; Wong, A.; Nowacki, M.P.; Abt, M.; Burris, H.; Carrato, A.; Cassidy, J.; Cervantes, A.; Fagerberg, J.; Georgoulias, V.; et al. Capecitabine as adjuvant treatment for stage III colon cancer. N. Engl. J. Med. 2005, 352, 2696–2704. [Google Scholar] [CrossRef] [PubMed]
- Suto, T.; Ishiguro, M.; Hamada, C.; Kunieda, K.; Masuko, H.; Kondo, K.; Ishida, H.; Nishimura, G.; Sasaki, K.; Morita, T.; et al. Preplanned safety analysis of the JFMC37-0801 trial: A randomized phase III study of six months versus twelve months of capecitabine as adjuvant chemotherapy for stage III colon cancer. Int. J. Clin. Oncol. 2017, 22, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Haydon, A. Adjuvant chemotherapy in colon cancer: What is the evidence? Intern. Med. J. 2003, 33, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Andre, T.; Iveson, T.; Labianca, R.; Meyerhardt, J.A.; Souglakos, I.; Yoshino, T.; Paul, J.; Sobrero, A.; Taieb, J.; Shields, A.F.; et al. The IDEA (International Duration Evaluation of Adjuvant Chemotherapy) Collaboration: Prospective Combined Analysis of Phase III Trials Investigating Duration of Adjuvant Therapy with the FOLFOX (FOLFOX4 or Modified FOLFOX6) or XELOX (3 versus 6 months) Regimen for Patients with Stage III Colon Cancer: Trial Design and Current Status. Curr. CRC Rep. 2013, 9, 261–269. [Google Scholar] [CrossRef]
- Sadahiro, S.; Tsuchiya, T.; Sasaki, K.; Kondo, K.; Katsumata, K.; Nishimura, G.; Kakeji, Y.; Baba, H.; Sato, S.; Koda, K.; et al. Randomized phase III trial of treatment duration for oral uracil and tegafur plus leucovorin as adjuvant chemotherapy for patients with stage IIB/III colon cancer: Final results of JFMC33-0502. Ann. Oncol. 2015, 26, 2274–2280. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, H.; Shiroiwa, T.; Ishiguro, M.; Nakamura, M.; Hasegawa, J.; Yamaguchi, S.; Masuda, Y.; Sakamoto, J.; Tomita, N.; Fukuda, T. Cost-effectiveness of 12 months of capecitabine as adjuvant chemotherapy for stage III colon cancer: Preplanned cost-effectiveness analysis of the JFMC37-0801 study. Eur. J. Health Econ. 2022, 23, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Rödel, C.; Graeven, U.; Fietkau, R.; Hohenberger, W.; Hothorn, T.; Arnold, D.; Hofheinz, R.-D.; Ghadimi, M.; Wolff, H.A.; Lang-Welzenbach, M.; et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): Final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015, 16, 979–989. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total | 6 M | 12 M | p |
|---|---|---|---|---|
| (n = 350) | (n = 95) | (n = 255) | ||
| sex, n (%) | 0.922 | |||
| female | 147 (42) | 39 (41) | 108 (42) | |
| male | 203 (58) | 56 (59) | 147 (58) | |
| age, n (%) | 0.525 | |||
| ≤60 | 170 (49) | 43 (45) | 127 (50) | |
| >60 | 180 (51) | 52 (55) | 128 (50) | |
| location, n (%) | 0.569 | |||
| rectum | 260 (74) | 68 (72) | 192 (75) | |
| colon | 90 (26) | 27 (28) | 63 (25) | |
| differentiation, n (%) | 0.722 | |||
| low | 99 (28) | 26 (27) | 73 (29) | |
| medium | 243 (69) | 68 (72) | 175 (69) | |
| high | 8 (2) | 1 (1) | 7 (3) | |
| T (AJCC 8th), n (%) | 0.519 | |||
| T0-3 | 125 (36) | 37 (39) | 88 (35) | |
| T4 | 225 (64) | 58 (61) | 167 (65) | |
| N (AJCC 8th), n (%) | 0.378 | |||
| N0 | 215 (61) | 64 (67) | 151 (59) | |
| N1 | 104 (30) | 24 (25) | 80 (31) | |
| N2 | 31 (9) | 7 (7) | 24 (9) | |
| TNM (AJCC 8th), n (%) | 0.204 | |||
| II (high-risk) 1 | 215 (61) | 64 (67) | 151 (59) | |
| III | 135 (39) | 31 (33) | 104 (41) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Q.; Li, Z.; Liu, Y.; Luo, Y.; Fan, J.; Xie, P.; Cao, X.; Chen, X.; Wang, X. Effect of Different Durations of Adjuvant Capecitabine Monotherapy on the Outcome of High-Risk Stage II and Stage III Colorectal Cancer: A Retrospective Study Based on a CRC Database. Curr. Oncol. 2023, 30, 949-958. https://doi.org/10.3390/curroncol30010072
Yu Q, Li Z, Liu Y, Luo Y, Fan J, Xie P, Cao X, Chen X, Wang X. Effect of Different Durations of Adjuvant Capecitabine Monotherapy on the Outcome of High-Risk Stage II and Stage III Colorectal Cancer: A Retrospective Study Based on a CRC Database. Current Oncology. 2023; 30(1):949-958. https://doi.org/10.3390/curroncol30010072
Chicago/Turabian StyleYu, Qiao, Zhigui Li, Yuqing Liu, Yichen Luo, Jingya Fan, Peijun Xie, Xiaoman Cao, Xingyu Chen, and Xiaodong Wang. 2023. "Effect of Different Durations of Adjuvant Capecitabine Monotherapy on the Outcome of High-Risk Stage II and Stage III Colorectal Cancer: A Retrospective Study Based on a CRC Database" Current Oncology 30, no. 1: 949-958. https://doi.org/10.3390/curroncol30010072
APA StyleYu, Q., Li, Z., Liu, Y., Luo, Y., Fan, J., Xie, P., Cao, X., Chen, X., & Wang, X. (2023). Effect of Different Durations of Adjuvant Capecitabine Monotherapy on the Outcome of High-Risk Stage II and Stage III Colorectal Cancer: A Retrospective Study Based on a CRC Database. Current Oncology, 30(1), 949-958. https://doi.org/10.3390/curroncol30010072





