Neuroendocrine Carcinomas of the Uterine Cervix, Endometrium, and Ovary Show Higher Tendencies for Bone, Brain, and Liver Organotrophic Metastases
Abstract
1. Introduction
2. Materials and Methods
2.1. SEER Database
2.2. Institutional Database
2.3. Statistical Analysis
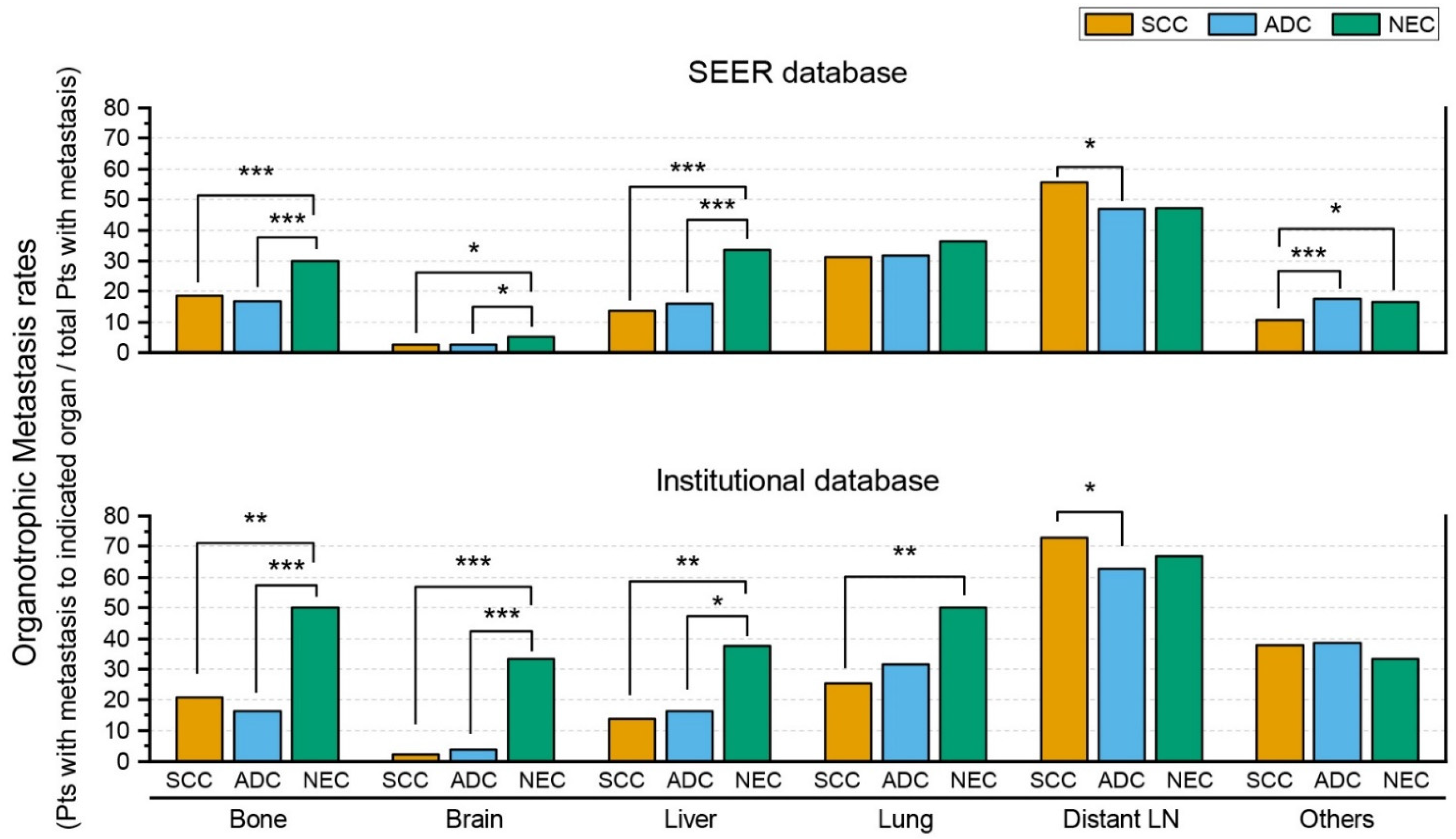
3. Results
3.1. SEER Database
3.2. Institutional Database
4. Discussion
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; de Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A Common Classification Framework for Neuroendocrine Neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) Expert Consensus Proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.K. Neuroendocrine Tumors of the Female Reproductive Tract: A Literature Review. J. Pathol. Transl. Med. 2015, 49, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Hermans, B.C.M.; de Vos-Geelen, J.; Derks, J.L.; Latten, L.; Liem, I.H.; van der Zwan, J.M.; Speel, E.J.M.; Dercksen, M.W.; Dingemans, A.M.C. Unique Metastatic Patterns in Neuroendocrine Neoplasms of Different Primary Origin. Neuroendocrinology 2021, 111, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Tan, Y.; Ge, W.; Ding, K.; Hu, H. Pattern and Risk Factors for Distant Metastases in Gastrointestinal Neuroendocrine Neoplasms: A Population-Based Study. Cancer Med. 2018, 7, 2699–2709. [Google Scholar] [CrossRef] [PubMed]
- Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence—SEER Research Data, 17 Registries, Nov 2021 Sub (2000–2019)—Linked to County Attributes—Time Dependent (1990–2019) Income/Rurality, 1969–2020 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2022, based on the November 2021 submission. Available online: www.seer.cancer.gov (accessed on 23 June 2022).
- Surveillance Research Program, National Cancer Institute. SEER*Stat Software, Version 8.4.0.1; National Cancer Institute: Bethesda, MD, USA, 2022. [Google Scholar]
- Tempfer, C.B.; Tischoff, I.; Dogan, A.; Hilal, Z.; Schultheis, B.; Kern, P.; Rezniczek, G.A. Neuroendocrine carcinoma of the cervix: A systematic review of the literature. BMC Cancer 2018, 18, 530. [Google Scholar] [CrossRef] [PubMed]
- Virarkar, M.; Vulasala, S.S.; Morani, A.C.; Waters, R.; Gopireddy, D.R.; Kumar, S.; Bhosale, P.; Lall, C. Neuroendocrine Neoplasms of the Gynecologic Tract. Cancers 2022, 14, 1835. [Google Scholar] [CrossRef] [PubMed]
- Riihimäki, M.; Hemminki, A.; Sundquist, K.; Sundquist, J.; Hemminki, K. The Epidemiology of Metastases in Neuroendocrine Tumors. Int. J. Cancer 2016, 139, 2679–2686. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.B.; Charo, L.M.; Mann, A.K.; Kapp, D.S.; Eskander, R.N.; Chan, J.K. Ovarian, Uterine, and Cervical Cancer Patients with Distant Metastases at Diagnosis: Most Common Locations and Outcomes. Clin. Exp. Metastasis 2020, 37, 107–113. [Google Scholar] [CrossRef]

| Histologic Subtype | p Value | |||||
|---|---|---|---|---|---|---|
| NEC | SCC | ADC | NEC vs. SCC | NEC vs. ADC | SCC vs. ADC | |
| SEER database | ||||||
| N | 495 | 19947 | 6517 | |||
| Patients with metastasis to the indicated organ/Patients with metastasis | ||||||
| Bone | 63/210 (30%) | 466/2502 (18.6%) | 109/647 (16.8%) | <0.001 | <0.001 | 0.297 |
| Brain | 11/210 (5.2%) | 66/2496 (2.6%) | 16/646 (2.5%) | 0.03 | 0.047 | 0.812 |
| Liver | 70/209 (33.5%) | 346/2512 (13.8%) | 104/654 (15.9%) | <0.001 | <0.001 | 0.165 |
| Lung | 76/209 (36.4%) | 777/2489 (31.2%) | 205/646 (31.7%) | 0.124 | 0.215 | 0.801 |
| Distant LN | 43/91 (47.3%) | 548/987 (55.5%) | 137/292 (46.9%) | 0.129 | 0.955 | 0.01 |
| Other | 35/214 (16.4%) | 275/2569 (10.7%) | 117/674 (17.4%) | 0.041 | 0.937 | <0.001 |
| Institutional database | ||||||
| N | 53 | 3206 | 670 | |||
| Patients with metastasis to the indicated organ/Patients with metastasis | ||||||
| Bone | 12/24 (50%) | 117/560 (20.9%) | 25/153 (16.3%) | 0.001 | <0.001 | 0.213 |
| Brain | 8/24 (33.3%) | 13/560 (2.3%) | 6/153 (3.9%) | <0.001 | <0.001 | 0.265 |
| Liver | 9/24 (37.5%) | 76/560 (13.6%) | 25/153 (16.3%) | 0.004 | 0.024 | 0.384 |
| Lung | 12/24 (50.0%) | 143/560 (25.5%) | 48/153 (31.4%) | 0.008 | 0.073 | 0.149 |
| distant LN | 16/24 (66.7%) | 408/560 (72.9%) | 96/153 (62.7%) | 0.505 | 0.711 | 0.015 |
| Other | 8/24 (33.3%) | 212/560 (37.9%) | 59/153 (38.6%) | 0.654 | 0.623 | 0.874 |
| Histologic Subtype | p Value | ||||||
|---|---|---|---|---|---|---|---|
| NEC | EC | MC | SC | NEC vs. EC | NEC vs. MC | NEC vs. SC | |
| Ovarian carcinoma | |||||||
| N | 242 | 4958 | 2700 | 24129 | |||
| Patients with metastasis to the indicated organ/Patients with metastasis | |||||||
| Bone | 13/84 (15.5%) | 13/247 (5.3%) | 26/260 (10.0%) | 157/7293 (2.2%) | 0.003 | 0.169 | <0.001 |
| Brain | 5/82 (6.1%) | 3/248 (1.2%) | 2/259 (0.8%) | 32/7283 (0.4%) | 0.025 | 0.01 | <0.001 |
| Liver | 40/83 (48.2%) | 68/248 (27.4%) | 78/264 (29.5%) | 1581/7297 (21.7%) | <0.001 | 0.002 | <0.001 |
| Lung | 19/82 (23.2%) | 50/247 (20.2%) | 56/258 (21.7%) | 1263/7271 (17.4%) | 0.573 | 0.78 | 0.169 |
| Distant LN | 8/33 (24.2%) | 12/84 (14.3%) | 11/97 (11.3%) | 735/3166 (23.2%) | 0.198 | 0.088 | 0.889 |
| Other | 16/33 (48.5%) | 50/84 (59.5%) | 61/100 (61.0%) | 2206/3216 (68.6%) | 0.279 | 0.207 | 0.013 |
| Endometrial carcinoma | |||||||
| N | 173 | 89637 | 702 | 10187 | |||
| Patients with metastasis to the indicated organ/Patients with metastasis | |||||||
| Bone | 14/72 (19.4%) | 314/2661 (11.8%) | 5/33 (15.2%) | 125/2243 (5.6%) | 0.049 | 0.596 | <0.001 |
| Brain | 6/72 (8.3%) | 102/2659 (3.8%) | 1/33 (3.0%) | 15/2238 (0.7%) | 0.063 | 0.429 | <0.001 |
| Liver | 18/72 (25.0%) | 349/2667 (13.1%) | 3/33 (9.1%) | 245/2242 (10.9%) | 0.003 | 0.058 | <0.001 |
| Lung | 17/71 (23.9%) | 862/2658 (32.4%) | 13/33 (39.4%) | 367/2233 (16.4%) | 0.131 | 0.106 | 0.095 |
| Distant LN | 15/32 (46.9%) | 303/1300 (23.3%) | 1/11 (9.1%) | 295/1215 (24.3%) | 0.002 | 0.033 | 0.004 |
| Other | 14/32 (43.8%) | 702/1309 (53.6%) | 8/12 (66.7%) | 899/1218 (73.8%) | 0.268 | 0.176 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.K. Neuroendocrine Carcinomas of the Uterine Cervix, Endometrium, and Ovary Show Higher Tendencies for Bone, Brain, and Liver Organotrophic Metastases. Curr. Oncol. 2022, 29, 7461-7469. https://doi.org/10.3390/curroncol29100587
Park HK. Neuroendocrine Carcinomas of the Uterine Cervix, Endometrium, and Ovary Show Higher Tendencies for Bone, Brain, and Liver Organotrophic Metastases. Current Oncology. 2022; 29(10):7461-7469. https://doi.org/10.3390/curroncol29100587
Chicago/Turabian StylePark, Hyung Kyu. 2022. "Neuroendocrine Carcinomas of the Uterine Cervix, Endometrium, and Ovary Show Higher Tendencies for Bone, Brain, and Liver Organotrophic Metastases" Current Oncology 29, no. 10: 7461-7469. https://doi.org/10.3390/curroncol29100587
APA StylePark, H. K. (2022). Neuroendocrine Carcinomas of the Uterine Cervix, Endometrium, and Ovary Show Higher Tendencies for Bone, Brain, and Liver Organotrophic Metastases. Current Oncology, 29(10), 7461-7469. https://doi.org/10.3390/curroncol29100587




