Current Clinical Practice of Precision Medicine Using Comprehensive Genomic Profiling Tests in Biliary Tract Cancer in Japan
Abstract
1. Introduction
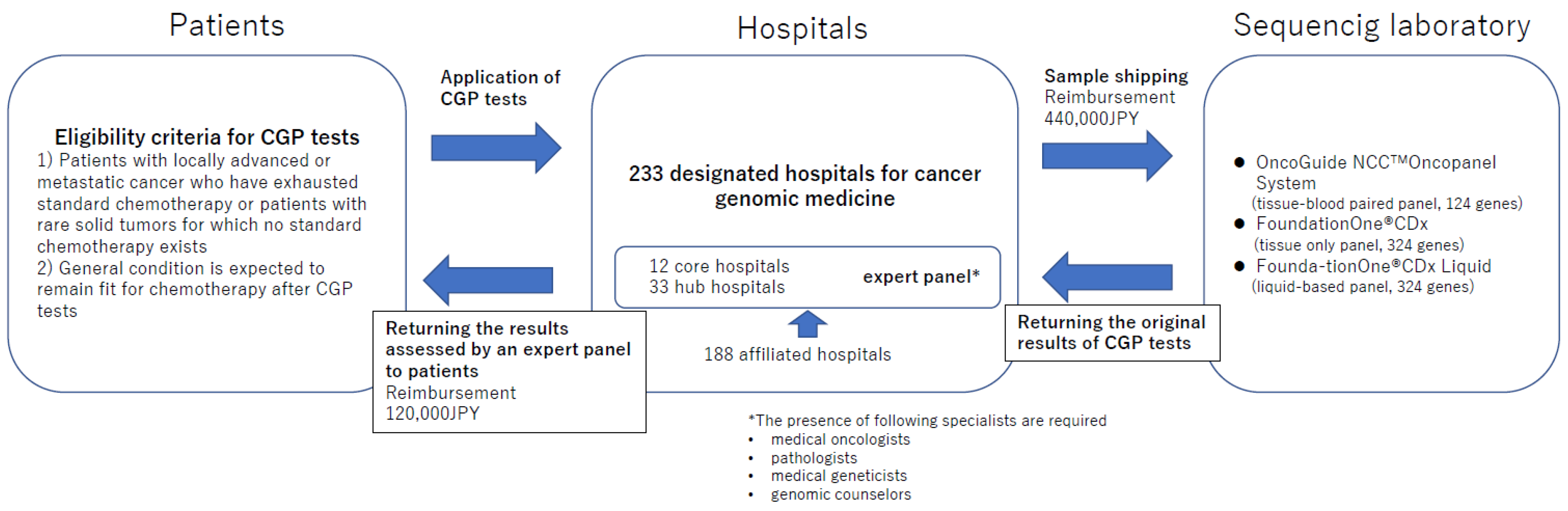
2. Principle Flow of CGP Tests in Japan
2.1. Reimbursed CGP Tests
2.2. Eligible Patients for CGP Tests
2.3. Requirements of Tissue Samples for Tissue-Based CGP Test
2.4. Assessment of CGP Test Results in an Expert Panel
2.5. Reimbursement System of CGP Tests in Japan
3. Optimal Timing for Ordering CGP Tests in Patients with Biliary Tract Cancer
4. Liquid-Based CGP Tests (Liquid Biopsy) in Biliary Tract Cancer
4.1. Eligible Patients for Liquid Biopsy
4.2. Performance of Liquid Biopsy in Patients with Biliary tract Cancer
4.3. Drawbacks of Liquid Biopsy
- (1)
- Results of MSI-H/TMB-H/copy number variations (e.g., ERBB2 amplifications) obtained by FoundationOne®CDx Liquid are not approved for clinical use by MHLW in Japan.
- (2)
- There is a risk of false negative results due to low levels of circulating tumor DNA (ctDNA), especially when total tumor volume is low.
- (3)
- There is a risk of false positive results due to the contamination of clonal hematopoiesis with indeterminate potential (CHIP).
4.4. Possible Application of Liquid Biopsy Technology for Early Detection of Biliary Tract Cancer
5. Representative Druggable Biomarkers in Biliary Tract Cancer
5.1. MSI-H
5.2. NTRK Fusion
5.3. Tumor Mutation Burden (TMB)-H
5.4. FGFR2 Fusion/Rearrangement
5.5. BRAF V600E
5.6. IDH1 Mutation
5.7. HER2 Overexpression
5.8. RET Fusion
5.9. KRAS G12C
6. Germline Pathogenic Variants Identified in CGP Tests
7. Conclusions
Funding
Conflicts of Interest
References
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.Y.; Zhu, A.X. Biliary tract cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef]
- Available online: https://ganjoho.jp/public/qa_links/report/statistics/2022_en.html (accessed on 21 August 2022).
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Morizane, C.; Okusaka, T.; Mizusawa, J.; Katayama, H.; Ueno, M.; Ikeda, M.; Ozaka, M.; Okano, N.; Sugimori, K.; Fukutomi, A.; et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: The FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann. Oncol. 2019, 30, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Sakai, D.; Kanai, M.; Kobayashi, S.; Eguchi, H.; Baba, H.; Seo, S.; Taketomi, A.; Takayama, T.; Yamaue, H.; Ishioka, C.; et al. Randomized phase III study of Gemcitabine, Cisplatin plus S-1 (GCS) versus Gemcitabine, Cisplatin (GC) for Advanced Biliary Tract Cancer (KHBO1401-MITSUBA). J. Hepato-Biliary Pancreat. Sci. 2022. [Google Scholar] [CrossRef]
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. J. Clin. Oncol. 2022, 40, 378. [Google Scholar] [CrossRef]
- Massa, A.; Varamo, C.; Vita, F.; Tavolari, S.; Peraldo-Neia, C.; Brandi, G.; Rizzo, A.; Cavalloni, G.; Aglietta, M. Evolution of the Experimental Models of Cholangiocarcinoma. Cancers 2020, 12, 2308. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Dageforde, L.A. Cholangiocarcinoma. Surg. Clin. N. Am. 2019, 99, 315–335. [Google Scholar] [CrossRef]
- Nakamura, H.; Arai, Y.; Totoki, Y.; Shirota, T.; ElZawahry, A.; Kato, M.; Hama, N.; Hosoda, F.; Urushidate, T.; Ohashi, S.; et al. Genomic spectra of biliary tract cancer. Nat. Genet. 2015, 47, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Jusakul, A.; Cutcutache, I.; Yong, C.H.; Lim, J.Q.; Ni Huang, M.; Padmanabhan, N.; Nellore, V.; Kongpetch, S.; Ng, A.W.T.; Ng, L.M.; et al. Whole-Genome and Epigenomic Landscapes of Etiologically Distinct Subtypes of Cholangiocarcinoma. Cancer Discov. 2017, 7, 1116–1135. [Google Scholar] [CrossRef]
- Wardell, C.P.; Fujita, M.; Yamada, T.; Simbolo, M.; Fassan, M.; Karlic, R.; Polak, P.; Kim, J.; Hatanaka, Y.; Maejima, K.; et al. Genomic characterization of biliary tract cancers identifies driver genes and predisposing mutations. J. Hepatol. 2018, 68, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Kendall, T.; Verheij, J.; Gaudio, E.; Evert, M.; Guido, M.; Goeppert, B.; Carpino, G. Anatomical, histomorphological and molecular classification of cholangiocarcinoma. Liver Int. 2019, 39 (Suppl. S1), 7–18. [Google Scholar] [CrossRef]
- Lowery, M.A.; Ptashkin, R.; Jordan, E.; Berger, M.F.; Zehir, A.; Capanu, M.; Kemeny, N.E.; O’Reilly, E.M.; El-Dika, I.; Jarnagin, W.R.; et al. Comprehensive Molecular Profiling of Intrahepatic and Extrahepatic Cholangiocarcinomas: Potential Targets for Intervention. Clin. Cancer Res. 2018, 24, 4154–4161. [Google Scholar] [CrossRef] [PubMed]
- Kou, T.; Kanai, M.; Yamamoto, Y.; Kamada, M.; Nakatsui, M.; Sakuma, T.; Mochizuki, H.; Hiroshima, A.; Sugiyama, A.; Nakamura, E.; et al. Clinical sequencing using a next-generation sequencing-based multiplex gene assay in patients with advanced solid tumors. Cancer Sci. 2017, 108, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Sunami, K.; Ichikawa, H.; Kubo, T.; Kato, M.; Fujiwara, Y.; Shimomura, A.; Koyama, T.; Kakishima, H.; Kitami, M.; Matsushita, H.; et al. Feasibility and utility of a panel testing for 114 cancer-associated genes in a clinical setting: A hospital-based study. Cancer Sci. 2019, 110, 1480–1490. [Google Scholar] [CrossRef]
- Kondo, T.; Matsubara, J.; Quy, P.N.; Fukuyama, K.; Nomura, M.; Funakoshi, T.; Doi, K.; Sakamori, Y.; Yoshioka, M.; Yokoyama, A.; et al. Comprehensive genomic profiling for patients with chemotherapy-naive advanced cancer. Cancer Sci. 2021, 112, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Ebi, H.; Bando, H. Precision Oncology and the Universal Health Coverage System in Japan. JCO Precis. Oncol. 2019, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://for-patients.c-cat.ncc.go.jp/knowledge/cancer_genomic_medicine/get_tested.html#get_tested (accessed on 21 August 2022).
- Available online: https://for-patients.c-cat.ncc.go.jp/library/statistics/ (accessed on 21 August 2022).
- NCCN Clinical Practice Guideline: Biliary Tract Cancers. Ver 1. 2022. Available online: https://wwwnccnorg/professionals/physician_gls/pdf/hepatobiliarypdf (accessed on 21 August 2022).
- Naito, Y.; Aburatani, H.; Amano, T.; Baba, E.; Furukawa, T.; Hayashida, T.; Hiyama, E.; Ikeda, S.; Kanai, M.; Kato, M.; et al. Clinical practice guidance for next-generation sequencing in cancer diagnosis and treatment (edition 2.1). Int. J. Clin. Oncol. 2021, 26, 233–283. [Google Scholar] [CrossRef] [PubMed]
- Quy, P.N.; Kanai, M.; Fukuyama, K.; Kou, T.; Kondo, T.; Yamamoto, Y.; Matsubara, J.; Hiroshima, A.; Mochizuki, H.; Sakuma, T.; et al. Association Between Preanalytical Factors and Tumor Mutational Burden Estimated by Next-Generation Sequencing-Based Multiplex Gene Panel Assay. Oncologist 2019, 24, e1401–e1408. [Google Scholar] [CrossRef]
- Matsumori, T.; Uza, N.; Shiokawa, M.; Maruno, T.; Nishikawa, Y.; Morita, T.; Kuwada, T.; Marui, S.; Okada, H.; Taura, K.; et al. Clinical impact of a novel device delivery system in the diagnosis of bile duct lesions: A single-center experience. J. Gastroenterol. Hepatol. 2022, 37, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Zill, O.A.; Greene, C.; Sebisanovic, D.; Siew, L.M.; Leng, J.; Vu, M.; Hendifar, A.E.; Wang, Z.; Atreya, C.E.; Kelley, R.K.; et al. Cell-Free DNA Next-Generation Sequencing in Pancreatobiliary Carcinomas. Cancer Discov. 2015, 5, 1040–1048. [Google Scholar] [CrossRef]
- Sunami, K.; Naito, Y.; Aimono, E.; Amano, T.; Ennishi, D.; Kage, H.; Kanai, M.; Komine, K.; Koyama, T.; Maeda, T.; et al. The initial assessment of expert panel performance in core hospitals for cancer genomic medicine in Japan. Int. J. Clin. Oncol. 2021, 26, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.mhlw.go.jp/content/12404000/000846288.pdf (accessed on 21 August 2022).
- Dumonceau, J.-M.; Kapral, C.; Aabakken, L.; Papanikolaou, I.S.; Tringali, A.; Vanbiervliet, G.; Beyna, T.; Dinis-Ribeiro, M.; Hritz, I.; Mariani, A.; et al. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2020, 52, 127–149. [Google Scholar] [CrossRef] [PubMed]
- Parikh, A.R.; Leshchiner, I.; Elagina, L.; Goyal, L.; Levovitz, C.; Siravegna, G.; Livitz, D.; Rhrissorrakrai, K.; Martin, E.E.; Van Seventer, E.E.; et al. Liquid versus tissue biopsy for detecting acquired resistance and tumor heterogeneity in gastrointestinal cancers. Nat. Med. 2019, 25, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Taniguchi, H.; Ikeda, M.; Bando, H.; Kato, K.; Morizane, C.; Esaki, T.; Komatsu, Y.; Kawamoto, Y.; Takahashi, N.; et al. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat. Med. 2020, 26, 1859–1864. [Google Scholar] [CrossRef]
- Mody, K.; Kasi, P.M.; Yang, J.; Surapaneni, P.K.; Bekaii-Saab, T.; Ahn, D.H.; Mahipal, A.; Sonbol, M.B.; Starr, J.S.; Roberts, A.; et al. Circulating Tumor DNA Profiling of Advanced Biliary Tract Cancers. JCO Precis. Oncol. 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Gupta, R.; Othman, T.; Chen, C.; Sandhu, J.; Ouyang, C.; Fakih, M. Guardant360 Circulating Tumor DNA Assay Is Concordant with FoundationOne Next-Generation Sequencing in Detecting Actionable Driver Mutations in Anti-EGFR Naive Metastatic Colorectal Cancer. Oncologist 2020, 25, 235–243. [Google Scholar] [CrossRef]
- Woodhouse, R.; Li, M.; Hughes, J.; Delfosse, D.; Skoletsky, J.; Ma, P.; Meng, W.; Dewal, N.; Milbury, C.; Clark, T.; et al. Clinical and analytical validation of FoundationOne Liquid CDx, a novel 324-Gene cfDNA-based comprehensive genomic profiling assay for cancers of solid tumor origin. PLoS ONE 2020, 15, e0237802. [Google Scholar] [CrossRef]
- Lee, J.K.; Hazar-Rethinam, M.; Decker, B.; Gjoerup, O.; Madison, R.W.; Lieber, D.S.; Chung, J.H.; Schrock, A.B.; Creeden, J.; Venstrom, J.M.; et al. The Pan-Tumor Landscape of Targetable Kinase Fusions in Circulating Tumor DNA. Clin. Cancer Res. 2022, 28, 728–737. [Google Scholar] [CrossRef]
- Imai, M.; Nakamura, Y.; Sunami, K.; Kage, H.; Komine, K.; Koyama, T.; Amano, T.; Ennishi, D.; Kanai, M.; Kenmotsu, H.; et al. Expert Panel Consensus Recommendations on the Use of Circulating Tumor DNA Assays for Patients with Advanced Solid Tumors. Cancer Sci. 2022. [Google Scholar] [CrossRef]
- Willis, J.; Lefterova, M.I.; Artyomenko, A.; Kasi, P.M.; Nakamura, Y.; Mody, K.; Catenacci, D.V.T.; Fakih, M.; Barbacioru, C.; Zhao, J.; et al. Validation of Microsatellite Instability Detection Using a Comprehensive Plasma-Based Genotyping Panel. Clin. Cancer Res. 2019, 25, 7035–7045. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Tukachinsky, H.; Lee, J.K.; Sokol, E.; Pavlick, D.C.; Aiyer, A.; Fabrizio, D.; Venstrom, J.M.; Mishima, S.; Nakamura, Y.; et al. Genomic immunotherapy (IO) biomarkers detected on comprehensive genomic profiling (CGP) of tissue and circulating tumor DNA (ctDNA). J. Clin. Oncol. 2021, 39, 2541. [Google Scholar] [CrossRef]
- Razavi, P.; Li, B.T.; Brown, D.N.; Jung, B.; Hubbell, E.; Shen, R.; Abida, W.; Juluru, K.; De Bruijn, I.; Hou, C.; et al. High-intensity sequencing reveals the sources of plasma circulating cell-free DNA variants. Nat. Med. 2019, 25, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 2014, 6, 224ra224. [Google Scholar] [CrossRef]
- Han, X.; Han, Y.; Tan, Q.; Huang, Y.; Yang, J.; Yang, S.; He, X.; Zhou, S.; Song, Y.; Pi, J.; et al. Tracking longitudinal genetic changes of circulating tumor DNA (ctDNA) in advanced Lung adenocarcinoma treated with chemotherapy. J. Transl. Med. 2019, 17, 339. [Google Scholar] [CrossRef]
- Jaiswal, S. Clonal hematopoiesis and nonhematologic disorders. Blood 2020, 136, 1606–1614. [Google Scholar]
- Jensen, K.; Konnick, E.Q.; Schweizer, M.T.; Sokolova, A.O.; Grivas, P.; Cheng, H.H.; Klemfuss, N.M.; Beightol, M.; Yu, E.Y.; Nelson, P.S.; et al. Association of Clonal Hematopoiesis in DNA Repair Genes with Prostate Cancer Plasma Cell-free DNA Testing Interference. JAMA Oncol. 2021, 7, 107–110. [Google Scholar] [CrossRef]
- Corcoran, R.B.; Chabner, B.A. Application of Cell-free DNA Analysis to Cancer Treatment. N. Engl. J. Med. 2018, 379, 1754–1765. [Google Scholar] [CrossRef]
- Lennon, A.M.; Buchanan, A.H.; Kinde, I.; Warren, A.; Honushefsky, A.; Cohain, A.T.; Ledbetter, D.H.; Sanfilippo, F.; Sheridan, K.; Rosica, D.; et al. Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science 2020, 369, eabb9601. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D.; Tavolari, S.; Brandi, G. Circulating Tumor DNA in Biliary Tract Cancer: Current Evidence and Future Perspectives. Cancer Genom. Proteom. 2020, 17, 441–452. [Google Scholar] [CrossRef]
- Gou, Q.; Zhang, C.; Sun, Z.; Wu, L.; Chen, Y.; Mo, Z.; Mai, Q.; He, J.; Zhou, Z.; Shi, F.; et al. Cell-free DNA from bile outperformed plasma as a potential alternative to tissue biopsy in biliary tract cancer. ESMO Open 2021, 6, 100275. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Zhu, B.; Zhang, W.; Nian, B.; Xu, X.; Yu, L.; Ruan, X.; Chen, S.; Liu, Y.; Cao, X.; et al. Comprehensive Evaluation and Application of a Novel Method to Isolate Cell-Free DNA Derived from Bile of Biliary Tract Cancer Patients. Front. Oncol. 2022, 12, 891917. [Google Scholar] [CrossRef]
- Massard, C.; Michiels, S.; Ferté, C.; Le Deley, M.-C.; Lacroix, L.; Hollebecque, A.; Verlingue, L.; Ileana, E.; Rosellini, S.; Ammari, S.; et al. High-Throughput Genomics and Clinical Outcome in Hard-to-Treat Advanced Cancers: Results of the MOSCATO 01 Trial. Cancer Discov. 2017, 7, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Verlingue, L.; Malka, D.; Allorant, A.; Massard, C.; Ferté, C.; Lacroix, L.; Rouleau, E.; Auger, N.; Ngo, M.; Nicotra, C.; et al. Precision medicine for patients with advanced biliary tract cancers: An effective strategy within the prospective MOSCATO-01 trial. Eur. J. Cancer 2017, 87, 122–130. [Google Scholar] [CrossRef]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Akagi, K.; Oki, E.; Taniguchi, H.; Nakatani, K.; Aoki, D.; Kuwata, T.; Yoshino, T. Nationwide large-scale investigation of microsatellite instability status in more than 18,000 patients with various advanced solid cancers. J. Clin. Oncol. 2020, 38, 803. [Google Scholar] [CrossRef]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N.; et al. Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef]
- Yoshino, T.; Pentheroudakis, G.; Mishima, S.; Overman, M.J.; Yeh, K.-H.; Baba, E.; Naito, Y.; Calvo, F.; Saxena, A.; Chen, C.-T.; et al. JSCO-ESMO-ASCO-JSMO-TOS: International expert consensus recommendations for tumour-agnostic treatments in patients with solid tumours with microsatellite instability or NTRK fusions. Ann. Oncol. 2020, 31, 861–872. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- VanderWalde, A.; Spetzler, D.; Xiao, N.; Gatalica, Z.; Marshall, J. Microsatellite instability status determined by next-generation sequencing and compared with PD-L1 and tumor mutational burden in 11,348 patients. Cancer Med. 2018, 7, 746–756. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- Maruki, Y.; Morizane, C.; Arai, Y.; Ikeda, M.; Ueno, M.; Ioka, T.; Naganuma, A.; Furukawa, M.; Mizuno, N.; Uwagawa, T.; et al. Molecular detection and clinicopathological characteristics of advanced/recurrent biliary tract carcinomas harboring the FGFR2 rearrangements: A prospective observational study (PRELUDE Study). J. Gastroenterol. 2021, 56, 250–260. [Google Scholar] [CrossRef]
- Subbiah, V.; Lassen, U.; Elez, E.; Italiano, A.; Curigliano, G.; Javle, M.; de Braud, F.; Prager, G.W.; Greil, R.; Stein, A.; et al. Dabrafenib plus trametinib in patients with BRAF(V600E)-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef]
- Goeppert, B.; Frauenschuh, L.; Renner, M.; Roessler, S.; Stenzinger, A.; Klauschen, F.; Warth, A.; Vogel, M.N.; Mehrabi, A.; Hafezi, M.; et al. BRAF V600E-specific immunohistochemistry reveals low mutation rates in biliary tract cancer and restriction to intrahepatic cholangiocarcinoma. Mod. Pathol. 2014, 27, 1028–1034. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Boscoe, A.N.; Rolland, C.; Kelley, R.K. Frequency and prognostic significance of isocitrate dehydrogenase 1 mutations in cholangiocarcinoma: A systematic literature review. J. Gastrointest. Oncol. 2019, 10, 751–765. [Google Scholar] [CrossRef]
- Javle, M.; Borad, M.J.; Azad, N.S.; Kurzrock, R.; Abou-Alfa, G.K.; George, B.; Hainsworth, J.; Meric-Bernstam, F.; Swanton, C.; Sweeney, C.J.; et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): A multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021, 22, 1290–1300. [Google Scholar] [CrossRef]
- Jain, A.; Javle, M. Molecular profiling of biliary tract cancer: A target rich disease. J. Gastrointest. Oncol. 2016, 7, 797–803. [Google Scholar] [CrossRef]
- Subbiah, V.; Cassier, P.A.; Siena, S.; Garralda, E.; Paz-Ares, L.; Garrido, P.; Nadal, E.; Vuky, J.; Lopes, G.; Kalemkerian, G.P.; et al. Pan-cancer efficacy of pralsetinib in patients with RET fusion-positive solid tumors from the phase 1/2 ARROW trial. Nat. Med. 2022, 28, 1640–1645. [Google Scholar] [CrossRef]
- Yurgelun, M.B.; Hampel, H. Recent Advances in Lynch Syndrome: Diagnosis, Treatment, and Cancer Prevention. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 101–109. [Google Scholar] [CrossRef]
- Takamizawa, S.; Morizane, C.; Tanabe, N.; Maruki, Y.; Kondo, S.; Hijioka, S.; Ueno, H.; Sugano, K.; Hiraoka, N.; Okusaka, T. Clinical characteristics of pancreatic and biliary tract cancers associated with Lynch syndrome. J. Hepato-Biliary Pancreat. Sci. 2022, 29, 377–384. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- McGrail, D.; Pilié, P.; Rashid, N.; Voorwerk, L.; Slagter, M.; Kok, M.; Jonasch, E.; Khasraw, M.; Heimberger, A.; Lim, B.; et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann. Oncol. 2021, 32, 661–672. [Google Scholar] [CrossRef]
- Rousseau, B.; Foote, M.B.; Maron, S.B.; Diplas, B.H.; Lu, S.; Argilés, G.; Cercek, A.; Diaz, L.A. The Spectrum of Benefit from Checkpoint Blockade in Hypermutated Tumors. N. Engl. J. Med. 2021, 384, 1168–1170. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D.; Brandi, G. Pemigatinib: Hot topics behind the first approval of a targeted therapy in cholangiocarcinoma. Cancer Treat. Res. Commun. 2021, 27, 100337. [Google Scholar] [CrossRef]
- Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-dabrafenib-combination-trametinib-unresectable-or-metastatic-solid (accessed on 21 August 2022).
- Boerner, T.; Drill, E.; Pak, L.M.; Nguyen, B.; Sigel, C.S.; Doussot, A.; Shin, P.; Goldman, D.A.; Gonen, M.; Allen, P.J.; et al. Genetic Determinants of Outcome in Intrahepatic Cholangiocarcinoma. Hepatology 2021, 74, 1429–1444. [Google Scholar] [CrossRef]
- Ohba, A.; Morizane, C.; Ueno, M.; Kobayashi, S.; Kawamoto, Y.; Komatsu, Y.; Ikeda, M.; Sasaki, M.; Okano, N.; Furuse, J.; et al. Multicenter phase II trial of trastuzumab deruxtecan for HER2-positive unresectable or recurrent biliary tract cancer: HERB trial. Futur. Oncol. 2022, 18, 2351–2360. [Google Scholar] [CrossRef]
- Available online: https://www.cbioportal.org/ (accessed on 21 August 2022).
- Bekaii-Saab, T.S.; Spira, A.I.; Yaeger, R.; Buchschacher, G.L.; McRee, A.J.; Sabari, J.K.; Johnson, M.L.; Barve, M.A.; Hafez, N.; Velastegui, K.; et al. KRYSTAL-1: Updated activity and safety of adagrasib (MRTX849) in patients (Pts) with unresectable or metastatic pancreatic cancer (PDAC) and other gastrointestinal (GI) tumors harboring a KRAS(G12C) mutation. J. Clin. Oncol. 2022, 40. [Google Scholar] [CrossRef]
- Kalia, S.S.; Adelman, K.; Bale, S.J.; Chung, W.K.; Eng, C.; Evans, J.P.; Herman, G.E.; Hufnagel, S.B.; Klein, T.E.; Korf, B.R.; et al. Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): A policy statement of the American College of Medical Genetics and Genomics. Genet. Med. 2017, 19, 249–255. [Google Scholar] [CrossRef]
- Miller, D.T.; Lee, K.; Chung, W.K.; Gordon, A.S.; Herman, G.E.; Klein, T.E.; Stewart, D.R.; Amendola, L.M.; Adelman, K.; Bale, S.J.; et al. ACMG SF v3.0 list for reporting of secondary findings in clinical exome and genome sequencing: A policy statement of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2021, 23, 1381–1390. [Google Scholar] [CrossRef]
- Mandelker, D.; Donoghue, M.; Talukdar, S.; Bandlamudi, C.; Srinivasan, P.; Vivek, M.; Jezdic, S.; Hanson, H.; Snape, K.; Kulkarni, A.; et al. Germline-focussed analysis of tumour-only sequencing: Recommendations from the ESMO Precision Medicine Working Group. Ann. Oncol. 2019, 30, 1221–1231. [Google Scholar] [CrossRef]
- Kondo, T.; Yamamoto, Y.; Fukuyama, K.; Kanai, M.; Yamada, A.; Matsubara, J.; Quy, P.N.; Yoshioka, M.; Yamada, T.; Minamiguchi, S.; et al. Germline sequencing for presumed germline pathogenic variants via tumor-only comprehensive genomic profiling. Int. J. Clin. Oncol. 2022, 27, 1256–1263. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Fukuyama, K.; Kanai, M.; Kondo, T.; Yoshioka, M.; Kou, T.; Quy, P.N.; Kimura-Tsuchiya, R.; Yamada, T.; Matsumoto, S.; et al. Prevalence of pathogenic germline variants in the circulating tumor DNA testing. Int. J. Clin. Oncol. 2022. [Google Scholar] [CrossRef]
- Sunami, K.; Naito, Y.; Komine, K.; Amano, T.; Ennishi, D.; Imai, M.; Kage, H.; Kanai, M.; Kenmotsu, H.; Koyama, T.; et al. Chronological improvement in precision oncology implementation in Japan. Cancer Sci. 2022. [Google Scholar] [CrossRef]
| OncoGuide NCC™Oncopanel System | FoundationOne®CDx | FoundationOne®CDx Liquid | |
|---|---|---|---|
| covered genes | 124 | 324 | 324 |
| platform | tissue and blood paired panel | tissue-only panel | blood-based panel |
| Results of MSI-H | available for clinical use | not available for clinical use | |
| Results of TMB-H | |||
| Results of CNV | |||
| Secondary findings | no additional testing is required | confirmatory germline testing is required | |
| Reimbursement cost | 560,000 JPY | ||
| Approval date for reimbursement | June 2019 | August 2021 | |
| Matched Drug | ORR | OS | FDA Approval | MHLW Approval | Prevalence in Biliary Tract Cancer | |
|---|---|---|---|---|---|---|
| MSI-H | Pembrolizumab | 40.9% (9/20) [51] | 24.3 months [51] | Yes | Yes | 1.6% (23/1017) [52] |
| NTRK fusion | Entrectinib Larotrectinib | 79% (121/153) [53] | not reached [53] | Yes | Yes | 0.15% (6/3905) [54] |
| TMB-H | Pembrolizumab | 29% (30/102) [55] | 11.7 months [55] | Yes | Yes | 3.4% (6/177) [56] |
| FGFR2 fusion/ rearrangement | Pemigatinib | 35.5% (38/107) [57] | 21.1 months [57] | Yes | Yes | intrahepatic 7.4% (20/272) extrahepatic 2.0% (3/151) [58] |
| BRAF V600E | Dabrafenib+ Trametinib | 46.5% (20/43) [59] | 14 months [59] | Yes | No | intrahepatic 3% (5/159) extrahepatic 0% (0/218) [60] |
| IDH1 mutaion | Ivosidenib | 2% (3/124) [61] | 10.8 months [61] | Yes | No | intrahepatic 13% (552/4214) extrahepatic 0.8% (9/1123) [62] |
| HER2 overexpression | Pertuzumab+ Trasutuzumab | 23% (9/39) [63] | 10.9 months [63] | No | No | intrahepatic 3% (7/224) extrahepatic 14% (14/97) [64] |
| RET fusion | Pralsetinib | 57% (13/23) [65] | 14 months [65] | No | No | very low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanai, M. Current Clinical Practice of Precision Medicine Using Comprehensive Genomic Profiling Tests in Biliary Tract Cancer in Japan. Curr. Oncol. 2022, 29, 7272-7284. https://doi.org/10.3390/curroncol29100573
Kanai M. Current Clinical Practice of Precision Medicine Using Comprehensive Genomic Profiling Tests in Biliary Tract Cancer in Japan. Current Oncology. 2022; 29(10):7272-7284. https://doi.org/10.3390/curroncol29100573
Chicago/Turabian StyleKanai, Masashi. 2022. "Current Clinical Practice of Precision Medicine Using Comprehensive Genomic Profiling Tests in Biliary Tract Cancer in Japan" Current Oncology 29, no. 10: 7272-7284. https://doi.org/10.3390/curroncol29100573
APA StyleKanai, M. (2022). Current Clinical Practice of Precision Medicine Using Comprehensive Genomic Profiling Tests in Biliary Tract Cancer in Japan. Current Oncology, 29(10), 7272-7284. https://doi.org/10.3390/curroncol29100573




