Abstract
Chinese national guidelines recommend various systemic therapies for patients with advanced hepatocellular carcinoma (HCC), but optimal treatment selection remains uncertain. To summarize the evidence supporting the systemic treatment of Chinese patients with advanced HCC, we performed a systematic review using a literature search of PubMed, Embase, China National Knowledge Infrastructure, and the Chinese Scientific Journal Database between 1 January 2009 and 15 June 2021, and abstracts from ASCO 2020, ASCO GI 2021, ESMO 2020, and ESMO GI 2020. The inclusion criteria were: Chinese patients aged ≥18 years with advanced HCC; first- or second-line systemic therapy; an evaluation of the efficacy or safety outcomes; and a randomized controlled, non-randomized controlled, prospective, or retrospective design. Thirty reports were identified for the following therapies: the single-agent tyrosine kinase inhibitor (TKI; n = 10), single-agent programmed death-1 (PD-1) inhibitor (n = 4), chemotherapy (n = 5), PD-1/programmed death-ligand 1 (PD-L1) inhibitor plus TKI (n = 6), PD-1/PD-L1 inhibitor plus bevacizumab or biosimilar (n = 4), and PD-1/PD-L1 inhibitor plus chemotherapy (n = 1). The heterogeneity between the studies precluded statistical analysis and the data were summarized using tables. In the first-line setting, evidence supported the use of atezolizumab or sintilimab plus bevacizumab or a biosimilar. There remains insufficient evidence to determine the optimal approved TKI-based therapeutic option, and active controlled trials in the second-line setting were lacking.
1. Introduction
Hepatocellular carcinoma (HCC) is the sixth most common type of cancer in adults and the fourth most common cause of cancer-related mortality worldwide [1]. The pattern of HCC occurrence and mortality shows a significant geographical imbalance, predominantly due to differences in the prevalence of risk factors associated with HCC, including infection with hepatitis B virus (HBV) and hepatitis C virus (HCV) [1,2]. In China, the prevalence of HCC is particularly high, accounting for over 50% of the global HCC cases and HCC-related deaths [3]. Approximately 80% of liver cancer cases in China were attributed to chronic infection of HBV and HCV [1,4].
The therapeutic options for HCC can be divided into potentially curative (e.g., surgical tumor resection or locoregional therapy) and noncurative interventions (e.g., systemic chemotherapy). The selection of treatment is based on the stage of disease, tumor characteristics, and the presence and severity of comorbidities (e.g., liver dysfunction), among other factors [2,5]. The majority of patients with early-stage HCC are eligible for curative surgical resection, percutaneous local ablation, or liver transplantation, and those with intermediate-stage HCC can often be treated with locoregional therapies, such as transarterial chemoembolization (TACE). However, about half of patients with HCC are diagnosed with advanced-stage disease [6], equivalent to the Barcelona clinic liver cancer (BCLC) stage C or Chinese National Liver Cancer (CNLC) stage IIIa–IIIb, and characterized by vascular invasion or extrahepatic spread [7]. Patients with advanced or metastatic HCC are usually only eligible to receive systemic therapy with chemotherapy, tyrosine kinase inhibitors (TKIs), or immune checkpoint inhibitors, such as programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors [2,8,9]. Most international treatment guidelines recommend systemic therapy for the first- and second-line treatment of patients with advanced HCC [10]. However, differences exist between Chinese and Western patients with advanced HCC in terms of epidemiology, genetics, clinical management, and outcomes in clinical trials [11,12]. The Chinese national guidelines recommend a broader range of treatments for these patients, including locoregional therapies for selected patients [7,13].
Despite accumulating literature on the efficacy and safety of systemic treatments in Chinese patients with HCC, decisions about which systemic treatment should be selected as optimal based on benefit–risk considerations are not variable [13]. In this article, we analyzed the findings of a systematic collation and synthesis of the results of the studies addressing the efficacy and safety of the different systemic treatments in Chinese patients with CNLC stage IIIa–IIIb HCC. The aim of this review was to provide a summary of the current evidence supporting systemic treatments for Chinese patients with advanced HCC. This will help guide evidence-based clinical decision making on the optimal treatment of Chinese patients with advanced HCC, as well as enabling the identification of future research priorities in the field.
2. Materials and Methods
This systematic review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 guidelines [14]; the PRISMA checklist and checklist for abstracts can be found in Supplementary Files S1 and S3. The research protocol was registered at the International Prospective Register of Systematic Reviews (PROSPERO; http://www.crd.york.ac.uk/PROSPERO; protocol ID: CRD42021251536; date of registration: 5 June 2021).
2.1. Information Sources and Search Strategy
We searched the literature using the following databases: PubMed, Embase, China National Knowledge Infrastructure (CNKI), and the Chinese Scientific Journal Database. We also searched abstracts from ASCO 2020, ASCO GI 2021, ESMO 2020, and ESMO GI 2020. The search included English- or Chinese-language studies published from 1 January 2009 to present. A Chinese-language translated version of the search terms was used to search Chinese-language databases. The dates when each source was last searched are as follows: PubMed 20 April 2021; Embase 27 May 2021; CNKI 28 April 2021; Chinese Scientific Journal Database 15 June 2021; ESMO/ASCO June 2021.
The PubMed search strategy was “hepatocellular carcinoma” AND “intervention” (detailed in Table 1) NOT “review” NOT “preclinical” NOT “mouse” NOT “rat” AND “clinical” NOT “case” NOT “cohort” AND ““1 January 2009” [Date-Publication]: “present” [Date-Publication]”. The same search strategy was used for Embase and CNKI with the publication date settings as “2009–2021”. The search strategies for ASCO and ESMO were simplified because of the scope of these databases and involved searching “hepatocellular carcinoma” AND “intervention” (detailed in Table 1). The Chinese Scientific Journal Database search was conducted in Chinese with the translations of the search terms shown in Table 2 and Table 3. The detailed search strategies are listed in Supplementary File S2.

Table 1.
Search terms for Chinese National Knowledge Infrastructure.

Table 2.
Interventions for Chinese National Knowledge Infrastructure search.

Table 3.
Interventions included in literature searches.
2.2. Study Selection
Records collected during the search were independently assessed for inclusion by two reviewers against predefined inclusion and exclusion criteria. Disagreements were resolved by discussion. The inclusion criteria were: (1) Chinese patients aged ≥18 years with HCC CNCLC IIIa–IIIb or BCLC stage C; (2) first- or second-line treatment with the interventions described in Table 1; (3) study/treatment of ≥1 year duration; (4) evaluation of ≥1 of the following outcomes: overall response rate (ORR), progression-free survival (PFS), overall survival (OS), duration of response (DOR), or safety; (5) randomized controlled trials (RCTs), non-randomized controlled trials, prospective or retrospective comparative studies, and single-arm studies (if comparative studies were not available). Studies were excluded if they met any of the following criteria: (1) reviews, case series, case reports, editorials, and letters; (2) data on Chinese patients could not be extracted from pooled results; (3) patients with HCC not classified as CNCLC stage IIIa–IIIb or BCLC stage C.
2.3. Data Collection and Data Items
Full-text articles were obtained for records that met the inclusion criteria, and two reviewers independently extracted data from the full-text articles of the included studies. Data from the relevant publications were extracted using standardized data extraction tables. We extracted the following data from included articles: (1) author names, year of publication, country of publication, and geographical setting; (2) study design; (3) study size (number of centers and patients/participants); (4) patient demographics and characteristics (including age, sex, HCC stage); (5) interventions (treatment, dosage, and duration); (6) outcomes and follow-up time points; and (7) data for quality and risk of bias assessment. Disagreements were resolved by discussion and with assistance from a third party (Jake Burrell), if required, until a consensus was formed. Data from studies reported in Chinese language were extracted by two native Chinese speakers.
2.4. Assessment of the Risk of Bias and Certainty in Individual Studies
Characteristics of the studies used to assess bias included random sequence generation (risk of selection bias), allocation concealment (risk of selection bias), incomplete outcome data (risk of attrition bias), selective outcome reporting, blinding of participants and personnel (performance bias and detection bias), and heterogeneity in baseline characteristics and outcome measurements. We evaluated risk of bias at the study level using the Cochrane Collaboration’s tools for assessing the risk of bias [15,16]. Two reviewers independently assessed the risk of bias in each individual study. Disagreements were resolved by discussion, with support from a third party (Jake Burrell) if required.
Risk of bias for RCTs was evaluated using the RoB2 tool, which included five domains: (1) method of randomization, (2) deviations from intended interventions involving (a) the effect of assignment to the invention and (b) the effect of adhering to the intervention, (3) missing outcome data, (4) measurement of the outcome, and (5) selection of the reported result. For each domain, the studies were ranked as low, some concerns, or high. For non-randomized trials with ≥2 arms, the ROBINS-I tool was used, which evaluates the risk of bias based on seven factors: (1) confounding, (2) selection of participants, (3) classification of interventions, (4) intended interventions, (5) missing data, (6) measurement of outcomes, and (7) selection of reported result. For each factor, the studies were classified as low, moderate, serious, or critical. Single-arm trials were not formally evaluated for bias, as they do not compare outcomes.
2.5. Measurements of Effect
We organized results from trials by intervention type and treatment effect/outcome. The main outcomes were: (1) ORR (proportion of patients with a complete or partial response by imaging assessment using the response evaluation criteria in solid tumors (RECIST) or modified RECIST (mRECIST) criteria); (2) PFS (time from randomization or assignment to treatment to disease progression or death from any cause); (3) DOR (time from disease response to disease progression); (4) OS (time from randomization or inclusion to death from any cause); (5) severe (grade ≥ 3) adverse events per the National Cancer Institute Common Terminology Criteria for Adverse Events. ORR data were summarized as proportion of patients and 95% confidence intervals (CIs). PFS, OS, and DOR were summarized as medians and 95% CIs. Safety data were summarized as number and percentage of patients with treatment-related adverse events (TRAEs) or treatment-emergent adverse events (TEAEs).
2.6. Data Synthesis
Due to the significant variation in patient characteristics, clinical settings, interventions, and reported outcomes among the included studies, we did not quantitively combine data in a meta-analysis. Instead, we conducted a qualitative synthesis by summarizing the extracted data in tables. Along with the narrative report of the outcome data of each study, we also summarized the quality and potential for bias of each data source.
3. Results
3.1. Study Selection
The searches of the PubMed, Embase, CNKI, VIP, ASCO 2020, ASCO GI 2021, ESMO 2020, and ESMO GI 2020 databases returned a total of 2934 records (Figure 1). Of these, 1083 records were duplicates and were removed before screening. Of the remaining 1851 records screened, 1685 records were discarded after assessing the titles and abstracts for inclusion (Figure 1). The full-text articles of the remaining 166 citations were sought for retrieval for a detailed evaluation, and 156 full-text reports (including congress abstracts) could be retrieved. Of these, 35 lacked extractable Chinese patient subgroup data, 48 met the exclusion criteria upon detailed evaluation, and 48 were excluded due to a serious risk of bias. Finally, 30 reports were included in the systematic review, including 5 that were added during the writing of this review (Table 4). No further studies were identified by screening the references of the included articles.
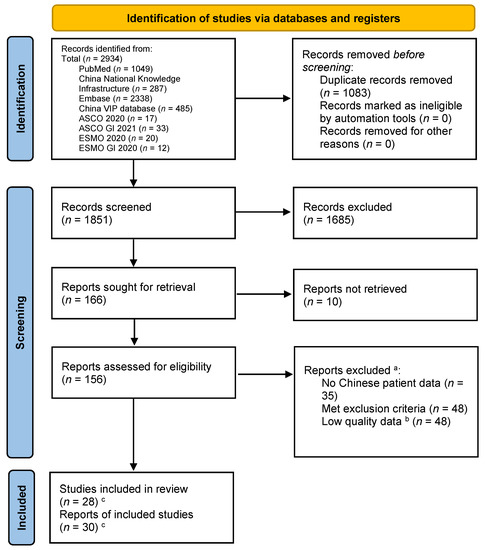
Figure 1.
PRISMA flow diagram of study identification and selection. a Studies could have been excluded for more than one reason. b The Chinese language searches returned a large volume of studies with small numbers of patients and with designs likely to have introduced a high level of bias and imprecision. For each treatment with data available, only the highest quality studies were selected for inclusion in this review. c Five studies were added during the writing of the review, because from the title and abstract, they did not appear to meet the inclusion criteria but on inspection of the full text were suitable for inclusion or were published after the initial literature search was completed. Two studies had multiple reports.

Table 4.
Characteristics of the included studies.
3.2. Study Characteristics
The key characteristics of the 30 reports included in this systematic review are shown in Table 4, and the details of the interventions used are summarized in Table 5. Overall, 28 studies were included as 2 studies were reported twice in two separate publications. Overall, 36% (10/28) of the studies were randomized, 50% (14/28) included an active control arm, and 2 were placebo controlled, while 64% (18/28) were non-randomized, 54% (15/28) were single arm, 43% (12/28) were single center, and 64% (18/28) included a sample of <100 patients. The mean/median patient ages were between 46 and 60 years, and the proportion of male patients ranged between 74.1 and 100%.

Table 5.
Intervention details.
3.3. Effects and Safety of Interventions
The treatment outcomes and safety for all included studies are summarized in Table 6.

Table 6.
Outcome data.
3.3.1. TKI Monotherapy
Based on the global multicenter studies that included Chinese patients, sorafenib, lenvatinib, and regorafenib have been approved as first- and second-line treatments for advanced stage HCC. In this review, nine studies of TKI monotherapy in Chinese patients were identified, including one study with two separate reports [17,18,19,20,21,22,23,24,25,26]. Of these, 33% (3/9) were randomized, 56% (5/9) were single arm, and 89% (8/9) had a prospective design. Across all studies, where reported, the ORR ranged from 4.6 to 40.9% (by RECIST or RECIST 1.1), the median PFS ranged from 3.0 to 6.8 months, and the median OS ranged from 5.0 to 12.1 months. When considered by individual TKIs, the ORR and OS, respectively, where available, were 10.7–40.9% and 8.7 months with apatinib [17,22,25], 22% and not reported with lenvatinib [19], 7.8% and 5 to 11.3 months with sorafenib [20,21,26], 9.1% and 5.36 months with cabozantinib [23], and 4.6–4.8% and 12.1 months with donafenib [18,24].
There was one head-to-head comparison of different TKIs [18]. In this study, donafenib demonstrated a superior median OS compared with sorafenib as a first-line treatment for patients with unresectable or metastatic HCC (12.1 vs. 10.3 months; HR 0.831; 95% CI 0.699, 0.988; p = 0.0245) [18]. However, the ORR (4.6 vs. 2.7%; p = 0.2488) and median PFS (3.7 vs. 3.6 months; HR 0.909; 95% CI 0.763, 1.082; p = 0.0570) were similar between the two arms. The incidence of grade ≥ 3 TRAEs was significantly lower with donafenib compared with sorafenib (38 vs. 50%; p = 0.0018).
In a randomized, placebo-controlled study, apatinib significantly prolonged the PFS (4.5 vs. 1.9 months; HR 0.471; 95% CI 0.369–0.601; p < 0.0001) and OS (8.7 vs. 6.8 months; HR 0.785; 95% CI 0.617–0.998; p = 0.048) compared with the placebo in patients who had received at least one line of systemic therapy [22]. The incidence of grade 3 or 4 TRAEs was 77% with apatinib versus 19% with placebo [22].
3.3.2. PD-1/PD-L1 Inhibitor Monotherapy
Four articles were identified reporting outcomes of immune checkpoint inhibitor monotherapy, of which two reported results from a multicenter phase II trial of two dosing regimens of camrelizumab in patients who had previously received systemic treatment [27,28]. In the total population, the ORR was 14.7% and the median OS was 13.8 months [27]. The incidence of grade 3 or 4 TRAEs was 22%. A longer-term follow-up analysis of this study demonstrated a median OS of 14.2 months [28].
Two articles were recent congress abstracts. The first abstract reported findings from a phase III study of pembrolizumab as a second-line therapy (KEYNOTE-394) [29]. Pembrolizumab compared with placebo improved the median OS (14.6 vs. 13.0 months; HR 0.79; 95% CI 0.63–0.99; p = 0.0180), median PFS (2.6 vs. 2.3 months; HR 0.74; 95% CI 0.60–0.92; p = 0.0032), and ORR (13.7 versus 1.3%). Pembrolizumab compared with placebo had a higher incidence of any TRAEs (66.9 vs. 49.7%) and grade ≥ 3 TRAEs (14.4 vs. 5.9%). The second abstract reported findings from a phase II, open-label study of tislelizumab as a second- or third-line treatment [30]. Tislelizumab was associated with a median OS of 13.5 months, median PFS of 2.7 months, and ORR of 13.6%. The incidences of any and grade ≥ 3 TRAEs were 62.6 and 13.6%, respectively.
3.3.3. Chemotherapy
Of the five identified studies of chemotherapy alone [31,32,33,34,35], two randomized patients to two different chemotherapy regimens, two had a single-arm design, and one compared different doses of pegylated arginine deiminase (ADI-PEG 20). All studies reported an ORR, which ranged from 0% with ADI-PEG 20 to 20% with FOLFOX4. The median PFS ranged from 1.8 to 2.9 months, and the median OS ranged from 5.0 to 9.7 months. In the four studies investigating oxaliplatin-based chemotherapies, the ORRs ranged from 8.2 to 20.0%, suggesting a similar efficacy among these various regimens [32,33,34,35]. In one study, there was a trend toward an increased OS with FOLFOX4 compared with doxorubin (6.4 vs. 5.0 months; HR 0.80; 95% CI 0.63–1.02; p = 0.07), as well as a significant improvement in PFS (2.9 vs. 1.8 months; HR 0.62; 95% CI 0.49–0.79; p < 0.001) and ORR (8.2 vs. 2.7%; p = 0.02) [35].
3.3.4. PD-1/PD-L1 Inhibitor plus Tyrosine Kinase Inhibitor
No randomized prospective controlled studies investigating the efficacy and safety of PD-1/PD-L1 in combination with a TKI were identified. However, six studies investigating the combination of a PD-1/PD-L1 inhibitor plus a TKI were retrieved, including four single-arm prospective studies [36,37,38,39] and two retrospective studies [40,41]. It should be noted that the study sample sizes were small and further robust studies with larger patient populations are warranted. Across all studies, the ORR ranged from 21.4% (by mRECIST) to 42.9% (by RECIST). A median PFS was reported from four studies and ranged from 5.5 to 8.8 months [37,39,40,41]. In a study of sintilimab plus anlotinib, the PFS rate at 6 months was 78.8% and the median PFS was not reached [36]. The available data suggest that the responses were durable. OS was reported from two studies of camrelizumab plus apatinib [37,41]. In the first, a multicenter, prospective study, the 18-month OS rates were 58.1 and 56.5% with first- and second-line treatment, respectively [37]. The second, a multicenter, retrospective study, reported a 12-month OS rate of 62.3% with second-line treatment [41]. In the only study with a comparator arm, camrelizumab plus sorafenib improved the ORR (by mRECIST, 24.0 vs. 4.0%; p = 0.025) and median PFS (8.0 vs. 6.4 months; p = 0.040) compared with sorafenib alone, while the median OS was similar between the two groups (7.4 vs. 7.0 months; p = 0.513) [40]. Across the four studies with available data, the incidences of grade ≥ 3 TRAEs varied considerably, ranging from 19.4% with penpulimab plus anlotinib [39] and 77.4% with camrelizumab plus apatinib [37]. This variation probably reflects the small study sample sizes.
3.3.5. PD-1/PD-L1 Inhibitor plus Bevacizumab or Biosimilar
The efficacy and safety data were recently reported from the Chinese subpopulation of the randomized, phase III IMbrave150 trial of atezolizumab plus bevacizumab versus sorafenib in patients with systemic treatment-naïve unresectable HCC [42]. In this analysis, atezolizumab plus bevacizumab was associated with clinically meaningful improvements compared with sorafenib in terms of the OS (median, not reached versus 11.4 months; stratified HR 0.44; 95% CI 0.25–0.76) and PFS (median, 5.7 versus 3.2 months; stratified HR 0.60; 95% CI 0.40–0.90). The ORR was 24.6 versus 6.7% (by RECIST 1.1), respectively. Any grade TRAEs (90.2 vs. 93.1%) and grade 3 or 4 TRAEs (43.9 vs. 37.9%) occurred at a similar frequency in the atezolizumab plus bevacizumab versus sorafenib groups, respectively.
In addition, three studies assessed sintilimab plus a bevacizumab biosimilar (IBI305) in the first-line setting [43,44,45]. In a large, randomized phase II/III study, sintilimab plus IBI305 compared with sorafenib monotherapy was associated with a higher ORR (21 versus 4%; by RECIST 1.1, p < 0.0001) and longer median PFS (4.6 versus 2.8 months; HR 0.56; 95% CI 0.46–0.70; p < 0.0001) and median OS (not reached versus 10.4 months; HR 0.57; 95% CI 0.43–0.75; p < 0.0001) [43]. The incidence of grade ≥3 TEAEs was similar in the sintilimab plus IBI305 and sorafenib groups (54.0 vs. 47.0%). These findings were supported by preliminary data from the two smaller phase I/II studies [44,45].
3.3.6. PD-1/PD-L1 Inhibitor plus Chemotherapy
A single-arm phase Ib/II study of camrelizumab plus FOLFOX4 as a first-line systemic therapy in advanced HCC patients was identified [46]. The ORR was 29.4%, and the median DOR was 6.9 months (range, 3.3–11.5). The PFS and OS were 7.4 months (95% CI, 3.9–9.2) and 11.7 months (95% CI, 8.2–22.0), respectively. Grade ≥ 3 TRAEs were reported at an incidence of 85.3%.
3.3.7. Chemotherapy plus Targeted Agents
There were no published articles on chemotherapy plus targeted agents that met the inclusion criteria, highlighting the lack of high-quality evidence for this treatment modality in advanced HCC.
4. Discussion
This systematic review provides a summary of the available evidence concerning the efficacy and safety of different systemic treatments in Chinese patients with advanced unresectable HCC. Of the various systemic treatments, the largest number of studies were retrieved for TKIs administered as monotherapy, but most lacked a comparator arm. One active-controlled study suggested that donafenib may offer superior OS outcomes and a lower incidence of grade ≥3 TRAEs compared with sorafenib [18]. A placebo-controlled study also indicated survival benefits with apatinib in the second-line setting [22]. It should be noted that this was the only placebo-controlled study identified in our search. Although placebo arms are generally not included in cancer treatment trials for ethical reasons, there was no approved standard of care in the second-line setting at the time this study was being conducted. The findings from global phase III trials that included Asian patients but did not meet the eligibility criteria for this review also support the use of TKIs in Chinese patients with advanced HCC [8,9,47,48].
We found three studies assessing immune checkpoint inhibitors as monotherapy, which suggested encouraging efficacy with camrelizumab, pembrolizumab, or tislelizumab as the second-line treatment [27,28,29,30]. The international phase I/II CheckMate 040 study, which included centers in Asia, demonstrated the efficacy of nivolumab monotherapy as a second-line treatment in patients with advanced HCC [49]. However, the phase III CheckMate 459 study showed that nivolumab did not significantly improve overall survival compared with sorafenib in the first-line setting [50]. The phase II KEYNOTE-224 study, which did not include Chinese patients, initially showed efficacy of pembrolizumab monotherapy in the second-line setting [51]. However, in the phase III KEYNOTE-240 study, which compared second-line pembrolizumab monotherapy with placebo in patients with advanced HCC, results from the primary endpoints (OS and PFS) did not reach the prespecified criteria for statistical significance, although the benefit-to-risk ratio for pembrolizumab was favorable [52]. A post hoc analysis of KEYNOTE-240 showed a trend toward a greater efficacy benefit in the Asian subpopulation versus the overall cohort [53], consistent with the significant improvements in the OS, PFS, and ORR observed with pembrolizumab versus placebo in the KEYNOTE-394 study in Asian patients [29].
Several studies on single chemotherapy regimens were retrieved, which supported the use of oxaliplatin-based chemotherapies in advanced HCC, with no clear advantages in favor of a particular regimen. Among the studies investigating combinations of different treatment types, the strongest evidence was provided by the sub-analysis of the phase III IMbrave150 study, which demonstrated improved efficacy outcomes with atezolizumab plus bevacizumab compared with sorafenib in Chinese patients [42]. Moreover, a large, randomized phase II/III study (ORIENT 32) showed improved efficacy outcomes with a similar type of treatment combination, sintilimab plus IBI305, over sorafenib in the first-line setting [43]. In addition, promising clinical activity with PD-1/PD-L1 inhibitors combined with TKIs in the first- or second-line setting was suggested in single-arm studies [36,37,38,39,40,41]. However, these findings need to be confirmed in randomized studies with larger sample sizes. A small study also suggested the potential antitumor activity of camrelizumab combined with FOLFOX4 for the first-line treatment of advanced HCC [46].
Other than the results from the Chinese sub-analysis of the phase III IMbrave 150 trial [42] and the phase II/III ORIENT 32 trial [43], no randomized controlled evidence was available for combination strategies, such as PD-1 antibodies in combination with a TKI or chemotherapy in Chinese patients. Evidence from single-arm, studies with a small sample size have limited strength, and further phase III randomized studies are warranted for robust evidence. In this review, a data synthesis was precluded due to the clinical heterogeneity detected between studies during the feasibility assessment, and therefore it is not possible to draw any definitive conclusions regarding the relative efficacy of different treatment types from this qualitative review. However, the compiled efficacy and safety information can be used as a reference for clinical practice. In general, the available data in Chinese patients with advanced HCC support the use of combination therapy with a PD-1/PD-L1 inhibitor (atezolizumab or sintilimab) and bevacizumab in the first-line setting compared with sorafenib.
Although the BCLC staging system is widely preferred for staging HCC, the restrictive criteria for treatment recommendation and allocation have been challenged [54,55]. Unlike the BCLC staging system, which categorizes patients with advanced HCC into one category (stage C), the CNLC system divides these patients into two subclasses (stages IIIa and IIIb) [7]. Reflecting these differences in disease classification, the range of recommended treatments for advanced HCC is more restrictive in international guidelines compared with Chinese national guidelines. The Chinese guidelines provide a variety of treatment options based on the disease stage and individual characteristics of patients [7,13].
According to international guidelines, systemic therapy with atezolizumab plus bevacizumab is the preferred first-line treatment for patients with advanced HCC and Child–Pugh class A liver function [10,56,57]. This recommendation was based on results from the phase III IMbrave150 trial, which demonstrated a significant OS benefit for atezolizumab plus bevacizumab compared with sorafenib in this setting (median OS, 19.2 vs. 13.4 months; HR, 0.66; 95% CI 0.52–0.85; descriptive p < 0.001) [58]. The median PFS was also significantly prolonged with atezolizumab plus bevacizumab (6.9 vs. 4.3 months; HR 0.65; 95% CI 0.53–0.81; descriptive p < 0.001), and the ORR (RECIST v1.1) was 27.3 versus 11.9%, respectively (p < 0.001) [59]. If there are contraindications to atezolizumab plus bevacizumab, the guidelines state that sorafenib or lenvatinib may be offered as an alternative first-line treatment [10,56,57]. For patients with disease progression on first-line therapy, recommended second-line options usually involve TKI therapy with sorafenib, lenvatinib, regorafenib, ramucirumab, or cabozantinib, while immune checkpoint inhibitors may be considered for patients with progression on or intolerance to TKIs.
In contrast, Chinese guidelines endorse a wider range of treatments for patients with advanced HCC, including systemic therapy with TKIs, FOLFOX4, or PD-1 inhibitors; TACE for CNLC stage IIIa and select IIIb cases; and resection with or without radiotherapy for CNLC stage IIIa cases [7,13]. TACE in combination with TKIs or immunotherapy is also recommended [7]. The recommended first-line systemic treatments in China consist of sorafenib, lenvatinib, or oxaliplatin-based chemotherapy, while regorafenib or PD-1 inhibitors are recommended in the second-line setting [13]. In addition, donafenib and apatinib, which were independently developed in China, have recently been approved by the China National Medical Products Administration (NMPA) as first- and second-line treatments for advanced HCC. Combined immuno-oncology options, including a PD-1 inhibitor (sintilimab) and a PD-L1 inhibitor (atezolizumab) in combination with bevacizumab or biosimilar, have been approved as first-line options by the NMPA.
A key limitation of this review was the considerable proportion of the included studies that were published only as conference abstracts, of which the data collected had therefore not undergone peer review and the risk of reporting bias was increased due to missing results. Another limitation was that some studies did not specify whether the systemic therapy under investigation was given in the first- or second-line setting or included patients regardless of therapy line.
Clinical decisions for the treatment of HCC are complex, integrating the tumor burden, disease stage and aggressiveness, and patient characteristics, such as age, existing comorbidities, and liver dysfunction. This is particularly true for the treatment of advanced HCC using systemic interventions, which can aggravate underlying liver conditions. Variability in the available treatment options and level of expertise and resources further complicates the management of patients with advanced HCC [2]. There is a clear need for further head-to-head studies in Chinese patients to guide clinical decisions given the range of available systemic treatment choices, as well as evidence regarding the optimal sequencing of therapies.
5. Conclusions
The available evidence in Chinese patients with advanced HCC supports the first-line use of atezolizumab or sintilimab plus bevacizumab or a biosimilar, as these regimens have shown superior efficacy versus sorafenib in this patient population. However, TKIs and oxaliplatin-based chemotherapy have demonstrated survival benefits and remain as options for first-line treatment, depending on individual patient characteristics. There is currently insufficient evidence to determine a preferred second-line systemic treatment, which should be selected according to individual patient situations. Although the heterogeneity of the data precluded conducting a meta-analysis, this review provides a summary of the landscape of the available evidence for systemic treatment in Chinese patients with advanced HCC, which will support clinical decision making and inform future research. Further head-to-head controlled trials between different regimens in different populations, including first-line, and TKI- or immuno-oncology- exposed second-line patients, are encouraged.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/curroncol29100575/s1, File S1: PRISMA Checklist, File S2: Methods, File S3: PRISMA Checklist for Abstracts.
Author Contributions
Conceptualization, X.Z. and Z.R.; methodology, formal analysis, data curation, writing—original draft preparation, writing—review and editing, L.Z., J.S., K.W., H.Z., X.Z. and Z.R.; supervision, Z.R.; project administration, funding acquisition, X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MSD China.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All the data, protocol, and other materials used in the review are publicly available and can be found in the information sources stated in the methods.
Acknowledgments
Editorial assistance was provided by Jake Burrell and Sharon Gladwin (Rude Health Consulting). This assistance was funded by MSD China.
Conflicts of Interest
Eisai Inc. and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, entered into a strategic collaboration for the worldwide co-development and co-commercialization of lenvatinib, which is used in the treatment of unresectable hepatocellular carcinoma in China. Xijie Zhang is an employee of MRL Global Medical Affairs, MSD China.
References
- Rawla, P.; Sunkara, T.; Muralidharan, P.; Raj, J.P. Update in global trends and aetiology of hepatocellular carcinoma. Contemp. Oncol. 2018, 22, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Qu, C.; Zhang, S.; Zeng, H.; Sun, K.; Gu, X.; Xia, C.; Yang, Z.; Li, H.; Wei, W.; et al. Liver cancer incidence and mortality in China: Temporal trends and projections to 2030. Chin. J. Cancer Res. 2018, 30, 571–579. [Google Scholar] [CrossRef]
- Mak, L.-Y.; Cruz-Ramón, V.; Chinchilla-López, P.; Torres, H.A.; LoConte, N.K.; Rice, J.P.; Foxhall, L.E.; Sturgis, E.M.; Merrill, J.K.; Bailey, H.H.; et al. Global epidemiology, prevention, and management of hepatocellular carcinoma. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 262–279. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Chen, M.; Colombo, M.; Roberts, L.; Schwartz, M.; Chen, P.-J.; Kudo, M.; Johnson, P.; Wagner, S.; Orsini, L.S.; et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: The bridge study. Liver Int. 2015, 35, 2155–2166. [Google Scholar] [CrossRef]
- Xie, D.-Y.; Ren, Z.-G.; Zhou, J.; Fan, J.; Gao, Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: Updates and insights. Hepatobiliary Surg. Nutr. 2020, 9, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.-H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology; Hepatocellular Cancers; National Comprehensive Cancer Network (NCCN): Plymouth Meeting, PA, USA, 2021; Version 1. [Google Scholar]
- Choo, S.P.; Tan, W.L.; Goh, B.K.P.; Tai, W.M.; Zhu, A.X. Comparison of hepatocellular carcinoma in Eastern versus Western populations. Cancer 2016, 122, 3430–3446. [Google Scholar] [CrossRef]
- Lee, V.; Seong, J.; Yoon, S.; Wong, T.; Wang, B.; Zhang, J.; Chiang, C.; Ho, P.; Dawson, L. Contrasting some differences in managing advanced unresectable hepatocellular carcinoma between the east and the west. Clin. Oncol. 2019, 31, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition). Zhonghua Gan Zang Bing Za Zhi 2020, 28, 112–128. [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H. Clinical efficacy and safety of Apatinib in first-line treatment for a hepatocellular carcinoma: A prospective study. Hepatol. Int. 2019, 13 (Suppl. S1), S173. [Google Scholar]
- Qin, S.; Bi, F.; Gu, S.; Bai, Y.; Chen, Z.; Wang, Z.; Ying, J.; Lu, Y.; Meng, Z.; Pan, H.; et al. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: A randomized, open-label, parallel-controlled phase II-III trial. J. Clin. Oncol. 2021, 39, 3002–3011. [Google Scholar] [CrossRef]
- Wang, D.-X.; Yang, X.; Lin, J.-Z.; Bai, Y.; Long, J.-Y.; Yang, X.-B.; Seery, S.; Zhao, H.-T. Efficacy and safety of lenvatinib for patients with advanced hepatocellular carcinoma: A retrospective, real-world study conducted in China. World J. Gastroenterol. 2020, 26, 4465–4478. [Google Scholar] [CrossRef]
- Ye, S.-L.; Chen, X.; Yang, J.; Bie, P.; Zhang, S.; Liu, F.; Liu, L.; Zhou, J.; Dou, K.; Yip, C.S.; et al. Evaluation of sorafenib in Chinese unresectable hepatocellular carcinoma patients with prior surgery and portal vein tumor thrombosis: A subset analysis of GIDEON study data. Tumor Biol. 2017, 39, 1010428317695030. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.-L.; Chen, X.; Yang, J.; Bie, P.; Zhang, S.; Liu, F.; Liu, L.; Zhou, J.; Dou, K.; Hao, C.; et al. Safety and efficacy of sorafenib therapy in patients with hepatocellular carcinoma: Final outcome from the Chinese patient subset of the GIDEON study. Oncotarget 2016, 7, 6639–6648. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Li, Q.; Gu, S.; Chen, X.; Lin, L.; Wang, Z.; Xu, A.; Chen, X.; Zhou, C.; Ren, Z.; et al. Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 559–568. [Google Scholar] [CrossRef]
- Dong, Y.; Leung, T.W.-T.; Kwok, G.W.; Tang, V.; Li, B.; Leung, R.C.-Y.; Chiu, J.W.Y.; Wong, J.S.L.; Ma, K.W.; She, W.H.; et al. The use of cabozantinib in advanced hepatocellular carcinoma (HCC): Hong Kong multi-center experience. J. Clin. Oncol. 2021, 39, 316. [Google Scholar] [CrossRef]
- Bi, F.; Qiu, M.; Chai, X.; Niu, J.; Ding, Y.; Bai, Y.; Wu, L.; Shentu, J.; Hao, P.; Chen, J.; et al. A multicenter phase II study of donafenib in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 2017, 35, e15682. [Google Scholar] [CrossRef]
- Kong, Y.; Sun, L.; Hou, Z.; Zhang, Y.; Chen, P.; Cui, Y.; Zhu, X.; Song, T.; Li, Q.; Li, H.; et al. Apatinib is effective for treatment of advanced hepatocellular carcinoma. Oncotarget 2017, 8, 105596–105605. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Chan, P.; Ng, K.K.; Chok, S.H.; Cheung, T.T.; Fan, S.T.; Poon, R.T. Phase 2 open-label study of single-agent sorafenib in treating advanced hepatocellular carcinoma in a hepatitis B-endemic Asian population: Presence of lung metastasis predicts poor response. Cancer 2009, 115, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Ren, Z.; Meng, Z.; Chen, Z.; Chai, X.; Xiong, J.; Bai, Y.; Yang, L.; Zhu, H.; Fang, W.; et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: A multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 571–580. [Google Scholar] [CrossRef]
- Ren, Z.; Qin, S.; Meng, Z.; Chen, Z.; Chai, X.; Xiong, J.; Bai, Y.; Yang, L.; Zhu, H.; Fang, W.; et al. 985P A phase II study of camrelizumab for advanced hepatocellular carcinoma: Two-year outcomes and continued treatment beyond RECIST-defined progression. Ann. Oncol. 2020, 31, S689–S690. [Google Scholar] [CrossRef]
- Qin, S.; Chen, Z.; Fang, W.; Ren, Z.; Xu, R.; Ryoo, B.-Y.; Meng, Z.; Bai, Y.; Chen, X.; Liu, X.; et al. Pembrolizumab plus best supportive care versus placebo plus best supportive care as second-line therapy in patients in Asia with advanced hepatocellular carcinoma (HCC): Phase 3 KEYNOTE-394 study. J. Clin. Oncol. 2022, 40, 383. [Google Scholar] [CrossRef]
- Edeline, J.; Merle, P.; Fang, W.; Assenat, E.; Pan, H.; Rimassa, L.; Li, Z.; Blanc, J.-F.; Yen, C.-J.; Ross, P.J.; et al. Clinical outcomes associated with tislelizumab in patients (pts) with advanced hepatocellular carcinoma (HCC) who have been previously treated with sorafenib (SOR) or lenvatinib (LEN) in RATIONALE-208. J. Clin. Oncol. 2022, 40, 420. [Google Scholar] [CrossRef]
- Yang, T.-S.; Lu, S.-N.; Chao, Y.; Sheen, I.-S.; Lin, C.-C.; Wang, T.-E.; Chen, S.-C.; Wang, J.-H.; Liao, L.-Y.; Thomson, J.A.; et al. A randomised phase II study of pegylated arginine deiminase (ADI-PEG 20) in Asian advanced hepatocellular carcinoma patients. Br. J. Cancer 2010, 103, 954–960. [Google Scholar] [CrossRef]
- Zhu, H.; Sun, P. Clinical study of FOLFOX4 regimen for patients of advanced hepatocellular carcinoma. Chin.-German J. Clin. Oncol. 2012, 11, 134–137. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, J.; Li, W.; Yan, W.; Jia, G. Comparison of XELOX and FOLFOX4 regimens for treatment of advanced hepatocellular carcinoma. J. Chin. Oncol. 2017, 23, 868–871. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, P.; Wu, Y.; Xiao, J.; Peng, J. Clinical observation of advanced primary hepatocarcinoma with FOLFOX6 regimen. Chin. Clin. Oncol. 2010, 15, 70–74. [Google Scholar] [CrossRef]
- Qin, S.; Bai, Y.; Lim, H.Y.; Thongprasert, S.; Chao, Y.; Fan, J.; Yang, T.-S.; Bhudhisawasdi, V.; Kang, W.K.; Zhou, Y.; et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J. Clin. Oncol. 2013, 31, 3501–3508. [Google Scholar] [CrossRef]
- Chen, X.; Li, W.; Wu, X.; Zhao, F.; Wu, H.; Gu, Y.; Li, X.; Qian, X.; Hu, J.; Li, C.; et al. 170P Sintilimab plus anlotinib as first-line therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann. Oncol. 2020, 31, S1305. [Google Scholar] [CrossRef]
- Xu, J.; Shen, J.; Gu, S.; Zhang, Y.; Wu, L.; Wu, J.; Shao, G.; Zhang, Y.; Xu, L.; Yin, T.; et al. Camrelizumab in combination with Apatinib in patients with advanced hepatocellular carcinoma (RESCUE): A nonrandomized, open-label, phase II trial. Clin. Cancer Res. 2021, 27, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, J.; You, N.; Wu, K.; Wang, Z.; Zhu, Y.; Wang, L.; Zheng, L. P-129 Camrelizumab combined with sorafenib versus sorafenib alone in patients with advanced hepatocellular carcinoma: A retrospective study. Ann. Oncol. 2020, 31, S131–S132. [Google Scholar] [CrossRef]
- Lin, H.; Ma, J.; Zhuo, M.; Zhang, C.; Luo, J.; Zhuang, X.; Zeng, Z.; Yang, L. Updated results of the phase II ALTER-H003 trial: Anlotinib plus toripalimab as a first-line treatment for patients with unresectable hepatocellular carcinoma. J. Clin. Oncol. 2021, 39, e16130. [Google Scholar] [CrossRef]
- Han, C.; Ye, S.; Hu, C.; Shen, L.; Qin, Q.; Bai, Y.; Yang, S.; Bai, C.; Zang, A.; Jiao, S.; et al. Clinical activity and safety of penpulimab (Anti-PD-1) with anlotinib as first-line therapy for unresectable hepatocellular carcinoma: An open-label, multicenter, phase Ib/II trial (AK105-203). Front. Oncol. 2021, 11, 684867. [Google Scholar] [CrossRef]
- Yuan, G.S.; He, W.M.; Hu, X.Y.; Li, Q.; Zang, M.Y.; Cheng, X.; Huang, W.; Ruan, J.; Wang, J.J.; Hou, J.L.; et al. Clinical efficacy and safety analysis of camrelizumab combined with apatinib as a second-line therapy for unresectable hepatocellular carcinoma: A multicenter retrospective study. Zhonghua Gan Zang Bing Za Zhi 2021, 29, 326–331. (In Chinese) [Google Scholar] [CrossRef]
- Qin, S.; Ren, Z.; Feng, Y.-H.; Yau, T.; Wang, B.; Zhao, H.; Bai, Y.; Gu, S.; Li, L.; Hernandez, S.; et al. Atezolizumab plus bevacizumab versus sorafenib in the Chinese subpopulation with unresectable hepatocellular carcinoma: Phase 3 randomized, open-label imbrave150 study. Liver Cancer 2021, 10, 296–308. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, J.; Bai, Y.; Xu, A.; Cang, S.; Du, C.; Li, Q.; Lu, Y.; Chen, Y.; Guo, Y.; et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2–3 study. Lancet Oncol. 2021, 22, 977–990. [Google Scholar] [CrossRef]
- Jia, F.; Ren, Z.; Xu, J.; Shao, G.; Dai, G.; Liu, B.; Xu, A.; Yang, Y.; Wang, Y.; Zhou, H.; et al. 991P Sintilimab plus IBI305 as first-line treatment for advanced hepatocellular carcinoma. Ann. Oncol. 2020, 31, S692. [Google Scholar] [CrossRef]
- Zhang, W.; Bi, X.; Sun, Y.; Yu, Y.; Zhou, J.-G.; Zeng, H.; Wu, F.; Luo, Y.; Yang, Y.; Chen, M.; et al. Preliminary results of sintilimab plus different dose of IBI305 (anti-VEGF monoclonal antibody) in patients with advanced hepatocellular carcinoma: A phase Ib study. J. Clin. Oncol. 2020, 38, 3079. [Google Scholar] [CrossRef]
- Li, H.; Qin, S.; Liu, Y.; Chen, Z.; Ren, Z.; Xiong, J.; Meng, Z.; Zhang, X.; Wang, L.; Zhang, X.; et al. Camrelizumab combined with FOLFOX4 regimen as first-line therapy for advanced hepatocellular carcinomas: A sub-cohort of a multicenter phase Ib/II study. Drug Des. Dev. Ther. 2021, 15, 1873–1882. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Kang, Y.-K.; Chen, Z.; Tsao, C.-J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.-S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2008, 10, 25–34. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.-L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.-Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.-W.; et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H., 3rd; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Yau, T.; Park, J.-W.; Finn, R.S.; Cheng, A.-L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2021, 23, 77–90. [Google Scholar] [CrossRef]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.-Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase III trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef]
- Kudo, M.; Lim, H.Y.; Cheng, A.-L.; Chao, Y.; Yau, T.; Ogasawara, S.; Kurosaki, M.; Morimoto, N.; Ohkawa, K.; Yamashita, T.; et al. Pembrolizumab as second-line therapy for advanced hepatocellular carcinoma: A subgroup analysis of asian patients in the phase 3 KEYNOTE-240 trial. Liver Cancer 2021, 10, 275–284. [Google Scholar] [CrossRef]
- Saraswat, V.A.; Pandey, G.; Shetty, S. Treatment algorithms for managing hepatocellular carcinoma. J. Clin. Exp. Hepatol. 2014, 4, S80–S89. [Google Scholar] [CrossRef]
- Richani, M.; Kolly, P.; Knoepfli, M.; Herrmann, E.; Zweifel, M.; von Tengg-Kobligk, H.; Candinas, D.; Dufour, J.-F. Treatment allocation in hepatocellular carcinoma: Assessment of the BCLC algorithm. Ann. Hepatol. 2016, 15, 82–90. [Google Scholar] [CrossRef]
- Vogel, A.; Martinelli, E.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.; Meyer, T.; Nault, J.-C.; Neumann, U.; Ricke, J.; et al. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO clinical practice guidelines. Ann. Oncol. 2021, 32, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Gordan, J.D.; Kennedy, E.B.; Abou-Alfa, G.K.; Beg, M.S.; Brower, S.T.; Gade, T.P.; Goff, L.; Gupta, S.; Guy, J.; Harris, W.P.; et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J. Clin. Oncol. 2020, 38, 4317–4345. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2021, 76, 862–873. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).